Microwave-Assisted Synthesis of New Substituted Anilides of Quinaldic Acid †
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
| Comp. | Solvent | Catalyst | Conversion after 0.5 h | Conversion after 1 h | Conversion after 2 h | |||
| Amide 5c or 6 | Product 7 or 8 | Amide 5c or 6 | Product 7 or 8 | Amide 5c or 6 | Product 7 or 8 | |||
| 1a | – | – | 57% | 37% | –a | – a | –a | –a |
| 4a | – | – | – | – | – | – | 9% | – |
| 1b | – | – | 6% | – | 21% | – | 51% | – |
| 4b | – | – | – | – | – | – | – | – |
| 1c | – | – | 96% | – | 100% | – | –e | –e |
| 1a | DMF | – | – | – | – | – | – | – |
| 4a | DMF | – | – | – | – | – | – | – |
| 1b | DMF | – | – | – | – | – | – | – |
| 4b | DMF | – | – | – | – | – | – | – |
| 1a | PhCl | – | – | – | – | – | – | – |
| 4a | PhCl | – | – | – | – | – | 7% | – |
| 1b | PhCl | – | – | – | – | – | – | – |
| 4b | PhCl | – | – | – | – | – | – | – |
| 1a | – | PTSA | 53% | 46% | 55% | 44% | 33% b | 67% b |
| 4a | – | PTSA | 20% | – | 46% | – | 100% a | – a |
| 1b | – | PTSA | 35% | – | – a | – a | –a | –a |
| 4b | – | PTSA | – | – | – | – | – | – |
| 1a | DMF | PTSA | –c | 100% | –c | 100% | –c | 100% |
| 1b | DMF | PTSA | – | – | – | – | –c | – |
| 4b | DMF | PTSA | – | – | – | – | – | – |
| 1a | PhCl | PTSA | 19% | 24% | 28% | 25% | 32% | 30% |
| 4a | PhCl | PTSA | – | – | – | – | – | – |
| 1b | PhCl | PTSA | 20% | – | 25% | – | 34% | – |
| 4b | PhCl | PTSA | – | – | – | – | – | – |
| 1a | – | tBuOK | 59% | 41% | 61% | 39% | 62% | 38% |
| 4a | – | tBuOK | 11% | – | 20% | – | 44% | – |
| 1b | – | tBuOK | 26% | 74% | 43% a | 56% a | –a | –a |
| 4b | – | tBuOK | – | – | – | – | – | – |
| 1a | DMF | tBuOK | – | 100% | – | 100% | – | 100% |
| 4a | DMF | tBuOK | – | – | – | – | – | – |
