Synthesis and Insecticidal Activity of an Oxabicyclolactone and Novel Pyrethroids
Abstract
:1. Introduction
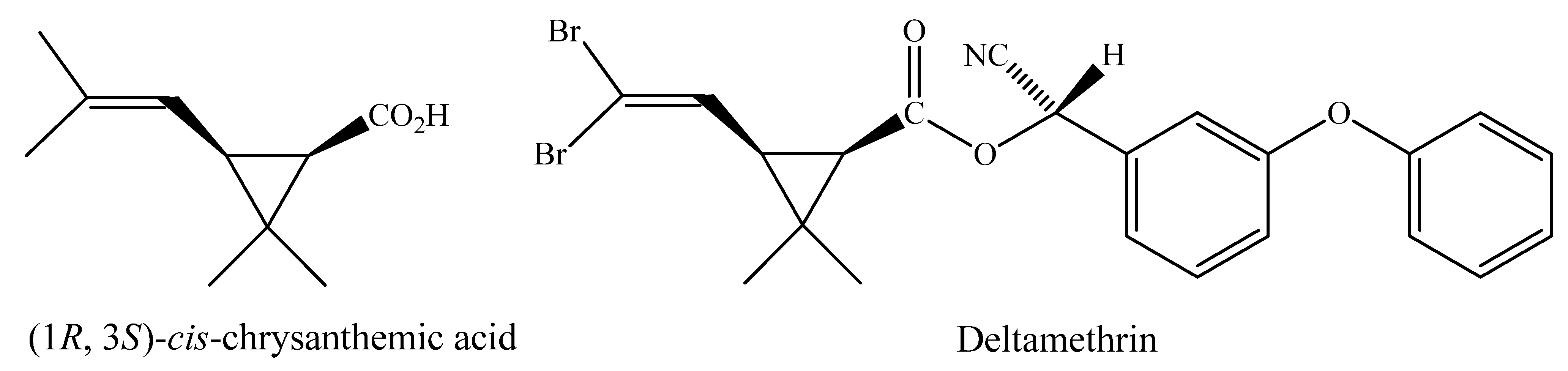
2. Results and Discussion
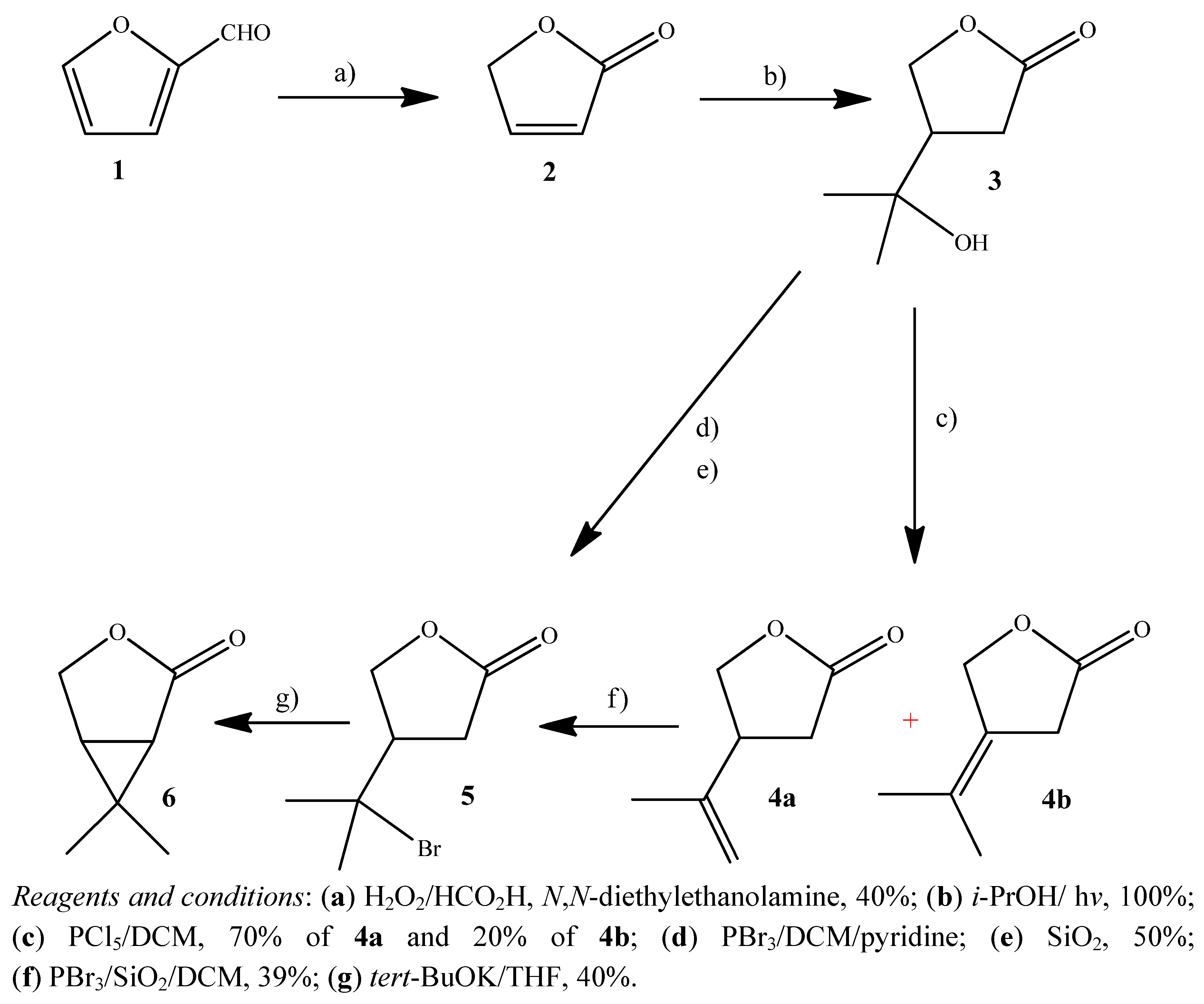
2.1. Synthesis
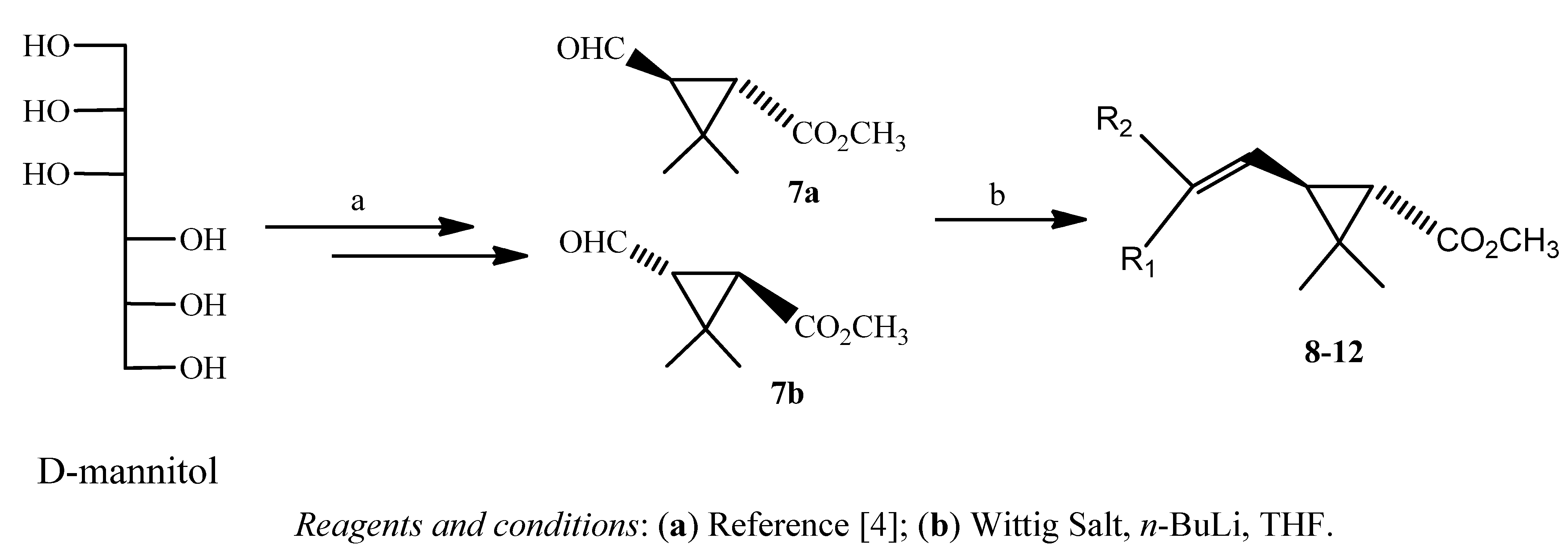
| Pyrethroids | Wittig salt | R1 | R2 | Proportion | Yield (%) |
|---|---|---|---|---|---|
| Z : E | |||||
| 8a | o-MeOPhCH2PPh3+Cl− | -H | o-CH3OPh | 1 : 1 | 46 |
| 8b | o-CH3OPh | -H | |||
| 9a | m-MeOPhCH2PPh3+Cl− | -H | m-CH3OPh | 1 : 1 | 45 |
| 9b | m-CH3OPh | -H | |||
| 10a | o-ClPhCH2PPh3+Br− | -H | o-ClPh | 2 : 1 | 64 |
| 10b | o-ClPh | -H | |||
| 11a | F5PhCH2PPh3+Br− | -H | F5Ph | 1 : 1 | 50 |
| 11b | F5Ph | -H | |||
| 12a | * p-EtOPhCH2PPh3+Cl− | -H | p-(CH3CH2O)Ph | 1 : 1 | 65 |
| 12b | p-(CH3CH2O)Ph | -H |
2.2. Bioassay
| Treatment (compound) | Mean percent mortality * | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A. obtectus | S. zeamais | A. monuste orseis | P. americana | ||||||||
| 12 h | 24 h | 12 h | 24 h | 12 h | 24 h | 12 h | 24 h | ||||
| Permethrin # | 100.0 aA | 100.0 aA | 85.5 bA | 90.0 aA | 100.0 aA | 100.0 aA | 87.5 bA | 97.5 aA | |||
| 6 | 100.0 aA | 100.0 aA | 85.00 aA | 90.00 aA | 100.00 aA | 100.00 aA | 100.00 aA | 100.00 aA | |||
| 7a | 100.0 aA | 100.0 aA | 45.00 bB | 47.50 bB | 27.50 cD | 47.50 bC | 79.33 aABC | 96.00 aAB | |||
| 7b | 90.00 aA | 97.50 aA | 42.50 cB | 45.00 cB | 50.00 cC | 67.50 bB | 67.00 bC | 71.00 aCD | |||
