The Sesquiterpenes β-Caryophyllene and Caryophyllene Oxide Isolated from Senecio salignus Act as Phytogrowth and Photosynthesis Inhibitors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sesquiterpene Isolation and ATP Synthesis Determination
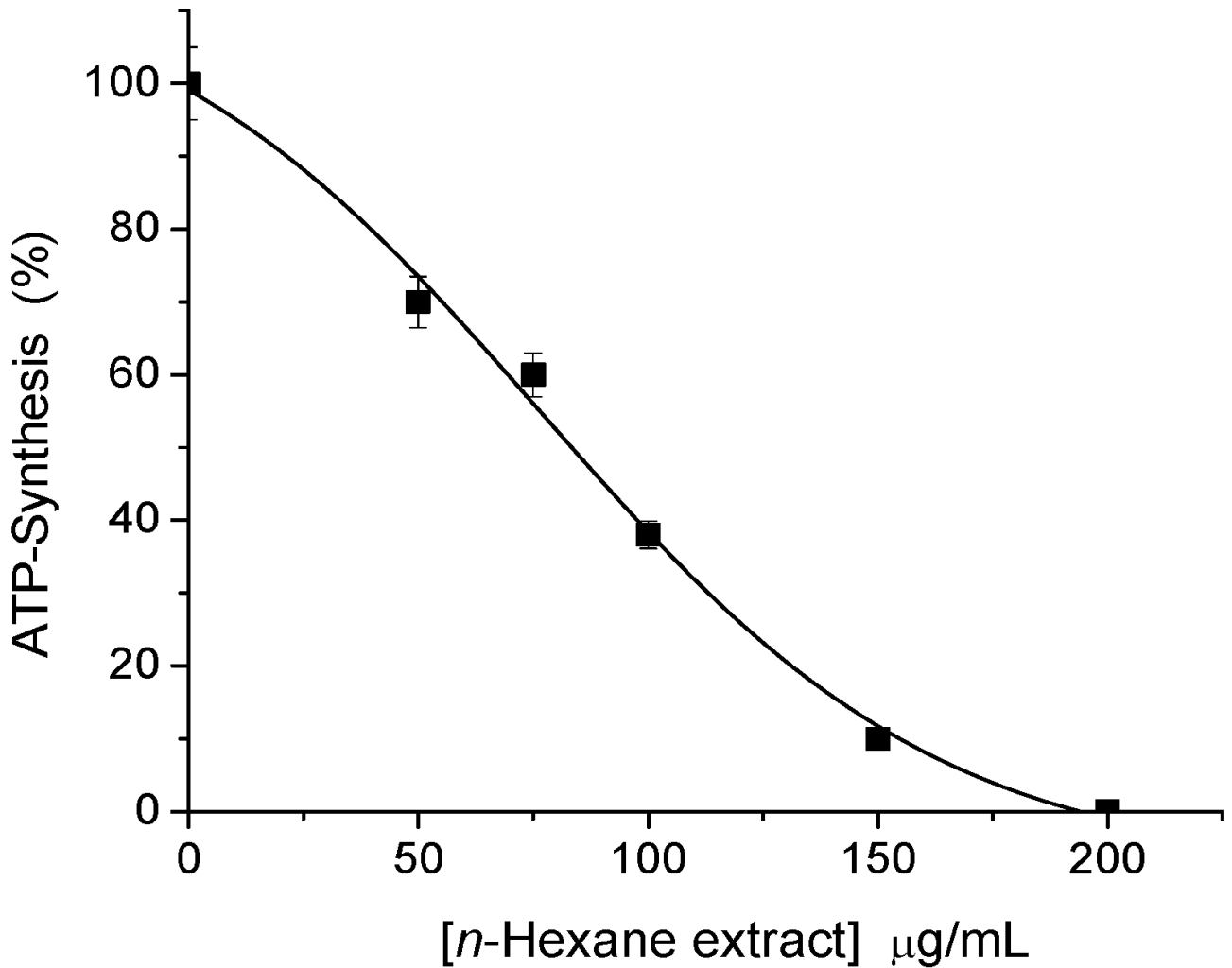
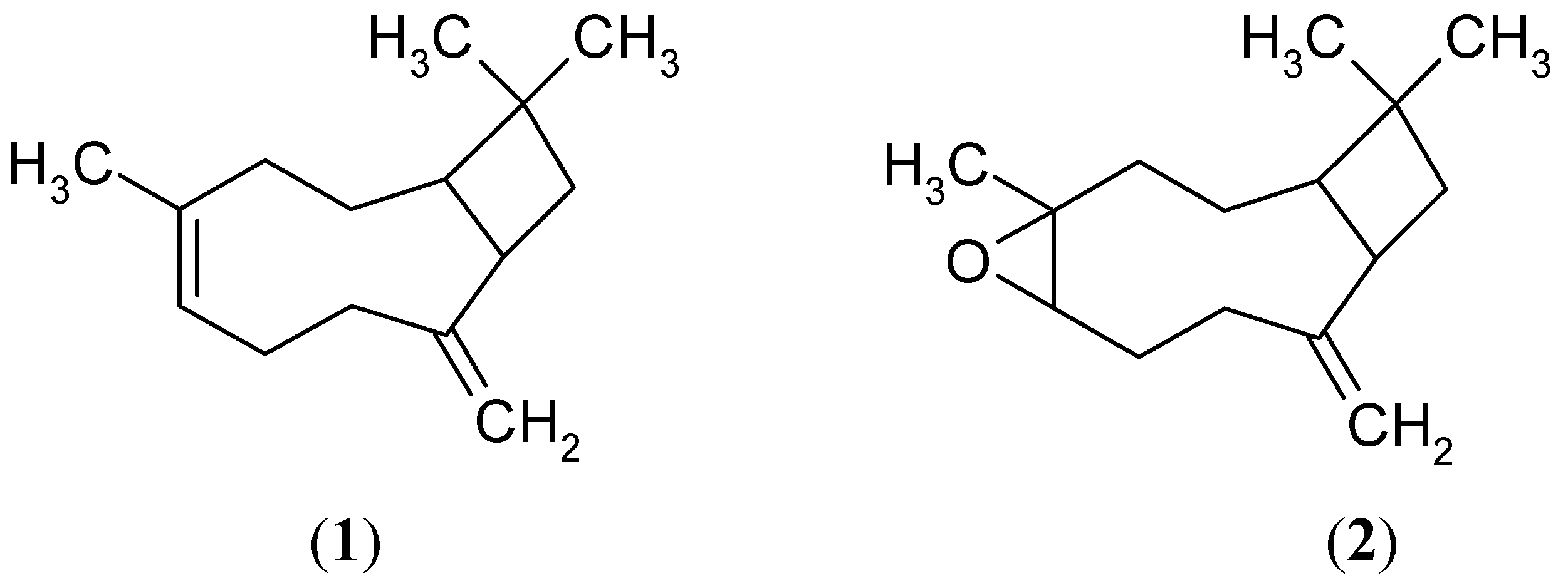
2.2. Effects of 1 and 2 on Germination and Root and Stem Growth of E. cruss-galli and P. ixocarpa
| Conc. [µg/mL] | Root Elongation (%) p | Shoot Elongation (%) p | Germination (%) | ||
|---|---|---|---|---|---|
| 50 | 42 | 0.0046 | 16 | 0.075 | 0 |
| 100 | 44 | 6.04 × 10−4 | 18 | 0.06 | 0 |
| 150 | 53 | 1.94 × 10−4 | 19 | 0.064 | 4 |
2.3. Postemergence Activity of 1 and 2 on Biomass Production of P. ixocarpa and L. perenne Plants
| P. ixocarpa | L. perenne | |||
|---|---|---|---|---|
| Comp / Conc. | G | % | g | % |
| Control | 0.7203 ± 0.184 | 100 | 1.068 ± 0.031 | 100 |
| DCMU | 0.533 ± 0.053 | 74 | 0.897 ± 0.054 | 84 |
| (1) / [µg/mL] | ||||
| 25 | 0.663 ± 0.03 | 92 | 1.107 ± 0.142 | 104 |
| 50 | 0.575 ± 0.021 | 80 | 0.879 ± 0.132 | 82 |
| 100 | 0.45 ± 0.104 | 63 | 0.908 ± 0.197 | 85 |
| (2) / [µg/mL] | ||||
| 25 | 0.685 ± 0.055 | 95 | 0.953 ± 0.111 | 89 |
| 50 | 0.654 ± 0.065 | 91 | 0.962 ± 0.117 | 90 |
| 100 | 0.657 ± 0.02 | 78 | 1.02 ± 0.111 | 96 |

2.4. Localization of the Target of 1 on Photosynthetic Electron Transport Chain Studied by the Fluorescence of Chl a in Vivo Technique on Leaves of P. ixocarpa and L. perenne Plants
