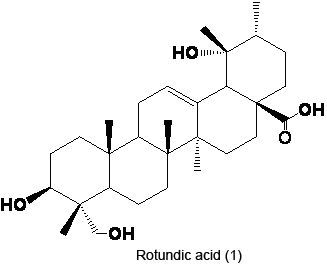
Synthesis, Characterization and Cytotoxicity of New Rotundic Acid Derivatives
Abstract
:1. Introduction
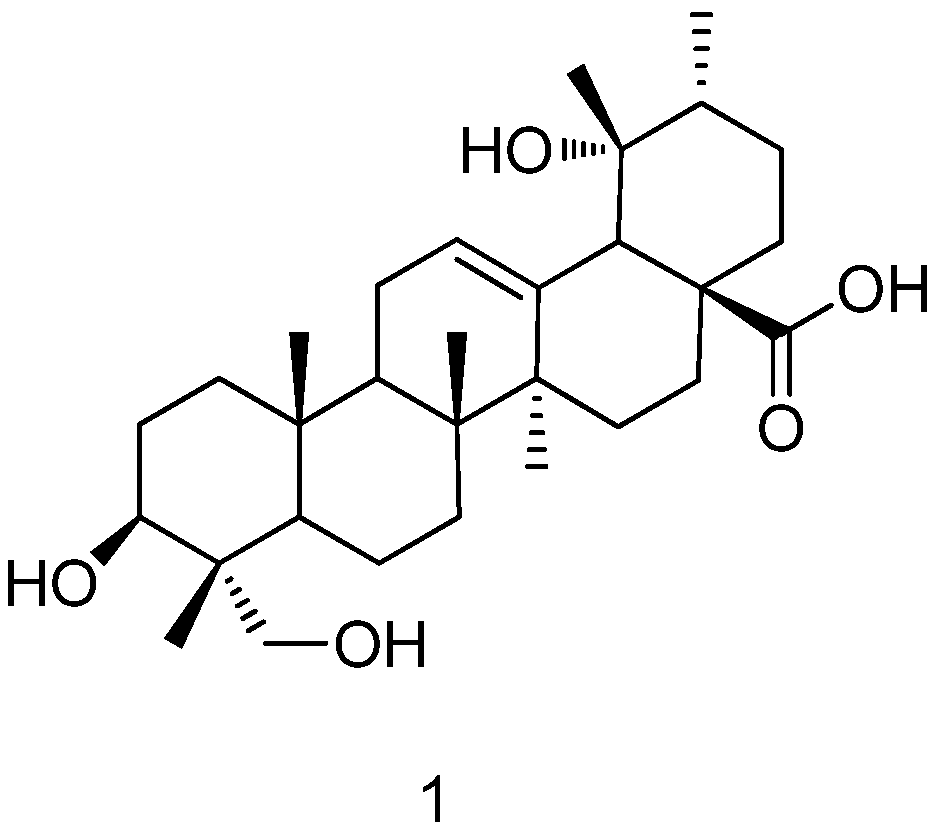
2. Results and Discussion
2.1. Preparation of RA
2.2. Structure Modification of RA
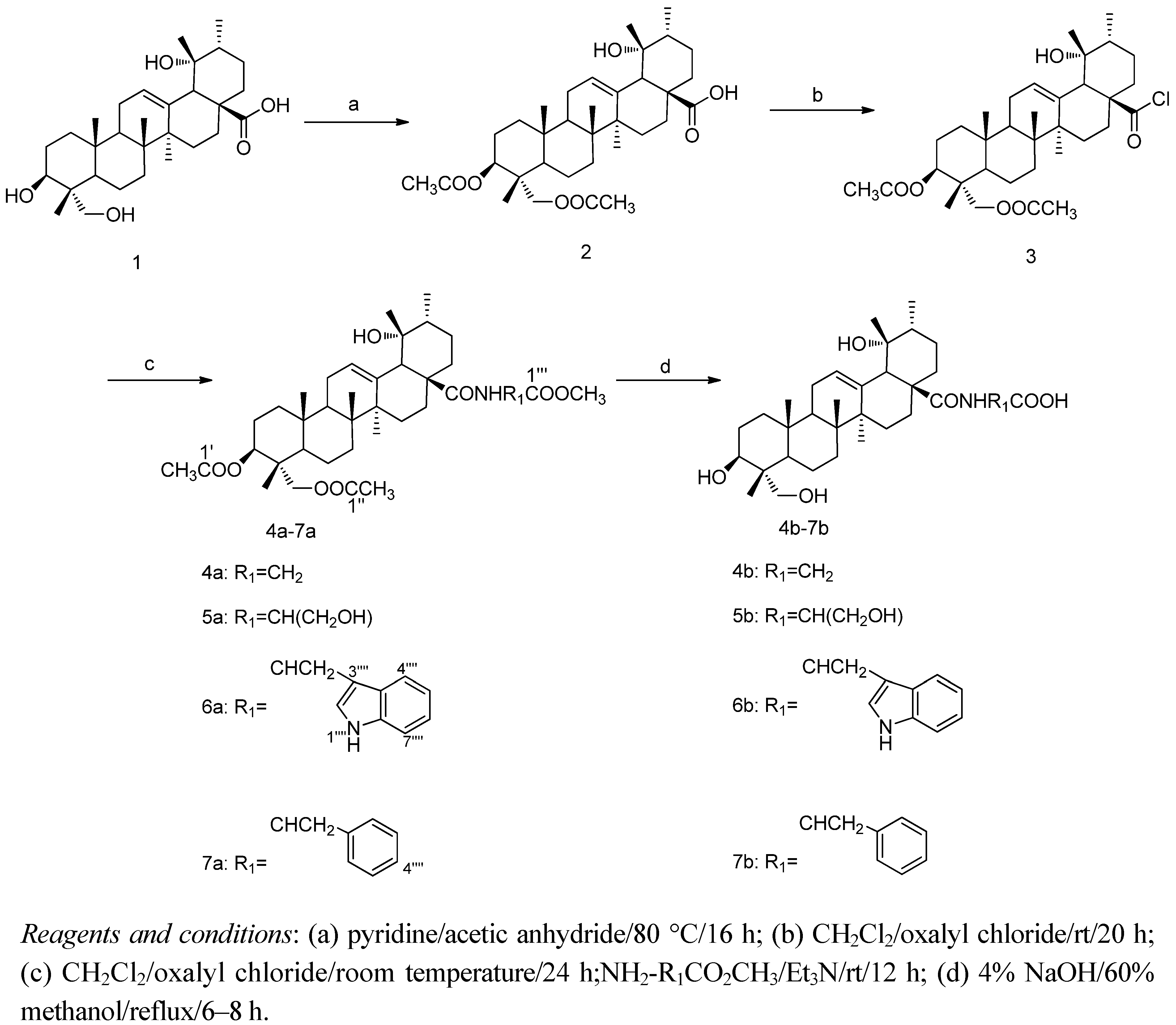
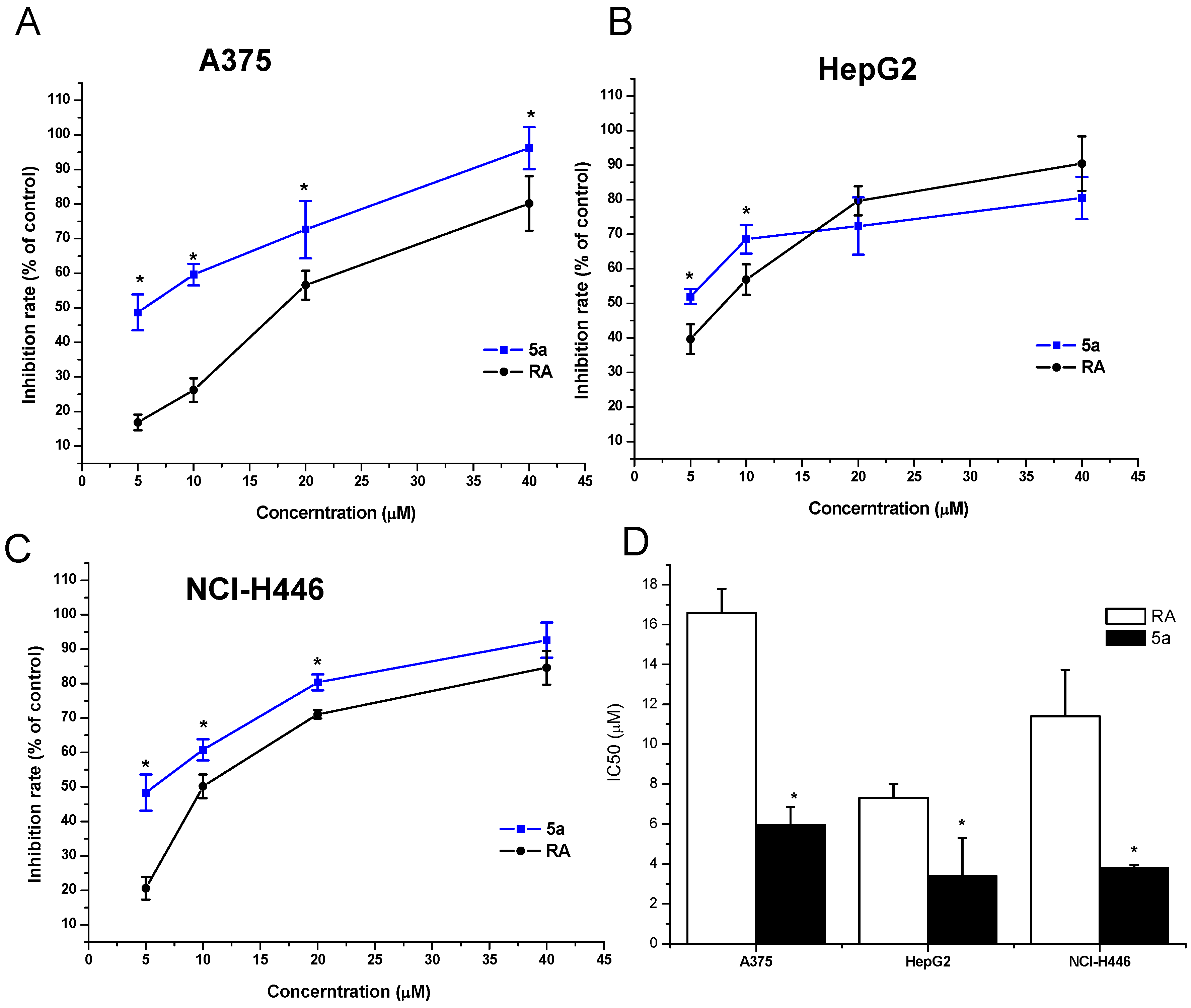
2.3. Biological Activity
| Compound | R1 | IC50 ± SD (µM) | ||
|---|---|---|---|---|
| A375 | HepG2 | NCI-H446 | ||
| RA | – | 16.58 ± 1.22 | 7.33 ± 0.68 | 11.40 ± 2.32 |
| 4a | CH2 | 27.97 ± 2.55 | 10.73 ± 1.69 | 14.79 ± 3.10 |
| 5a | CH(CH2OH) | 5.99 ± 0.88 * | 3.41 ± 1.89 * | 3.84 ± 0.12 * |
| 6a |  | 20.60 ± 0.67 | 44.39 ± 2.87 | 41.78 ± 2.36 |
| 7a | 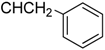 | 23.12 ± 1.23 | 85.70 ± 3.55 | 20.84 ± 3.69 |
| 4b | CH2 | >100 a | 46.67 ± 3.98 | 15.24 ± 1.58 |
| 5b | CH(CH2OH) | >100 a | 22.28 ± 2.25 | 82.79 ± 2.98 |
| 6b | 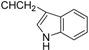 | 8.03 ± 0.87 * | 6.11 ± 1.00 | 11.32 ± 1.56 |
| 7b | 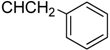 | 34.59 ± 1.96 | 14.19 ± 0.98 | 11.99 ± 1.48 |
3. Experimental
3.1. General
3.2. Extraction and Isolation of RA (1)
3.3. General Procedure for the Preparation of 19α-Hydroxy-3β, 23-diacetoxyurs-12-en-28-oic acid (2)
3.4. General Procedure for the Preparation of N-[3β,23-diacetoxy-19α-hydroxy urs-12-en-28-oyl]-amino acid methyl esters 4a–7a
3.4.1. Methyl N-[3β,23-diacetoxy-19α-hydroxy-urs-12-en-28-oyl]-2-amino acetate (4a,C37H57NO8, R1 = CH2)
3.4.2. Methyl N-[3β,23-diacetoxy-19α-hydroxy -urs-12-en-28-oyl]-2-amino-3-hydroxypropionate (5a, C38H59NO9, R1 = CH(CH2OH)