| 1b | DMF | tBuOK | – c | – | –c | – | –c | – |
| 4b | DMF | tBuOK | – | – | – | – | – | – |
| 1a | PhCl | tBuOK | 13% | 27% | 30% | 34% | 35% | 41% |
| 4a | PhCl | tBuOK | – | – | – | – | – | – |
| 1b | PhCl | tBuOK | 17% | – | 18% | – | 16% b | – |
| 4b | PhCl | tBuOK | – | – | – | – | – | – |
| 1a | – | silica gel | 63% | 37% | 17% | 83% | 53% | 47% |
| 4a | – | silica gel | – | – | 22% | – | 100% d | – |
| 1b | – | silica gel | 11% | – | 30% | – | 19% | 19% |
| 4b | – | silica gel | – | – | – | – | – | – |
| 1a | DMF | silica gel | – c | – | – c | – | –c | – |
| 4a | DMF | silica gel | – | – | – | – | – | – |
| 1b | DMF | silica gel | – | – | – | – | –c | – |
| 4b | DMF | silica gel | – | – | – | – | – | – |
| 1a | PhCl | silica gel | –c | 26% | 9% | 23% | 68% | 8% |
| 4a | PhCl | silica gel | – | – | – | – | – | – |
| 1b | PhCl | silica gel | – | – | 6% | – | 9% | – |
| 4b | PhCl | silica gel | – | – | – | – | – | – |
| 1a | – | KF/Al2O3 | – | – | – | – | 90% | 8% |
| 4a | – | KF/Al2O3 | – | – | –c | – | –c | – |
| 1b | – | KF/Al2O3 | 10% | – | 18% | – | 35% | – |
| 4b | – | KF/Al2O3 | – | – | – | – | – | – |
| 1a | DMF | KF/Al2O3 | – | 73% | – | 89% | – | 98% |
| 4a | DMF | KF/Al2O3 | – | – | – | – | – | – |
| 1b | DMF | KF/Al2O3 | – | – | – | – | – | – |
| 4b | DMF | KF/Al2O3 | – | – | – | – | – | – |
| 1a | PhCl | KF/Al2O3 | – | – | 7% | 24% | 20% | 20% |
| 4a | PhCl | KF/Al2O3 | – | – | – | – | – | – |
| 1b | PhCl | KF/Al2O3 | – | – | – | – | –c | – |
| 4b | PhCl | KF/Al2O3 | – | – | – | – | – | – |
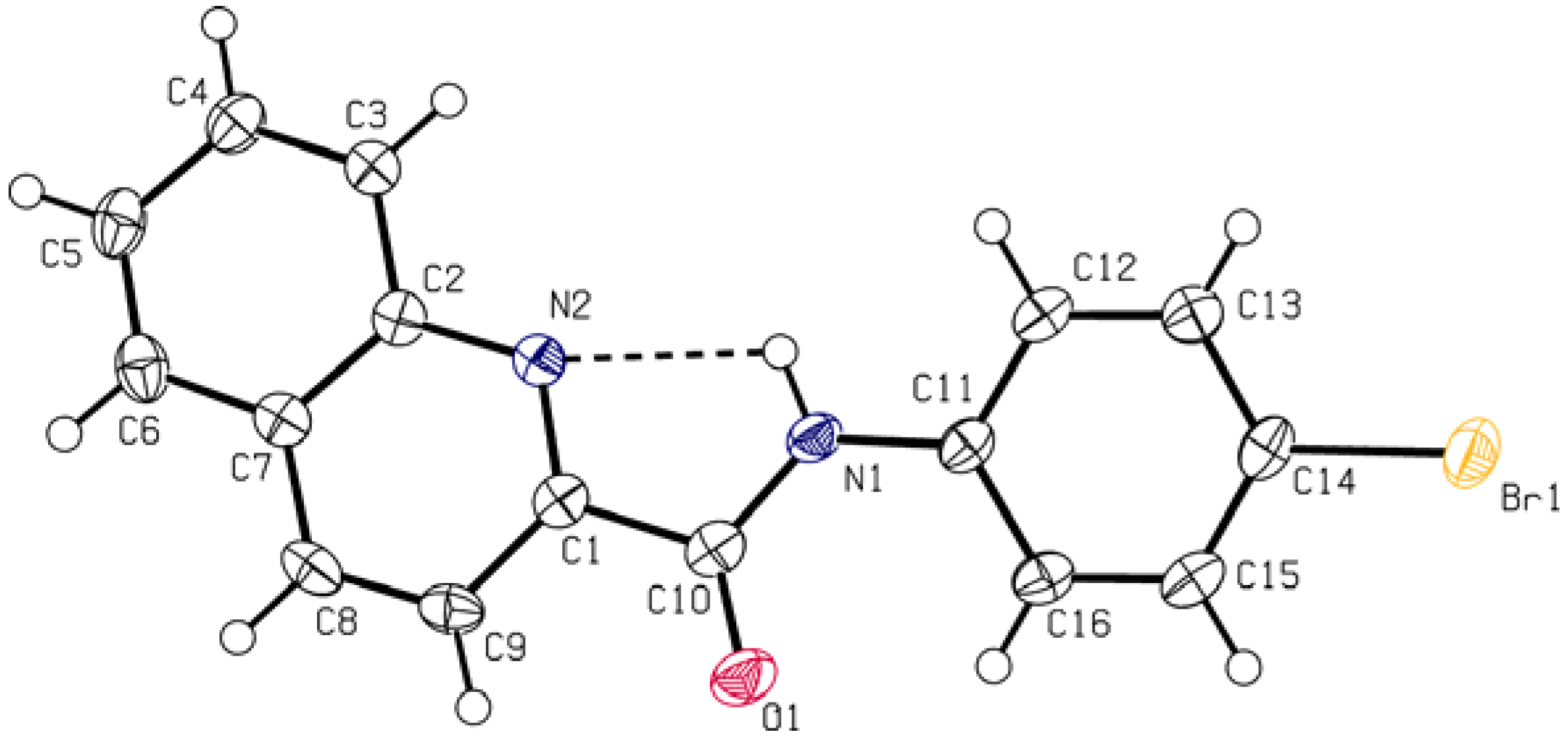
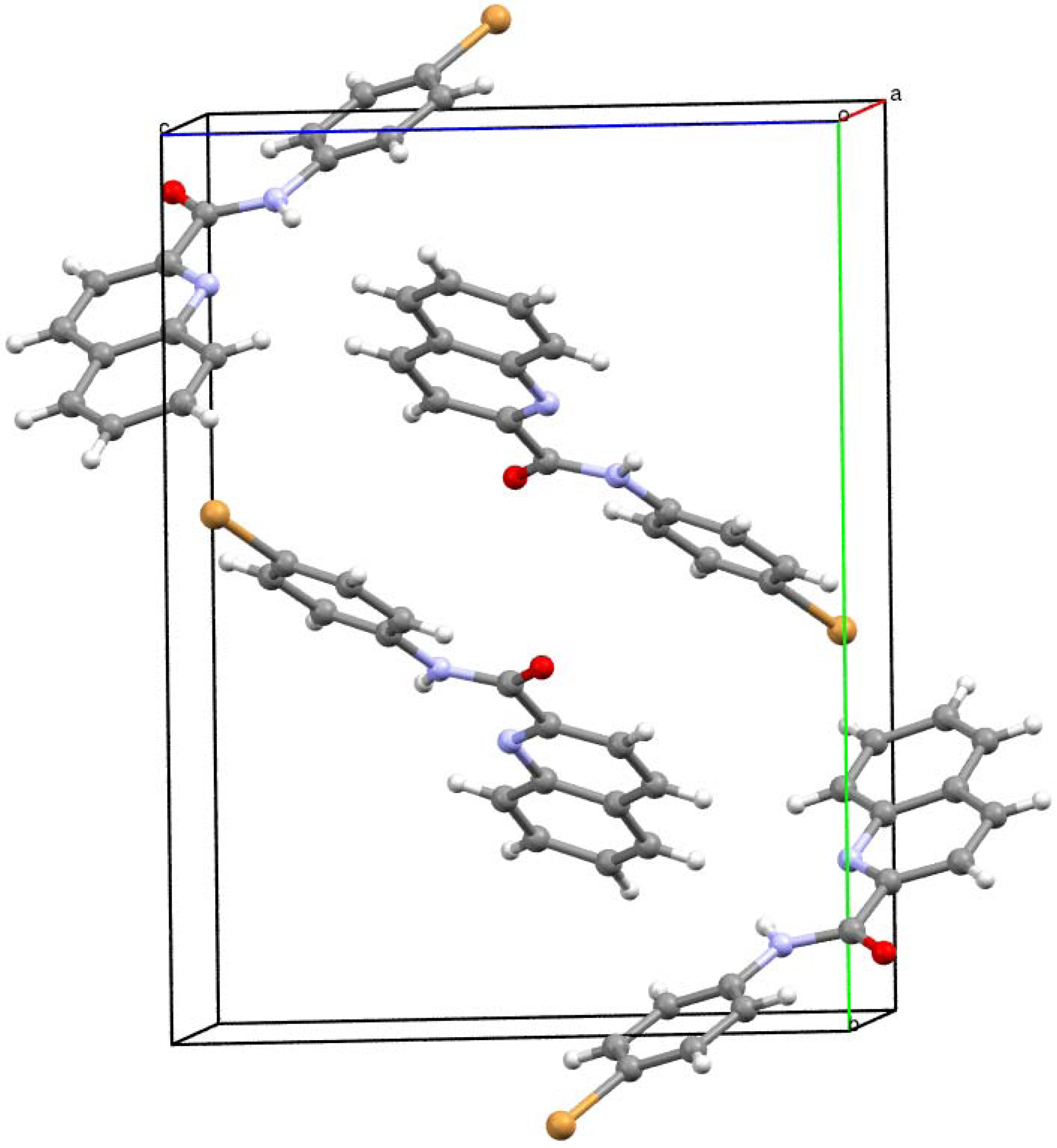
2.2. Crystallography
3. Experimental Section
3.1. General
3.2. Synthesis
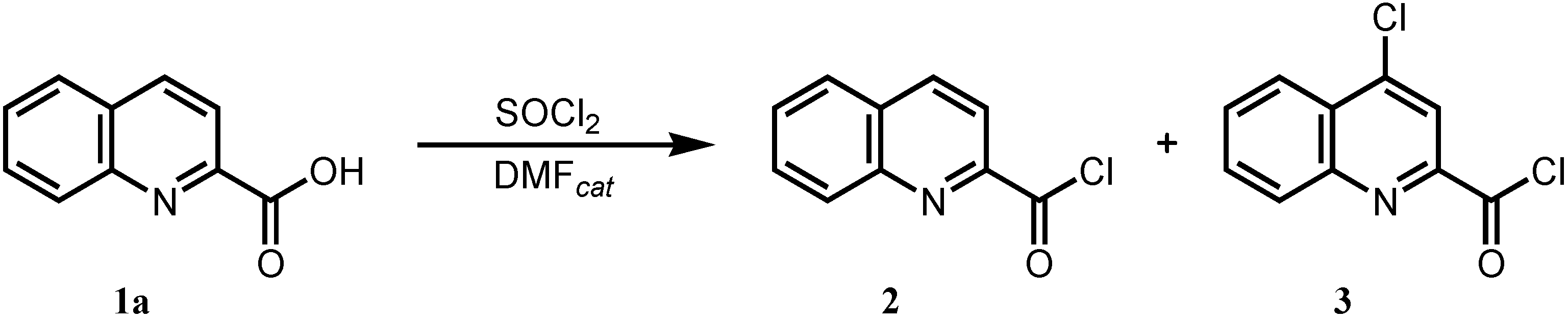