| 8 | 87.50 aA | 95.00 aA | 95.00 aA | 95.00 aA | 62.50 bBC | 95.00 aA | 100.00 aA | 100.00 aA | |||
| 9 | 82.50 aA | 92.50 aA | 92.50 aA | 97.50 aA | 75.00 aB | 95.00 aA | 92.00 aAB | 92.00 aABC | |||
| 10 | 97.50 aA | 97.50 aA | 82.50 aA | 90.00 aA | 100.00 aA | 100.00 aA | 67.00 bC | 67.00 bD | |||
| 11 | 100.0 aA | 100.0 aA | 97.50 aA | 100.00 aA | 100.00 aA | 100.00 aA | 100.00 aA | 100.00 aA | |||
| 12 | 97.50 aA | 97.50 aA | 97.50 aA | 100.00 aA | 75.00 aB | 92.50 aA | 75.00 aBC | 75.00 aBCD | |||
| Control § | 0.00 aB | 3.33 aB | 0.00 aC | 3.33 aC | 11.67 aD | 13.33 aD | 4.00 aD | 6.00 aE | |||
3. Experimental
3.1. General Procedures
3.2. Synthetic Procedures
 , cm−1): 3426, 2978, 2928, 1774, 1378, 1186, 1016, 956, 850. 1H-NMR (CDCl3) δ (m, l, J (Hz), atrib.): 1.28 (m, 6H, 2 × CH3), 2.61 (m, 3H, H3, H3' and H4), 2.8 (s, 1H, OH), 4.4 (m, 2H, H5 and H5'); 13C-NMR (CDCl3): δ 27.9 (CH3), 28.3 (CH3), 30.1 (C3), 46.1 (C4), 69.7 (C5), 70.0 (C-OH), 178.0 (C=O); MS, m/z (100%): [M•+] 144 (1.43), 126 (3.40), 111 (3.60), 85 (56.39), 59 (100.00).
, cm−1): 3426, 2978, 2928, 1774, 1378, 1186, 1016, 956, 850. 1H-NMR (CDCl3) δ (m, l, J (Hz), atrib.): 1.28 (m, 6H, 2 × CH3), 2.61 (m, 3H, H3, H3' and H4), 2.8 (s, 1H, OH), 4.4 (m, 2H, H5 and H5'); 13C-NMR (CDCl3): δ 27.9 (CH3), 28.3 (CH3), 30.1 (C3), 46.1 (C4), 69.7 (C5), 70.0 (C-OH), 178.0 (C=O); MS, m/z (100%): [M•+] 144 (1.43), 126 (3.40), 111 (3.60), 85 (56.39), 59 (100.00). , cm−1): 3083, 2975, 