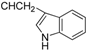
3.4.3. Methyl N-[3β,23-diacetoxy-19α-hydroxy-urs-12-en-28-oyl]-2-amino-3-(1H-indol-3-yl)propionate (6a, C46H64N2O8, R1 = ![Molecules 17 01278 i005]() )
)
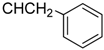
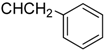
3.4.4. Methyl N-[3β,23-diacetoxy-19α-hydroxyurs-12-en-28-oyl]-2-amino-3-phenyl propionate (7a, C44H63NO8, R1 = ![Molecules 17 01278 i006]() )
)
3.5. General Procedure for the Preparation of N-[3β,19α,23-trihydroxy-urs-12-en-28-oyl] amino acids 4b–7b
3.5.1. N-[3β,19α,23-trihydroxyurs-12-en-28-oyl]-2-amino acetic acid (4b, C32H51NO6, R1 = CH2)
3.5.2. N-[3β,19α,23-trihydroxy urs-12-en-28-oyl]-2-amino-3-hydroxypropionic acid (5b, C33H53NO7, R1 = CH(CH2OH)
3.5.3. N-[3β,19α,23-trihydroxyurs-12-en-28-oyl]-2-amino-3-(1H-indol-3-yl)propionic acid (6b, C41H58N2O6, R1 = ![Molecules 17 01278 i007]() )
)
3.5.4. N-[3β,19α,23-trihydroxy urs-12-en-28-oyl]-2-amino-3-phenylpropionic acid (7b, C39H57NO6, R1 = ![Molecules 17 01278 i008]() )
)
3.6. In Vitro Anti-tumor Assays
4. Conclusions
Acknowledgements
References and Notes
- Sun, H.; Zhang, X.Q.; Cai, Y.; Han, W.L.; Wang, Y.; Ye, W.C. Study on chemical constituents of Ilex rotunda Thunb. Chem. Indus. Forest Prod. 2009, 295, 111–114, In Chinese. [Google Scholar]
- Xie, J.B.; Bi, Z.M.; Li, P. HPLC-ELSD determination of triterpenoids and triterpenoid saponins in Ilex pupurea leaves. Acta Pharmaceu. Sin. 2003, 38, 534–536, In Chinese. [Google Scholar]