3.2.1. Procedure for Classical Synthesis of Ring-substituted Quinoline- and 4-chloroquinoline-2-carboxanilides
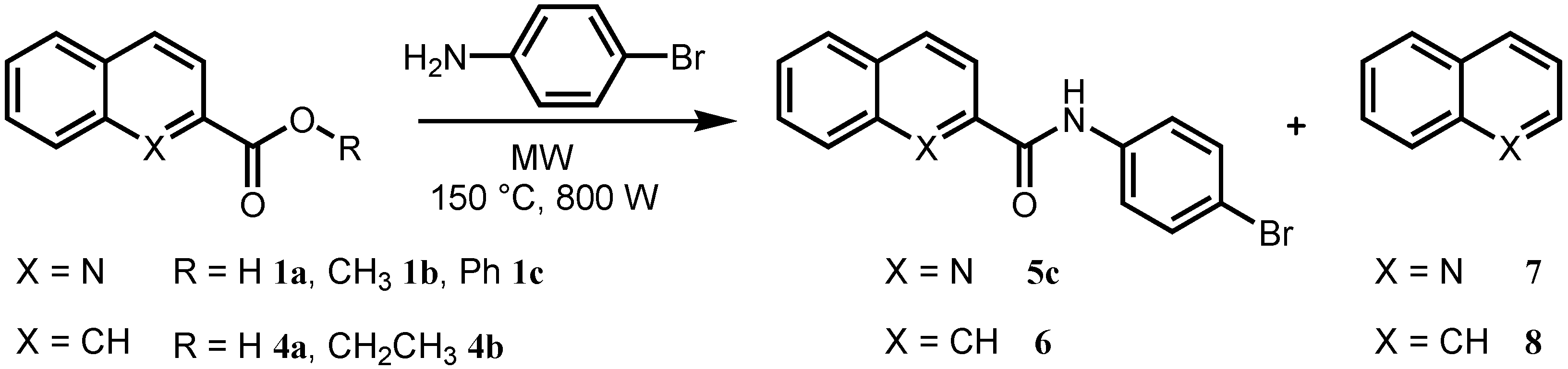
3.2.2. Procedure for the Optimization of Microwave-assisted Synthesis
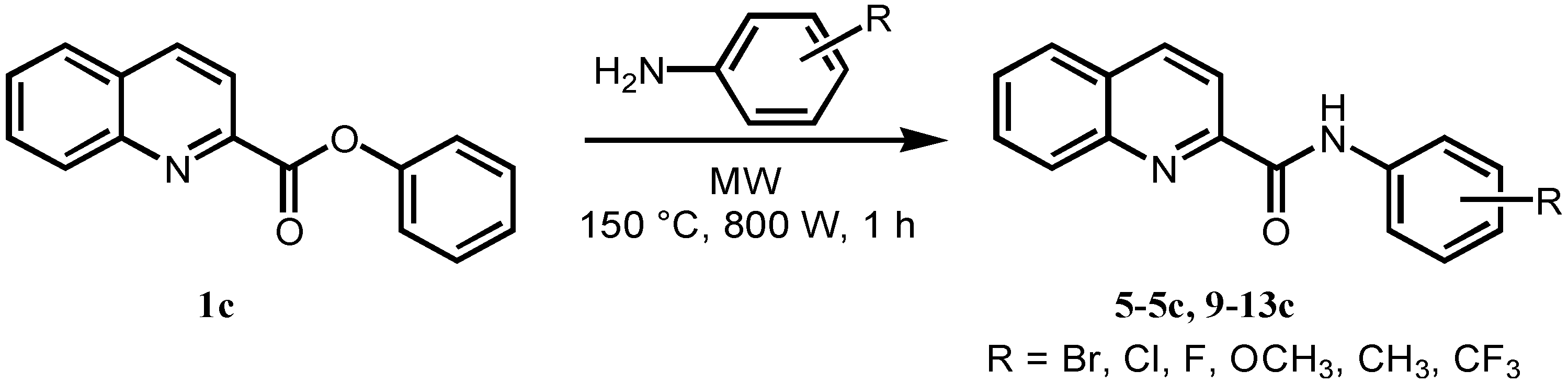
3.2.3. General Procedure for Microwave-assisted Synthesis of Ring-substituted Quinoline-2-carboxanilides
3.3. Crystallography
4. Conclusions
Supplementary Materials
Acknowledgements
- Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- Kharkar, P.S.; Deodhar, M.N.; Kulkarni, V.M. Design, synthesis, antifungal activity, and ADME prediction of functional analogues of terbinafine. Med. Chem. Res. 2009, 18, 421–432. [Google Scholar]
- Musiol, R.; Jampilek, J.; Buchta, V.; Niedbala, H.; Podeszwa, B.; Palka, A.; Majerz-Maniecka, K.; Oleksyn, B.; Polanski, J. Antifungal properties of new series of quinoline derivatives. Bioorg. Med. Chem. 2006, 14, 3592–3598. [Google Scholar] [CrossRef]
- Musiol, R.; Serda, M.; Hensel-Bielowka, S.; Polanski, J. Quinoline-based antifungals. Curr. Med. Chem. 2010, 17, 1960–1973. [Google Scholar]
- Nakamoto, K.; Tsukada, I.; Tanaka, K.; Matsukura, M.; Haneda, T.; Inoue, S.; Murai, N.; Abe, S.; Ueda, N.; Miyazaki, M.; et al. Synthesis and evaluation of novel antifungal agents-quinoline and pyridine amide derivatives. Bioorg. Med. Chem. Lett. 2010, 20, 4624–4626. [Google Scholar]
- Oliva, B.; Miller, K.; Caggiano, N.; O’Neill, A.J.; Cuny, G.D.; Hoemarm, M.Z.; Hauske, J.R.; Chopra, I. Biological properties of novel antistaphylococcal quinoline-indole agents. Antimicrob. Agents Chemother. 2003, 47, 458–466. [Google Scholar]