2916, 1794, 1650, 1179, 1021, 900; 1H-NMR (CDCl3) δ (m, l, J(Hz), atrib.): 1.73 (s, 3H, CH3), 2.43 (dd, 1H, Jgem = 17.1, J3–4 = 8.5, H3), 2.62 (dd, 1H, Jgem = 17.1 and J3'-4 = 8.5, H3), 3.15 (m, 1H, H4), 4.07 (dd, 1H, Jgem = 8.8 and J5–4 = 7.8, H5), 4.42 (dd, 1H, Jgem = 8.8 and J5'-4 = 7.8, H5'), 4.85 (m, 2H, =CH2); 13C-NMR (CDCl3): δ 20.5 (CH3), 33.2 (C3), 42.5 (C4), 71.9 (C5), 112.4 (=CH2), 176.9 (C=O); MS, m/z (100%): [M•+] 126 (12.36), 125 (28.86), 95 (22.98), 68 (88.19), 67 (100.00), 59 (34.65). Compound 4b: TLC: Rf = 0.68 (hexane-ethyl acetate, 1:1 v/v); IR (
, cm−1): 3083, 2975, 2916, 1794, 1650, 1179, 1021, 900; 1H-NMR (CDCl3) δ (m, l, J(Hz), atrib.): 1.73 (s, 3H, CH3), 2.43 (dd, 1H, Jgem = 17.1, J3–4 = 8.5, H3), 2.62 (dd, 1H, Jgem = 17.1 and J3'-4 = 8.5, H3), 3.15 (m, 1H, H4), 4.07 (dd, 1H, Jgem = 8.8 and J5–4 = 7.8, H5), 4.42 (dd, 1H, Jgem = 8.8 and J5'-4 = 7.8, H5'), 4.85 (m, 2H, =CH2); 13C-NMR (CDCl3): δ 20.5 (CH3), 33.2 (C3), 42.5 (C4), 71.9 (C5), 112.4 (=CH2), 176.9 (C=O); MS, m/z (100%): [M•+] 126 (12.36), 125 (28.86), 95 (22.98), 68 (88.19), 67 (100.00), 59 (34.65). Compound 4b: TLC: Rf = 0.68 (hexane-ethyl acetate, 1:1 v/v); IR (  , cm−1): 2974, 2912, 1784, 1650, 1362, 1180, 1025, 847, 558; 1H-NMR (CDCl3) δ (m, l, J(Hz), atrib.): 1.60 (m, 3H, CH3), 1.68 (m, 3H, CH3'), 3.13 (m, 2H, H3 e H3'), 4.83 (m, 2H, H5 and H5'); 13C-NMR (CDCl3): δ 19.6 (CH3), 21.6 (CH3'), 32.4 (C3), 71.6 (C5), 121.8 (C4), 126.8 (C6), 176.7 (C=O).