- Liao, L.P.; Bi, Z.M.; Li, P.; Xie, J.B.; Zhang, T.D. Triterpenoids from leaves of Ilex purpurea. Chin. J. Nat. Med. 2005, 3, 344–346, In Chinese. [Google Scholar]
- Haraguchi, H.; Kataoka, S.; Okamoto, S.; Hanafi, M.; Shibata, K. Antimicrobial triterpenes from Ilex integra and the mechanism of antifungal action. Phytother. Res. 1999, 13, 151–156. [Google Scholar] [CrossRef]
- Zhao, W.M.; Wolfender, J.L.; Hostettmann, K.; Cheng, K.F.; Xu, R.S.; Qin, G.W. Triterpenes and triterpenoid saponins from Mussaenda pubescens. Phytochemistry 1997, 45, 1073–1078. [Google Scholar] [CrossRef]
- Bhattacharyya, J.; Almeida, M.D. Isolation of the constituents of the root-bark of Guettarda platypoda. J. Nat. Prod. 1985, 48, 148. [Google Scholar] [CrossRef]
- Saimaru, H.; Orihara, Y.; Tahsakul, P.; Kang, Y.H.; Shibuya, M.; Ebizuka, Y. Production of triterpene acids by cell suspension cultures of Olea europaea. Chem. Pharm. Bull. 2007, 55, 784–788. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhang, H.S.; Xu, F.L.; Wu, Z.Y. Triterpene acids from the leaves of Planchonella duclitan (Blanco) Bakhuizan. J. Chin. Chem. Soc. 2005, 52, 1275–1280. [Google Scholar]
- Zhao, Q.C.; Nan, M.L.; He, Y.F.; Chen, S.W. Application of Rotundic Acid in the Cardiovascular Disease Prevention. CHN 201010204596.9, 2010. In Chinese. [Google Scholar]
- Xu, R. Studied on the Chemical Components and Antitumor Activity of Ilex rotunda Thunb. Ph.D. Thesis, Guangzhou University of Chinese Medicine, 2009. [Google Scholar]
- Zhao, Q.C.; Nan, M.L.; He, Y.F.; Chen, S.W. Application of Rotundic Acid in the Preparation of Lipid-lowering Drugs. CHN 201010204607.3, 2010. In Chinese. [Google Scholar]