- Upadhayaya, R.S.; Vandavasi, J.K.; Kardile, R.A.; Lahore, S.V.; Dixit, S.S.; Deokar, H.S.; Shinde, P.D.; Sarmah, M.P.; Chattopadhyaya, J. Novel quinoline and naphthalene derivatives as potent antimycobacterial agents. Eur. J. Med. Chem. 2010, 45, 1854–1867. [Google Scholar]
- Jampilek, J.; Musiol, R.; Pesko, M.; Kralova, K.; Vejsova, M.; Carroll, J.; Coffey, A.; Finster, J.; Tabak, D.; Niedbala, H.; et al. Ring-substituted 4-hydroxy-1H-quinolin-2-ones: Preparation and biological activity. Molecules 2009, 14, 1145–1159. [Google Scholar]
- Vaillancourt, V.A.; Cudahy, M.M.; Staley, S.A.; Brideau, R.J.; Conrad, S.J.; Knechtel, M.L.; Oien, N.L.; Wieber, J.L.; Yagi, Y.; Wathen, M.W. Naphthalene carboxamides as inhibitors of human cytomegalovirus DNA polymerization. Bioorg. Med. Chem. Lett. 2000, 10, 2079–2081. [Google Scholar]
- Brideau, R.J.; Knechtel, M.L.; Huang, A.; Vaillancourt, V.A.; Vera, E.E.; Oien, N.L.; Hopkins, T.A.; Wieber, J.L.; Wilkinson, K.F.; Rush, B.D.; et al. Broad-spectrum antiviral activity of PNU-183792, a 4-oxo-dihydroquinoline, against human and animal herpesviruses. Antivir. Res. 2002, 54, 19–28. [Google Scholar]
- Oien, N.L.; Brideau, R.J.; Hopkins, T.A.; Wieber, J.L.; Knechtel, M.L.; Shelly, J.A.; Anstadt, R.A.; Wells, P.A.; Poorman, R.A.; Huang, A.; et al. Broad-spectrum antiherpes activities of 4-hydroxyquinoline carboxamides, a novel class of herpesvirus polymerase inhibitors. Antimicrob. Agents Chemother. 2002, 46, 724–730. [Google Scholar]
- Podeszwa, B.; Niedbala, H.; Polanski, J.; Musiol, R.; Tabak, D.; Finster, J.; Serafin, K.; Wietrzyk, J.; Boryczka, S.; Mol, W.; et al. Investigating the antiproliferative activity of quinoline-5,8-dione analogues on tumour cell lines. Bioorg. Med. Chem. Lett. 2007, 17, 6138–6141. [Google Scholar]