, cm−1): 2974, 2912, 1784, 1650, 1362, 1180, 1025, 847, 558; 1H-NMR (CDCl3) δ (m, l, J(Hz), atrib.): 1.60 (m, 3H, CH3), 1.68 (m, 3H, CH3'), 3.13 (m, 2H, H3 e H3'), 4.83 (m, 2H, H5 and H5'); 13C-NMR (CDCl3): δ 19.6 (CH3), 21.6 (CH3'), 32.4 (C3), 71.6 (C5), 121.8 (C4), 126.8 (C6), 176.7 (C=O). , cm−1): 2973, 2917, 1779, 1373, 1175, 1115, 1027, 629; 1H-NMR (CDCl3) δ (m, l, J(Hz), atrib.): 1.75 (m, 6H, 2 × CH3), 2.64 (m, 3H, H3, H3' and H4), 4.29 (m, 1H, H5), 4.45 (m, 1H, H5'); 13C-NMR (CDCl3): δ 31.9 (C3), 32.1 (CH3), 32.4 (CH3), 48.3 (C4), 66.6 (C-Br), 70.6 (C5), 175.8 (C=O); MS, m/z (100%): [M•++ 2] 209 (1.63), [M•+] 207 (1.67), 126 (17.59), 111 (60.31), 83 (100.00), 68 (54.27).
, cm−1): 2973, 2917, 1779, 1373, 1175, 1115, 1027, 629; 1H-NMR (CDCl3) δ (m, l, J(Hz), atrib.): 1.75 (m, 6H, 2 × CH3), 2.64 (m, 3H, H3, H3' and H4), 4.29 (m, 1H, H5), 4.45 (m, 1H, H5'); 13C-NMR (CDCl3): δ 31.9 (C3), 32.1 (CH3), 32.4 (CH3), 48.3 (C4), 66.6 (C-Br), 70.6 (C5), 175.8 (C=O); MS, m/z (100%): [M•++ 2] 209 (1.63), [M•+] 207 (1.67), 126 (17.59), 111 (60.31), 83 (100.00), 68 (54.27). , cm−1): 2973, 2917, 1779, 1373, 1175, 1115, 1027, 629; 1H-NMR (CDCl3) δ (m, l, J(Hz), atrib.): 1.75 (m, 6H, 2 × CH3), 2.64 (m, 3H, H3, H3' e H4), 4.29 (m, 1H, H5), 4.45 (m, 1H, H5'); 13C-NMR (CDCl3): δ 31.8 (C3), 32.0 (CH3), 32.3 (CH3), 48.2 (C4), 66.5 (C-Br), 70.5 (C5), 175.7 (C=O); MS, m/z (100%): [M•++ 2] 209 (1.63), [M•+] 207 (1.67), 126 (17.59), 111 (60.31), 83(100.00), 68 (54.27).
, cm−1): 2973, 2917, 1779, 1373, 1175, 1115, 1027, 629; 1H-NMR (CDCl3) δ (m, l, J(Hz), atrib.): 1.75 (m, 6H, 2 × CH3), 2.64 (m, 3H, H3, H3' e H4), 4.29 (m, 1H, H5), 4.45 (m, 1H, H5'); 13C-NMR (CDCl3): δ 31.8 (C3), 32.0 (CH3), 32.3 (CH3), 48.2 (C4), 66.5 (C-Br), 70.5 (C5), 175.7 (C=O); MS, m/z (100%): [M•++ 2] 209 (1.63), [M•+] 207 (1.67), 126 (17.59), 111 (60.31), 83(100.00), 68 (54.27). , cm−1): 3068, 2961, 2909, 1774, 1368, 1186, 1046, 975, 892; 1H-NMR (CDCl3) δ (m, l, J(Hz), atrib.): 1.10 (s, 6H, 2 × CH3), 1.88 (dd, 1H, J3-4 = 6.3 e J3–5’ = 0.9, H3), 2.01 (ddd, 1H, J3–4 = 6.3, J4-5 = 5.2 e J4–5' = 1.2, H4), 4.10 (dt, 1H, Jgem = 9.9, J3–5' = 0.9 e J4–5' = 1.2, H5’), 4.31 (dd, 1H, Jgem = 9.9 and J4–5 = 5.2, H5); 13C-NMR (CDCl3): δ 14.6 (CH3), 23.3 [C(CH3)2], 25.4 (CH3), 30.3 (C4), 30.7 (C3), 66.8 (C5), 175.3 (C=O). MS, m/z (100%): [M•+] 126 (3.67), 125 (9.74), 111 (18.33), 97 (25.60), 85 (28.50), 83 (31.98), 71 (53.30), 69 (47.64), 57 (100.00), 55 (67.45).