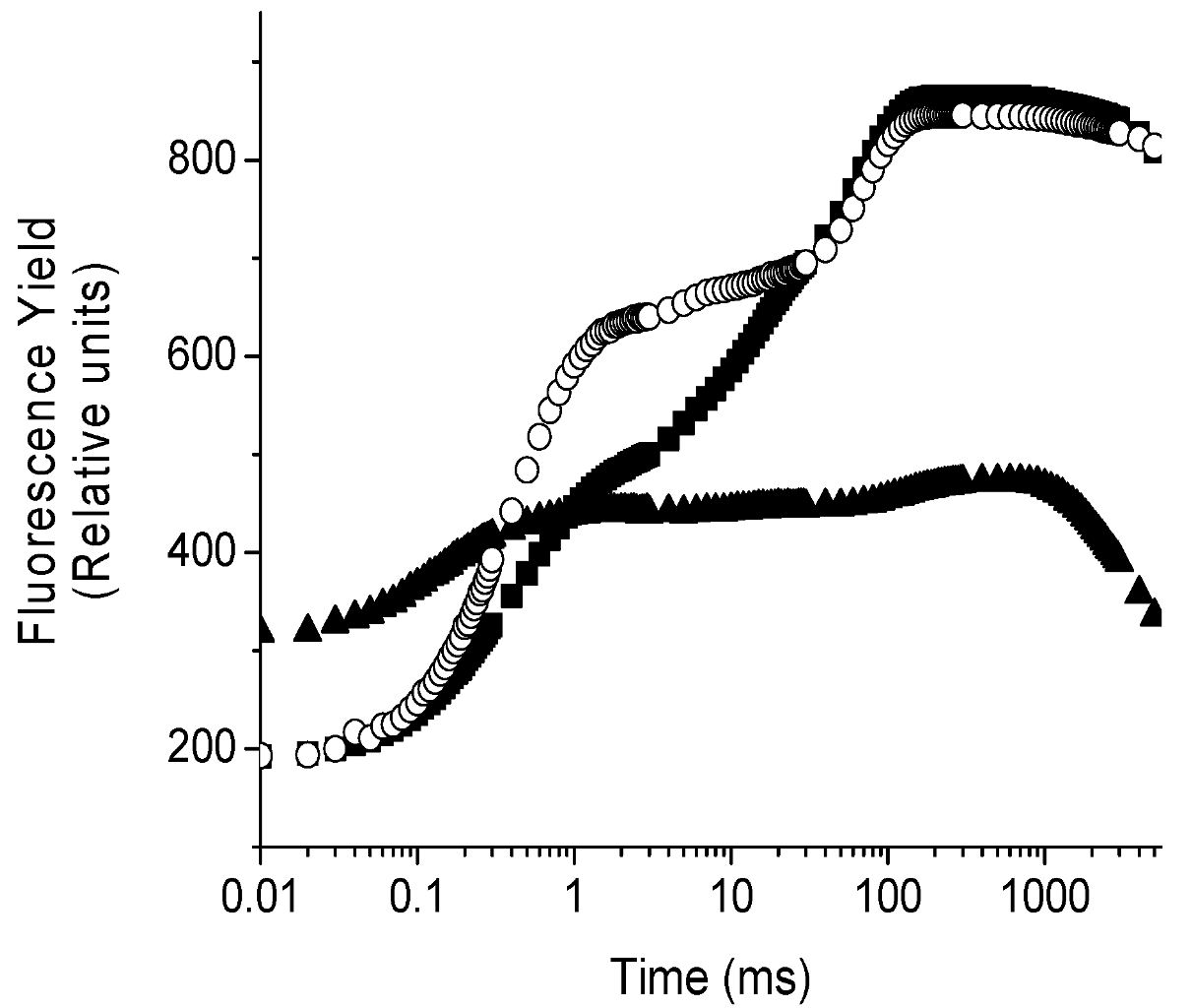
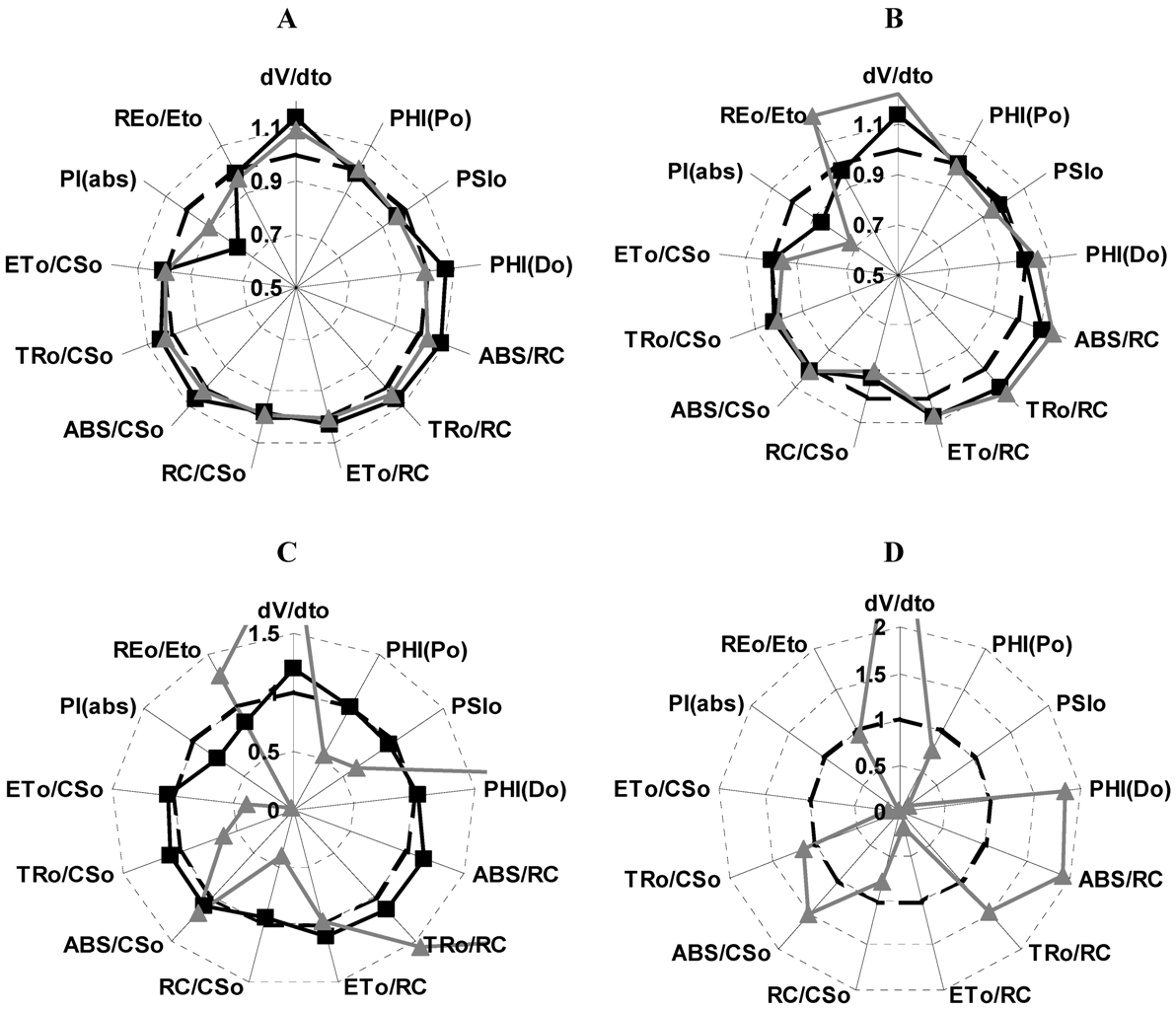
| 24 h | 48 h | 72 h | ||||
|---|---|---|---|---|---|---|
| (1) [µg/mL] | ABS/RC | Active RC (%) | ABS/RC | Active RC (%) | ABS/RC | Active RC (%) |
| 0 | 1.91 ± 0.19 | 100 | 2.053 ± 0.25 | 100 | 2.282 ± 0.27 | 100 |
| 25 | 1.736 ± 0.11 | 110 | 1.992 ± 0.14 | 103 | 1.888 ± 0.08 | 120 |
| 50 | 2.09 ± 0.22 | 91 | 2.273 ± 0.13 | 90 | 2.66 ± 0.5 | 86 |
| 100 | 1.98 ± .25 | 96 | 3.323 ± 1.49 | 62 | 16.13 ± 3.4 | 14 |
3. Experimental
3.1. General
3.1.1. Reagents
3.1.2. Methods
3.1.2.1. Tested Material
3.1.2.2. Chloroplast Isolation and Chlorophyll Determination
3.1.2.3. Determination of ATP Synthesis
3.1.2.4. Chlorophyll a Fluorescence of PS II
3.1.2.5. Seed Germination Bioassays
3.1.2.6. Plant Material for in Vivo Assays
4. Conclusions
Acknowledgments
References and Notes
- Romo de Vivar, A.; Pérez-Castorena, A.-L.; Arciniegas, A.; Villaseñor, J.L. Secondary metabolites from Mexican species of the Tribe senecioneae (Asteraceae). J. Mex. Chem. Soc. 2007, 51, 160–172. [Google Scholar]
- Rodríguez, J.; Tello, H.; Quijano, L.; Calderón, J.; Gómez, F.; Romo, J.; Ríos, T. Flavonoids of Mexican plants, isolation and structure of santin and glycoferide. Rev. Latinoamer. Quim. 1974, 5, 41–53. [Google Scholar]