- Shi, A.; Nguyen, T.A.; Battina, S.K.; Rana, S.; Takemoto, D.J.; Chiang, P.K.; Hua, D.H. Synthesis and anti-breast cancer activities of substituted quinolines. Bioorg. Med. Chem. Lett. 2008, 18, 3364–3368. [Google Scholar]
- Gakhar, G.; Shi, A.; Hua, D.H.; Nguyen, T.A. Antitumor effect of substituted quinolines in breast cancer cells. Drug Dev. Res. 2008, 69, 526–534. [Google Scholar]
- Mrozek-Wilczkiewicz, A.; Kalinowski, D.; Musiol, R.; Finster, J.; Szurko, A.; Serafin, K.; Knas, M.; Kamalapuram, S.K.; Kovacevic, Z.; Jampilek, J.; et al. Investigating anti-proliferative activity of styrylazanaphthalenes and azanaphthalenediones. Bioorg. Med. Chem. 2010, 18, 2664–2671. [Google Scholar]
- Bernzweig, J.; Heiniger, B.; Prasain, K.; Lu, J.; Hua, D.H.; Nguyen, T.A. Anti-breast cancer agents, quinolines, targeting gap junction author. Med. Chem. 2011, 7, 448–453. [Google Scholar]
- Foley, M.; Tilley, L. Quinoline antimalarials: Mechanisms of action and resistance and prospects for new agents. Pharmacol. Ther. 1998, 79, 55–87. [Google Scholar]
- Nakayama, H.; Loiseau, P.M.; Bories, C.; Torres de Ortiz, S.; Schinini, A.; Serna, E.; Rojas de Arias, A.; Fakhfakh, M.A.; Franck, X.; Figadere, B.; et al. Efficacy of orally administered 2-substituted quinolines in experimental murine cutaneous and visceral leishmaniases. Antimicrob. Agents Chemother. 2005, 49, 4950–4956. [Google Scholar]
- Kaur, K.; Jain, M.; Reddy, R.P.; Jain, R. Quinolines and structurally related heterocycles as antimalarials. Eur. J. Med. Chem. 2010, 45, 3245–3264. [Google Scholar]
- Rautio, J.; Kumpulainen, H.; Heimbach, T.; Oliyai, R.; Oh, D.; Jarvinen, T.; Savolainen, J. Prodrugs: Design and clinical applications. Nat. Rev. Drug Discov. 2008, 7, 255–270. [Google Scholar]
- Comprehensive Organic Synthesis; Trost, B.M.; Fleming, I.; Winterfeldt, E. (Eds.) Pergamon Press: Oxford, UK, 1991; Volume 6, pp. 301–399.