, cm−1): 3068, 2961, 2909, 1774, 1368, 1186, 1046, 975, 892; 1H-NMR (CDCl3) δ (m, l, J(Hz), atrib.): 1.10 (s, 6H, 2 × CH3), 1.88 (dd, 1H, J3-4 = 6.3 e J3–5’ = 0.9, H3), 2.01 (ddd, 1H, J3–4 = 6.3, J4-5 = 5.2 e J4–5' = 1.2, H4), 4.10 (dt, 1H, Jgem = 9.9, J3–5' = 0.9 e J4–5' = 1.2, H5’), 4.31 (dd, 1H, Jgem = 9.9 and J4–5 = 5.2, H5); 13C-NMR (CDCl3): δ 14.6 (CH3), 23.3 [C(CH3)2], 25.4 (CH3), 30.3 (C4), 30.7 (C3), 66.8 (C5), 175.3 (C=O). MS, m/z (100%): [M•+] 126 (3.67), 125 (9.74), 111 (18.33), 97 (25.60), 85 (28.50), 83 (31.98), 71 (53.30), 69 (47.64), 57 (100.00), 55 (67.45).3.3. General Method to Preparation of Alkenes 8 to 12
 , cm−1): 2950, 1724, 1598, 1438, 1244, 1167, 1029, 752; 1H-NMR (CDCl3) δ (m, l, J(Hz), atrib.): 1.20 (s, 6H, 2 × CH3), 1.25 (s, 3H, CH3), 1.30 (s, 3H, CH3), 1.57 (d, 1H, J1-3cis = 5.4, H1cis), 1.69 (d, 1H, J1-3trans = 5.4, H1trans), 2.26 (dd, 1H, J1-3trans = 5.4 e J3-4trans = 8.7, H3trans), 2.34 (dd, 1H, J1-3 cis = 5.4 e J3-4 cis = 9.0, H3cis), 3.65 (s, 3H, OCH3), 3.70 (s, 3H, OCH3), 3.83 (s, 3H, CH3O-Ar), 3.83 (s, 6H, CH3O-Ar), 5.45 (dd, 1H, J3-4cis = 9.0 e J4-5cis = 11.5, H4cis), 5.96 (dd, 1H, J3-4trans = 8.7 e J4-5trans = 15.9, H4trans), 6.68 (d, 1H, J4-5cis = 11.5, H5cis), 6.92 (m, 5H, H5trans, H3' e H5'), 7.30 (m, 4H, H4' e H6'); 13C-NMR (CDCl3): δ 20.4 (CH3), 20.7 (CH3), 22.4 (CH3), 22.5 (CH3), 29.7 [C(CH3)2], 28.8 [C(CH3)2], 33.7 (C3cis), 34.6 (C1trans), 35.7 (C1cis), 37.6 (C3trans), 51.7 (OCH3), 51.8 (OCH3), 55.7 (CH3OAr), 55.8 (CH3OAr), 110.5–130.2 (C4, C5 e Ar), 156.5 (C2'), 157.1 (C2'), 172.5 (C=O), 172.7 (C=O); MS, m/z (100%): [M•+] 260 (35.02), 201 (100.00), 185 (46.55), 121 (56.32), 91 (95.16), 77 (57.62), 41 (73.00).
, cm−1): 2950, 1724, 1598, 1438, 1244, 1167, 1029, 752; 1H-NMR (CDCl3) δ (m, l, J(Hz), atrib.): 1.20 (s, 6H, 2 × CH3), 1.25 (s, 3H, CH3), 1.30 (s, 3H, CH3), 1.57 (d, 1H, J1-3cis = 5.4, H1cis), 1.69 (d, 1H, J1-3trans = 5.4, H1trans), 2.26 (dd, 1H, J1-3trans = 5.4 e J3-4trans = 8.7, H3trans), 2.34 (dd, 1H, J1-3 cis = 5.4 e J3-4 cis = 9.0, H3cis), 3.65 (s, 3H, OCH3), 3.70 (s, 3H, OCH3), 3.83 (s, 3H, CH3O-Ar), 3.83 (s, 6H, CH3O-Ar), 5.45 (dd, 1H, J3-4cis = 9.0 e J4-5cis = 11.5, H4cis), 5.96 (dd, 1H, J3-4trans = 8.7 e J4-5trans = 15.9, H4trans), 6.68 (d, 1H, J4-5cis = 11.5, H5cis), 6.92 (m, 5H, H5trans, H3' e H5'), 7.30 (m, 4H, H4' e H6'); 13C-NMR (CDCl3): δ 20.4 (CH3), 20.7 (CH3), 22.4 (CH3), 22.5 (CH3), 29.7 [C(CH3)2], 28.8 [C(CH3)2], 33.7 (C3cis), 34.6 (C1trans), 35.7 (C1cis), 37.6 (C3trans), 51.7 (OCH3), 51.8 (OCH3), 55.7 (CH3OAr), 55.8 (CH3OAr), 110.5–130.2 (C4, C5 e Ar), 156.5 (C2'), 157.1 (C2'), 172.5 (C=O), 172.7 (C=O); MS, m/z (100%): [M•+] 260 (35.02), 201 (100.00), 185 (46.55), 121 (56.32), 91 (95.16), 77 (57.62), 41 (73.00). , cm−1): 2948, 1728, 1438, 1219, 767, 751; 1H-NMR (CDCl3) δ (m, l, J (Hz), atrib.): 1.24 (s, 6H, CH3 cis), 1.26 (s, 3H, CH3 trans), 1.28 (s, 6H, CH3 cis), 1.34 (s, 3H, CH3 trans), 1.60 (d, 2H, J1-3cis = 5.7, H1cis), 1.73 (d, 1H, J1-3trans = 5.7, H1trans), 2.27 (m, 3H, H3cis e H3trans), 3.67 (s, 6H, OCH3 cis), 3.71 (s, 3H, OCH3 trans), 5.55 (dd, 2H, J3-4cis = 9.0 e J4-5cis = 11.4, H4cis), 5.97 (dd, 1H, J3-4trans = 9.0 e J4-5trans= 15.9, H4trans), 6.67 (d, 2H, J4-5cis = 11,4, H5cis), 6.95 (d, 1H, J4-5trans = 15.9, H5trans), 7.25 (m, 12H, Arcis e Artrans)*; 13C-NMR (CDCl3): δ 20.4 (2 × CH3), 20.6 (CH3), 22.4 (CH3), 29.7 [2 × C(CH3)2], 33.2 (C3cis), 34.8 (C1trans), 35.7 (C1cis), 37.0 (C3trans), 51.8 (2 × OCH3), 126.5–135.3 (C4, C5 e Ar), 172.4 (2 × C=O); MS, m/z (100%): [M•+] 260 (26.10), 201 (61.42), 185 (58.24), 159 (60.84), 115 (77.52), 102 (100.00), 91 (67.98), 77 (68.16), 41 (72.87).