- Bohlmann, F.; Zdero, C. Naturally occurring terpene derivatives, LVI. New furoeremophilanes from Mexican Senecio species. Chem. Ber. 1976, 109, 819–825. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zdero, C.; Jakupovic, J.; Grenz, M.; Castro, V.; Kino, R.M.; Robinson, H.; Vincent, L.P.D. Further pyrrolizidine alkaloids and furoeremophilanes from Senecio species. Phytochemistry 1986, 25, 1151–1159. [Google Scholar] [CrossRef]
- Rzedowski, G.C.; de Rzedowski, J. Flora fanerogámica del valle de México. In Comisión nacional para el conocimiento y uso de la biodiversidad, 2nd ed; Instituto de ecologia, A.C. y: Michoacán, Mexico, 2005; p. 1406. [Google Scholar]
- Arrhenius, J.H.; Langenheim, L. Sesquiterpenes in leaf pocket resins of Capaifera species. Phytochemistry 1983, 22, 471–472. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Co.: Carol Stream, IL, USA, 1995; pp. 94–358. [Google Scholar]
- Wang, R.-L.; Staehelin, C.; Peng, S.-L.; Wang, W.-T.; Xie, X.-M.; Lu, H.-N. Responses of Mikania micrantha, an invasive weed to elevated CO2: Induction of β-caryophyllene synthase, changes in emission capability and allelopathic potential of β-caryophyllene. J. Chem. Ecol. 2010, 36, 1076–1082. [Google Scholar] [CrossRef]
- Perez-Vasquez, A.; Linares, E.; Bye, R.; Cerda-Garcia-Rojas, C.M.; Mata, R. Phytotoxic activity and conformational analysis of thymol analogs from Hofmeisteria schaffneri. Phytochemistry 2008, 69, 1339–1347. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Govindjee. Polyphasic chlorophyll a fluorescence transients in plants and cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of chlorophyll a fluorescence transient. In Chlorophyll Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; Chapter 12, pp. 321–362. [Google Scholar]