- He, Y.F.; Zhao, Q.C.; Nan, M.L.; Wang, H.L.; Ma, J.S.; Zhao, Y.W.; Wang, L.P. Application of Rotundic Acid and Its Derivatives in the Preparation of Anticancer Drugs. CHN, 201010607515.x, 2010; In Chinese. [Google Scholar]
- Nan, M.L.; Zhao, Q.C.; He, Y.F.; Chen, S.W.; Zhao, Y.W.; Wang, L.P. Pharmaceutical Compositions from Ilex rotunda Thunb. and Its Application. CHN 201010607550.1, 2010. In Chinese. [Google Scholar]
- Zhao, Q.C.; He, Y.F.; Nan, M.L.; Chen, S.W.; Zhao, Y.W.; Wang, L.P. Synthesis Method of Rotundic Acid Derivatives and Their Application in the Preparation of Cardiovascular Disease Prevention Drugs. CHN 20110030007.4, 2011. In Chinese. [Google Scholar]
- He, Y.F.; Nan, M.L.; Zhao, Q.C.; Zhao, Y.W.; Yue, F.G. Application of Amino Acid Modified Rotundic Acid Derivatives in the Preparation of Anticancer Drugs. CHN 201110351365.5, 2011. In Chinese. [Google Scholar]
- Lin, F.P.; Shao, J.W.; Du, H.D.; Dai, Y.C.; Wang, T. Synthesis, characterization and anti-tumor activity of ursolic acid derivatives. Chin. J. Appl. Chem. 2010, 27, 893–898, In Chinese. [Google Scholar]
- Liu, D.; Meng, Y.Q.; Zhao, J.; Chen, L.G. Synthesis and anti-tumor activity of novel amide derivatives of ursolic acid. Chem. Res. Chin. Univ. 2008, 24, 42–46. [Google Scholar] [CrossRef]
- Meng, Y.Q.; Liu, D.; Cai, L.L.; Chen, H.; Cao, B.; Wang, Y.Z. The synthesis of ursolic acid derivatives with cytotoxic activity and the investigation of their preliminary mechanism of action. Bioorg. Med. Chem. 2009, 17, 848–854. [Google Scholar] [CrossRef]