, cm−1): 2948, 1728, 1438, 1219, 767, 751; 1H-NMR (CDCl3) δ (m, l, J (Hz), atrib.): 1.24 (s, 6H, CH3 cis), 1.26 (s, 3H, CH3 trans), 1.28 (s, 6H, CH3 cis), 1.34 (s, 3H, CH3 trans), 1.60 (d, 2H, J1-3cis = 5.7, H1cis), 1.73 (d, 1H, J1-3trans = 5.7, H1trans), 2.27 (m, 3H, H3cis e H3trans), 3.67 (s, 6H, OCH3 cis), 3.71 (s, 3H, OCH3 trans), 5.55 (dd, 2H, J3-4cis = 9.0 e J4-5cis = 11.4, H4cis), 5.97 (dd, 1H, J3-4trans = 9.0 e J4-5trans= 15.9, H4trans), 6.67 (d, 2H, J4-5cis = 11,4, H5cis), 6.95 (d, 1H, J4-5trans = 15.9, H5trans), 7.25 (m, 12H, Arcis e Artrans)*; 13C-NMR (CDCl3): δ 20.4 (2 × CH3), 20.6 (CH3), 22.4 (CH3), 29.7 [2 × C(CH3)2], 33.2 (C3cis), 34.8 (C1trans), 35.7 (C1cis), 37.0 (C3trans), 51.8 (2 × OCH3), 126.5–135.3 (C4, C5 e Ar), 172.4 (2 × C=O); MS, m/z (100%): [M•+] 260 (26.10), 201 (61.42), 185 (58.24), 159 (60.84), 115 (77.52), 102 (100.00), 91 (67.98), 77 (68.16), 41 (72.87).  , cm−1): 2956, 2752, 1738, 1714, 1434, 1237, 1173, 1112, 975; 1H-NMR (CDCl3) δ (m, l, J(Hz), atrib.): 1.20 (s, 6H, 2 × CH3), 1.25 (s, 6H, 2 × CH3) 1.57 (d, 1H, J1-3cis = 5.4, H1cis), 1.67 (d, 1H, J1-3trans = 5.7, H1trans), 2.22 (dd, 1H, J1-3trans = 5.7 e J3-4trans = 8.4, H3trans), 2.45 (dd, 1H, J1-3cis = 5.4 e J3-4cis = 8.7, H3cis), 3.67 (s, 3H, OCH3), 3.69 (s, 3H, OCH3), 3.80 (s, 6H, 2 × CH3O-Ar), 5.40 (dd, 1H, J3-4cis = 8.7 e J4-5cis = 11.7, H4cis), 5.95 (dd, 1H, J3-4trans = 8.4 e J4-5trans = 15.9, H4trans), 6.54 (m, 2H, H5trans e H5cis), 6.90 (m, 6H, H2', H4' e H6'), 7.23 (m, 2H, H5’); 13C-NMR (CDCl3): δ 20.8 (CH3), 21.1 (CH3), 33.4 (C2), 34.3 (C1), 42.2 (C3), 52.2 (OCH3), 170.4 (COOCH3), 199.0 (HC=O); MS, m/z (100%): [M•++2] 266 (6.29), [M•+] 264 (19.09), 207 (28.14), 205 (100.00), 139 (43.93), 125 (84.02), 115 (31.50), 77 (25.86).