- Aguilar, M.I.; Romero, M.G.; Chávez, M.I.; King-Díaz, B.; Lotina-Hennsen, B. Biflavonoids isolated from Selaginella lepidophylla inhibit photosynthesis in spinach chloroplasts. J. Agric. Food Chem. 2008, 56, 6994–7000. [Google Scholar] [CrossRef]
- Mills, J.D.; Mitchell, P.; Schurrmann, P. Modulation of coupling ATPase activity in intact chloroplasts. FEBS Lett. 1980, 191, 144–148. [Google Scholar]
- Torres-Romero, D.; King-Díaz, B.; Strasser, R.J.; Jiménez, I.A.; Lotina-Hennsen, B.; Bazzocchi, I.L. Friedelane triterpenes from Celastrus vulcanicola as photosynthetic inhibitors. J. Agric. Food Chem. 2010, 58, 10847–10854. [Google Scholar] [CrossRef]
- Strain, H.H.; Cope, B.T.; Svec, W.A. Analytical procedures for the isolation, identification, estimation and investigation of the chlorophylls. Meth. Enzymol. 1971, 23, 452–466. [Google Scholar] [CrossRef]
- Dilley, R.A. Ion transport (H+, K+, Mg2+ exchange phenomena). Methods Enzymol. 1972, 24, 68–74. [Google Scholar] [CrossRef]
- Panda, D.; Rao, D.N.; Sharma, S.G.; Strasser, R.J.; Sarkar, R.K. Subemergence effects on rice genotypes during seedling stage: Probing of subemergence driven changes of photosystem II by chlorophyll a fluorescence induction O-J-I-P transients. Photosynthetica 2006, 44, 69–75. [Google Scholar] [CrossRef]