- Shibata, S.; Takahashi, K.; Yano, S.; Harada, M.; Saito, H.; Tamura, Y.; Kumagai, A.; Hirabayashi, K.; Yamamoto, M.; Nagata, N. Chemical modification of glycyrrhetinic acid in relation to the biological activities. Chem. Pharm. Bull. 1987, 35, 1910–1918. [Google Scholar] [CrossRef]
- Jain, S.M.; Atal, C.K. Synthesis of amino derivatives of ursolic acid. Indian J. Chem. 1986, 25B, 427–428. [Google Scholar]
- Saier, M.H.; Daniels, G.A.; Boerner, P.; Lin, J. Neutral amino acid transport systems in animal cells: Potential targets of oncogene action and regulators of cellular growth. J. Membr. Biol. 1988, 104, 1–20. [Google Scholar] [CrossRef]
- Eagle, H.; Oyama, V.; Levy, M. Amino acid requirements of normal and malignant human cells in tissue culture. Arch. Biochem. Biophys. 1957, 67, 432–446. [Google Scholar] [CrossRef]
- Xu, P.; Fu, Y.Q.; Zou, X.M. Compounds with the Function of Proteasome Inhibition and Their Preparation Methods and Applications. CHN 200610012149.7, 2006. In Chinese. [Google Scholar]
- Ohrai, K.; Adachi, M.; Toyama, K. Preparation of α-Amino Acid Derivatives as Dual Iohibitors of Aurora Kinase and Cyclin-Dependent Kinase (CDK). JPN 2008001883, 2008. [Google Scholar]
- Berst, F.; Grosche, P.; Janser, P.; Janwer, P.; Zecri, F.; Bloobuck, B. Preparation of N-biaryl (hetero) arylsulphonamide amino acid derivatives as sphingosine-1-phosphate recepctor type 1 antagonists useful in the treatment of diseases mediated by lymphocytes interactions. Swiss Patent Application 2008.