- Katritzky, A.R.; Suzuki, K.; Singh, S.K. N-Acylation in combinatorial chemistry. ARKIVOC 2004, i, 12–35. [Google Scholar]
- Lidstrom, P.; Tierney, J.P.; Wathey, B.; Westman, J. Microwave assisted organic synthesis—A review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar]
- Microwave Assisted Organic Synthesis; Tierney, J.P.; Lidstrom, P. (Eds.) Blackwell Publishing: Oxford, UK, 2005.
- de la Hoz, A.; Diaz-Ortiz, A.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar]
- Hayes, B.L. Microwave Synthesis: Chemistry at the Speed of Light; CEM Publishing: Matthews, NC, USA, 2002. [Google Scholar]
- Microwaves in Organic Synthesis; Loupy, A. (Ed.) Wiley-VCH: Weinheim, Germany, 2002.
- Varma, R.S. Advances in Green Chemistry: Chemical Syntheses using Microwave Irradiation; Varma, R.S., Ed.; AstraZeneca Research Foundation, Kavitha Printers: Bangalore, India, 2002. [Google Scholar]
- Bogdal, D. Microwave-assisted Organic Synthesis One Hundred Reaction Procedures; Elsevier: Oxford, UK, 2005. [Google Scholar]
- Bankston, D.; Dumas, J.; Natero, R.; Riedl, B.; Monahan, M.-K.; Sibley, R. A scaleable synthesis of BAY 43-9006: A potent Raf kinase inhibitor for the treatment of cancer. Org. Process. Res. Dev. 2002, 6, 777–781. [Google Scholar] [CrossRef]
- Perreux, L.; Loupy, A.; Volatron, F. Solvent-free preparation of amides from acids and primary amines under microwave irradiation. Tetrahedron 2002, 58, 2155–2162. [Google Scholar]
- Qi, J.Y.; Qiu, L.Q.; Yang, Q.Y.; Zhou, Z.Y.; Chan, A.S.C. N-(4-Iodophenyl)quinoline-2-carboxamide. Acta Crystallogr. E 2003, 59, o104–o105. [Google Scholar]