- Céspedes, C.L.; Calderón, J.S.; Salazar, J.R.; Lotina-Hennsen, B.; Segura, R. Plant-growth inhibitory activity of cedrelanolide from Cedrela salvadorensis. J. Chem. Ecol. 2001, 27, 137–149. [Google Scholar] [CrossRef]
- González-Ibarra, M.; Farfán, N.; Trejo, C.; Uribe, S.; Lotina-Hennsen, B. Selective herbicide activity of 2,5-di(benzylamine)-p-benzoquinone against the monocot weed Echinochloa crus-galli. An in vivo analysis of photosynthesis and growth. J. Agric. Food Chem. 2005, 53, 3415–3420. [Google Scholar]
- Dicke, M.; Sabelis, M.W.; Takabayashi, J.; Bruin, J.; Posthumus, M.A. Plant strategies of manipulating predator prey interactions through allelochemicals: Prospects for application in pest control. J. Chem. Ecol. 1990, 16, 3091–3118. [Google Scholar] [CrossRef]
- Duke, S.O. Inderjit. Ecophysiological aspects of allelopathy. Planta 2003, 217, 529–539. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kohli, R.K. Allelopathic interactions and allelochemicals new possibilities for sustainable weed management. Crit. Rev. Plant Sci. 2003, 22, 239–311. [Google Scholar] [CrossRef]
- Kegge, W.; Pierik, R. Biogenic volatile organic compounds and plant competition. Trends Plant Sci. 2010, 15, 126–132. [Google Scholar]
- Kil, B.S.; Han, D.M.; Lee, C.H.; Kim, Y.S.; Yun, K.Y.; Yoo, H.G. Allelopathic effects of Artemisia lavandulaefolia. Korean J. Ecol. 2000, 23, 149–155. [Google Scholar]
- Wang, R.L.; Peng, S.L.; Zeng, R.S.; Ding, L.W.; Xu, Z.F. Cloning, expression and wounding induction of β-caryophyllene synthase gene from Mikania micrantha H. B. K. and allelopathic potential of β-caryophyllene. Allelopathy J. 2009, 24, 35–44. [Google Scholar]