- Kim, N.C.; Desjardins, A.E.; Wu, C.D.; Kinghorn, A.D. Activity of triterpenoid glycosides from the root bark of Mussaenda macrophylla against two oral pathogens. J. Nat. Prod. 1999, 62, 1379–1384. [Google Scholar] [CrossRef]
- Nakatani, M.; Hatanaka, S.; Komura, H.; Kubota, T.; Hase, T. The structure of rotungenoside, a new bitter triterpene glucoside from Ilex rotunda. Bull. Chem. Soc. Jpn. 1989, 62, 469–473. [Google Scholar] [CrossRef]
- Zhang, A.L.; Ye, Q.; Li, B.G.; Qi, H.Y.; Zhang, G.L. Phenolic and triterpene glycosides from the stems of Ilex litseaefolia. J. Nat. Prod. 2005, 68, 1531–1535. [Google Scholar] [CrossRef]
- Xie, G.B.; Zhou, S.X.; Lu, Y.N.; Lei, L.D.; Tu, P.F. Triterpenoid glycosidesfrom the leaves of Ilex pernyi. Chem. Pharm. Bull. 2009, 57, 520–524. [Google Scholar] [CrossRef]
- Zhuo, R.X.; Fan, C.L.; Zhao, R.L. Synthesis and antitumor activity of 5-fluorouracil containing amino acid derivatives. Chem. J. Chin. Univ. 1986, 7, 508–512, In Chinese. [Google Scholar]
- Zhao, R.L.; Fan, C.L.; Zhuo, R.X. Synthesis and antitumor activity of polyphosphoramides and polyphosphates containing amino acid, 5-fluorouracil and phosphonoformate or phosphonoacetate. Funct. Polym. 1989, 2, 223–230, In Chinese. [Google Scholar]
- Zhuo, R.X.; Fan, C.L.; Zhuo, R.X. Synthesis and antitumor activity of polyphosphoramides and polyphosphates containing amino acid, 5-fluorouracil and nitrogen mustard. Funct. Polym. 1989, 2, 304–308, In Chinese. [Google Scholar]
- Zhuo, R.X.; Fan, C.L.; Zhuo, R.X. Synthesis of amino acid 5-fluorouracil esters derivatives and study on their antitumor activity. Chem. J. Chin.Univ. 1989, 10, 605–608, In Chinese. [Google Scholar]
- Zhuo, R.X.; Liu, G.W.; Peng, P.P. The synthesis and antitumor activity of 5-Fluorouracil-N1-carbonyl aminoacids and oligopeptides. Chem. J. Chin. Univ. 1991, 12, 555–559, In Chinese. [Google Scholar]
- Sun, H.; Hu, C.; Fang, W.S. Synthesis, water solubility and antitumor activity of amino acid conjugates of 3-oxooleanolic acid. Chin. J. Med. Chem. 2008, 18, 11–15. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983, 139, 55–63. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds RA, 4a–7a, 4b–7b are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
He, Y.-F.; Nan, M.-L.; Sun, J.-M.; Meng, Z.-J.; Yue, F.-G.; Zhao, Q.-C.; Yang, X.-H.; Wang, H. Synthesis, Characterization and Cytotoxicity of New Rotundic Acid Derivatives. Molecules 2012, 17, 1278-1291. https://doi.org/10.3390/molecules17021278
He Y-F, Nan M-L, Sun J-M, Meng Z-J, Yue F-G, Zhao Q-C, Yang X-H, Wang H. Synthesis, Characterization and Cytotoxicity of New Rotundic Acid Derivatives. Molecules. 2012; 17(2):1278-1291. https://doi.org/10.3390/molecules17021278
Chicago/Turabian StyleHe, Yu-Fang, Min-Lun Nan, Jia-Ming Sun, Zhao-Jie Meng, Fa-Gui Yue, Quan-Cheng Zhao, Xiao-Hong Yang, and Hui Wang. 2012. "Synthesis, Characterization and Cytotoxicity of New Rotundic Acid Derivatives" Molecules 17, no. 2: 1278-1291. https://doi.org/10.3390/molecules17021278
APA StyleHe, Y.-F., Nan, M.-L., Sun, J.-M., Meng, Z.-J., Yue, F.-G., Zhao, Q.-C., Yang, X.-H., & Wang, H. (2012). Synthesis, Characterization and Cytotoxicity of New Rotundic Acid Derivatives. Molecules, 17(2), 1278-1291. https://doi.org/10.3390/molecules17021278