, cm−1): 2956, 2752, 1738, 1714, 1434, 1237, 1173, 1112, 975; 1H-NMR (CDCl3) δ (m, l, J(Hz), atrib.): 1.20 (s, 6H, 2 × CH3), 1.25 (s, 6H, 2 × CH3) 1.57 (d, 1H, J1-3cis = 5.4, H1cis), 1.67 (d, 1H, J1-3trans = 5.7, H1trans), 2.22 (dd, 1H, J1-3trans = 5.7 e J3-4trans = 8.4, H3trans), 2.45 (dd, 1H, J1-3cis = 5.4 e J3-4cis = 8.7, H3cis), 3.67 (s, 3H, OCH3), 3.69 (s, 3H, OCH3), 3.80 (s, 6H, 2 × CH3O-Ar), 5.40 (dd, 1H, J3-4cis = 8.7 e J4-5cis = 11.7, H4cis), 5.95 (dd, 1H, J3-4trans = 8.4 e J4-5trans = 15.9, H4trans), 6.54 (m, 2H, H5trans e H5cis), 6.90 (m, 6H, H2', H4' e H6'), 7.23 (m, 2H, H5’); 13C-NMR (CDCl3): δ 20.8 (CH3), 21.1 (CH3), 33.4 (C2), 34.3 (C1), 42.2 (C3), 52.2 (OCH3), 170.4 (COOCH3), 199.0 (HC=O); MS, m/z (100%): [M•++2] 266 (6.29), [M•+] 264 (19.09), 207 (28.14), 205 (100.00), 139 (43.93), 125 (84.02), 115 (31.50), 77 (25.86). , cm−1): 2952, 1734, 1651, 1521, 1495, 1219, 1171, 1002, 869; 1H-NMR (CDCl3) δ (m, l, J(Hz), atrib.): 1.23 (s, 6H, 2 × CH3), 1.34 (s, 6H, 2 × CH3) 1.65 (d, 1H, J1-3cis = 5.4, H1cis), 1.75 (d, 1H, J1-3trans = 5.4, H1trans), 2.16 (m, 1H, H3cis), 2.23 (m, 1H, H3trans), 3.65 (s, 3H, OCH3), 3.67 (s, 3H, OCH3), 5.75 (dd, 1H, J3-4cis = 9.6 e J4-5cis = 11.1, H4cis), 6.14 (d, 1H, J4-5cis = 11.1, H5cis), 6.27 (dd, 1H, J3-4trans = 9.0 e J4-5trans = 16.2, H4trans), 6.46 (d, 1H, J4-5trans = 16.2, H5trans); 13C-NMR (CDCl3): δ 20.5 (2 × CH3), 22.3 (CH3), 22.5 (CH3), 29.8 [C(CH3)2], 30.0 [C(CH3)2], 34.1 (C3cis), 34.2 (C1trans), 35.7 (C1cis), 37.7 (C3trans), 51.9 (OCH3), 52.0 (OCH3), 114.7 (C5cis), 115.8 (C5trans), 137.4 (C4cis e C4trans), 172.0 (2 × C=O); MS, m/z (100%): [M•+] 320 (8.37), 261 (100.00), 195 (24.67), 181 (98.29), 139 (38.03), 59 (38.77), 41 (57.36).
, cm−1): 2952, 1734, 1651, 1521, 1495, 1219, 1171, 1002, 869; 1H-NMR (CDCl3) δ (m, l, J(Hz), atrib.): 1.23 (s, 6H, 2 × CH3), 1.34 (s, 6H, 2 × CH3) 1.65 (d, 1H, J1-3cis = 5.4, H1cis), 1.75 (d, 1H, J1-3trans = 5.4, H1trans), 2.16 (m, 1H, H3cis), 2.23 (m, 1H, H3trans), 3.65 (s, 3H, OCH3), 3.67 (s, 3H, OCH3), 5.75 (dd, 1H, J3-4cis = 9.6 e J4-5cis = 11.1, H4cis), 6.14 (d, 1H, J4-5cis = 11.1, H5cis), 6.27 (dd, 1H, J3-4trans = 9.0 e J4-5trans = 16.2, H4trans), 6.46 (d, 1H, J4-5trans = 16.2, H5trans); 13C-NMR (CDCl3): δ 20.5 (2 × CH3), 22.3 (CH3), 22.5 (CH3), 29.8 [C(CH3)2], 30.0 [C(CH3)2], 34.1 (C3cis), 34.2 (C1trans), 35.7 (C1cis), 37.7 (C3trans), 51.9 (OCH3), 52.0 (OCH3), 114.7 (C5cis), 115.8 (C5trans), 137.4 (C4cis e C4trans), 172.0 (2 × C=O); MS, m/z (100%): [M•+] 320 (8.37), 261 (100.00), 195 (24.67), 181 (98.29), 139 (38.03), 59 (38.77), 41 (57.36). , cm−1): 2979, 2949, 1725, 1607, 1510, 1245, 1167, 1048, 841, 732; 1H-NMR (CDCl3) δ (m, l, J(Hz), atrib.): 1.20 (s, 3H, CH3), 1.21 (s, 3H, CH3), 1.30 (s, 3H, CH3), 1.32 (s, 3H, CH3), 1.54 (d, 1H, J1-3cis = 5.4, H1cis), 1.64 (d, 1H, J1-3trans = 5.4, H1trans), 2.18 (m, 1H, H3trans), 2.40 (m, 1H, H3cis), 3.67 (s, 3H, OCH3), 3.69 (s, 3H, OCH3), 4.03 (m, 4H, 2 × OCH2), 5.30 (dd, 1H, J3-4cis = 8.4 e J4-5cis = 11.5, H4cis), 5.82 (dd, 1H, J3-4trans = 8.7 e J4-5trans = 15.6, H4trans), 6.46 (m, 2H, H5trans e H5cis), 6.85 ( m, 4H, H3'), 7.25 (m, 4H, H2'); 13C-NMR (CDCl3): δ 15.1 (2 × OCH2CH3), 20.4 (2 × CH3), 22.4 (2 × CH3), 29.5 [C(CH3)2], 39.8 [C(CH3)2], 33.4 (C3cis), 34.5 (C1trans), 35.7 (C1cis), 37.1 (C3trans), 51.8 (2 × OCH3), 63.36 (2 × OCH2), 114.4 (C3'), 114.7 (C3'), 125.0 (C4trans), 126.7 (C4cis), 127.2 (2 × C1'), 129.8 (C2'), 130.2 (C2'), 131.5 (C5), 131.6 (C5), 158.1 (C4'), 158.5 (C4'), 172.7 (2 × C=O); MS, m/z (100%): [M•+] 274 (51.44), 215 (100.00), 199 (35.28), 107 (50.20), 77 (44.56), 29 (62.83).