- Sample Availability: Not available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sánchez-Muñoz, B.A.; Aguilar, M.I.; King-Díaz, B.; Rivero, J.F.; Lotina-Hennsen, B. The Sesquiterpenes β-Caryophyllene and Caryophyllene Oxide Isolated from Senecio salignus Act as Phytogrowth and Photosynthesis Inhibitors. Molecules 2012, 17, 1437-1447. https://doi.org/10.3390/molecules17021437
Sánchez-Muñoz BA, Aguilar MI, King-Díaz B, Rivero JF, Lotina-Hennsen B. The Sesquiterpenes β-Caryophyllene and Caryophyllene Oxide Isolated from Senecio salignus Act as Phytogrowth and Photosynthesis Inhibitors. Molecules. 2012; 17(2):1437-1447. https://doi.org/10.3390/molecules17021437
Chicago/Turabian StyleSánchez-Muñoz, B. Arturo, Maria Isabel Aguilar, Beatriz King-Díaz, José Fausto Rivero, and Blas Lotina-Hennsen. 2012. "The Sesquiterpenes β-Caryophyllene and Caryophyllene Oxide Isolated from Senecio salignus Act as Phytogrowth and Photosynthesis Inhibitors" Molecules 17, no. 2: 1437-1447. https://doi.org/10.3390/molecules17021437
APA StyleSánchez-Muñoz, B. A., Aguilar, M. I., King-Díaz, B., Rivero, J. F., & Lotina-Hennsen, B. (2012). The Sesquiterpenes β-Caryophyllene and Caryophyllene Oxide Isolated from Senecio salignus Act as Phytogrowth and Photosynthesis Inhibitors. Molecules, 17(2), 1437-1447. https://doi.org/10.3390/molecules17021437