- Mocilac, P.; Lough, A.J.; Gallagher, J.F. Structures and conformational analysis of a 3 × 3 isomer grid of nine N-(fluorophenyl)pyridinecarboxamides. Cryst. Eng. Comm. 2011, 13, 1899–1999. [Google Scholar]
- Wilson, C.R.; Munro, O.Q. Unconventional hydrogen bonding and π-stacking in two substituted pyridine carboxamides. Acta Crystallogr. C 2010, 66, o513–o516. [Google Scholar] [CrossRef]
- Qi, J.Y.; Yang, Q.Y.; Lam, K.H.; Zhou, Z.Y.; Chan, A.S.C. N-(4-Bromophenyl)pyridine-2-carboxamide. Acta Crystallogr. E 2003, 59, o374–o375. [Google Scholar]
- Zhang, Q.; Zhang, S.P.; Shao, S.C. N-(4-Chlorophenyl)picolinamide. Acta Crystallogr. E 2006, 62, o4695–o4696. [Google Scholar]
- Schaefer, W.; Neubert, P. Mass spectra of heterocyclic carboxylic acid amides. I. Pyridine- and quinolinecarboxylic acid anilides. Tetrahedron 1969, 25, 315–327. [Google Scholar] [CrossRef]
- Davis, J.W., Jr. Studies with quinolines. I. Synthesis of quinaldic acid and some of its amide derivatives. J. Org. Chem. 1959, 24, 1691–1694. [Google Scholar] [CrossRef]
- Petrie, C.; Orme, M.W.; Baindur, N.; Robbins, K.G.; Harris, S.M.; Kontoyianni, M.; Hurley, L.H.; Kerwin, S.M.; Mundy, G.R. Compositions and Methods for Treating Bone Deficit Conditions. PCT Int. Appl. WO 9715308 A1, 1 May 1997. [Google Scholar]
- Chan, L.; Jin, H.; Stefanac, T.; Wang, W.; Lavallee, J.F.; Bedard, J.; May, S. Isoquinoline-6-carboxamides as potent and selective anti-human cytomegalovirus (HCMV) inhibitors. Bioorg. Med. Chem. Lett. 1999, 9, 2583–2586. [Google Scholar] [CrossRef]
- Kiselyov, A.S. Reaction of N-fluoropyridinium fluoride with isonitriles and TMSN3: A convenient one-pot synthesis of tetrazol-5-yl pyridines. Tetrahedron Lett. 2005, 46, 4851–4854. [Google Scholar] [CrossRef]
- Otwinowski, Z.; Minor, W. Processing of X-ray diffraction data collected in oscillation mode. In Methods in Enzymology 276: Macromolecular Crystallography, Part A; Carter, C.W., Jr., Sweet, R.M., Eds.; Academic Press: New York, NY, USA, 1997; pp. 307–326. [Google Scholar]
- Coppens, P. The Evaluation of Absorption and Extinction in Single Crystal Structure Analysis. In Crystallographic Computing; Ahmed, F.R., Hall, S.R., Huber, C.P., Eds.; Munksgaard: Copenhagen, Denmark, 1970; pp. 255–270. [Google Scholar]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A. Completion and refinement of crystal-structures with SIR92. J. Appl. Crystallogr. 1993, 26, 343–350. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-97; University of Gottingen: Gottingen, Germany, 1997. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bobal, P.; Sujan, J.; Otevrel, J.; Imramovsky, A.; Padelkova, Z.; Jampilek, J. Microwave-Assisted Synthesis of New Substituted Anilides of Quinaldic Acid. Molecules 2012, 17, 1292-1306. https://doi.org/10.3390/molecules17021292
Bobal P, Sujan J, Otevrel J, Imramovsky A, Padelkova Z, Jampilek J. Microwave-Assisted Synthesis of New Substituted Anilides of Quinaldic Acid. Molecules. 2012; 17(2):1292-1306. https://doi.org/10.3390/molecules17021292
Chicago/Turabian StyleBobal, Pavel, Josef Sujan, Jan Otevrel, Ales Imramovsky, Zdenka Padelkova, and Josef Jampilek. 2012. "Microwave-Assisted Synthesis of New Substituted Anilides of Quinaldic Acid" Molecules 17, no. 2: 1292-1306. https://doi.org/10.3390/molecules17021292
APA StyleBobal, P., Sujan, J., Otevrel, J., Imramovsky, A., Padelkova, Z., & Jampilek, J. (2012). Microwave-Assisted Synthesis of New Substituted Anilides of Quinaldic Acid. Molecules, 17(2), 1292-1306. https://doi.org/10.3390/molecules17021292