, cm−1): 2979, 2949, 1725, 1607, 1510, 1245, 1167, 1048, 841, 732; 1H-NMR (CDCl3) δ (m, l, J(Hz), atrib.): 1.20 (s, 3H, CH3), 1.21 (s, 3H, CH3), 1.30 (s, 3H, CH3), 1.32 (s, 3H, CH3), 1.54 (d, 1H, J1-3cis = 5.4, H1cis), 1.64 (d, 1H, J1-3trans = 5.4, H1trans), 2.18 (m, 1H, H3trans), 2.40 (m, 1H, H3cis), 3.67 (s, 3H, OCH3), 3.69 (s, 3H, OCH3), 4.03 (m, 4H, 2 × OCH2), 5.30 (dd, 1H, J3-4cis = 8.4 e J4-5cis = 11.5, H4cis), 5.82 (dd, 1H, J3-4trans = 8.7 e J4-5trans = 15.6, H4trans), 6.46 (m, 2H, H5trans e H5cis), 6.85 ( m, 4H, H3'), 7.25 (m, 4H, H2'); 13C-NMR (CDCl3): δ 15.1 (2 × OCH2CH3), 20.4 (2 × CH3), 22.4 (2 × CH3), 29.5 [C(CH3)2], 39.8 [C(CH3)2], 33.4 (C3cis), 34.5 (C1trans), 35.7 (C1cis), 37.1 (C3trans), 51.8 (2 × OCH3), 63.36 (2 × OCH2), 114.4 (C3'), 114.7 (C3'), 125.0 (C4trans), 126.7 (C4cis), 127.2 (2 × C1'), 129.8 (C2'), 130.2 (C2'), 131.5 (C5), 131.6 (C5), 158.1 (C4'), 158.5 (C4'), 172.7 (2 × C=O); MS, m/z (100%): [M•+] 274 (51.44), 215 (100.00), 199 (35.28), 107 (50.20), 77 (44.56), 29 (62.83).3.4. Bioassays
4. Conclusions
Acknowledgments
References
- Elliott, M.; Farnham, A.W.; Janes, N.F.; Needham, P.H.; Pulman, D.A. Synthetic insecticide with a new order of activity. Nature 1974, 248, 710–711. [Google Scholar] [CrossRef]
- Soderlund, D.M.; Clark, J.M.; Sheets, L.P.; Mullin, L.S.; Piccirillo, V.J.; Sargent, D.; Stevens, J.T.; Weiner, M.L. Mechanisms of pyrethroid neurotoxicity: Implications for cumulative risk assessment. Toxicology 2002, 171, 3–59. [Google Scholar]
- Elliott, M.; Janes, N.F. Synthetic pyrethroids - a new class of insecticide. Chem. Soc. Rev. 1978, 7, 473–505. [Google Scholar]
- Silvério, F.O.; de Alvarenga, E.S.; Moreno, S.C.; Picanço, M.C. Synthesis and insecticidal activity of new pyrethroids. Pest Manag. Sci. 2009, 65, 900–905. [Google Scholar] [CrossRef]
- Krief, A. Bicyclohexane derivatives and their use in preparing cyclopropane carboxylic acid. UK Pat. 2,246,129A, 22 January 1992. [Google Scholar]
- Funk, R.L.; Munger, J.D. The stereospecific total synthesis of (±) cis-chrysanthemic acid via the alicyclic Claisen rearrangement. J. Org. Chem. 1978, 50, 707–709. [Google Scholar]
- Krief, A.; Swinne, D. Novel synthesis of vinylcyclopropanecarboxylic acids: application to the synthesis of (d,l)- and (d)-cis-chrysanthemic acid. Tetrahedron Lett. 1996, 37, 7123–7126. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, W.; Wang, X.; Li, P. Immunoassay Development for the Class-Specific Assay for Types I and II Pyrethroid Insecticides in Water Samples. Molecules 2010, 15, 164–177. [Google Scholar] [CrossRef]
- Moreno, S.C.; Picanço, M.C.; Silvério, F.O.; de Alvarenga, E.S.; Carvalho, G.A. Toxicity of new pyrethroids to the social insects Protonectarina sylveirae, Solenopsis saevissima and Tetragonisca angustula. Sociobiology 2009, 54, 893–906. [Google Scholar]
- Jakovac, I.J.; Goodbrand, H.B.; Lok, K.P.; Jones, J.B. Enzymes in organic synthesis. 24. Preparations of enantiomerically pure chiral lactones via stereospecific horse liver alcohol dehydrogenase catalyzed oxidations of monocyclic meso diols. J. Am. Chem. Soc. 1982, 104, 4659–4665. [Google Scholar] [CrossRef]
- Doyle, M.P.; Pieters, R.J. High enantioselectivity in the intramolecular cyclopropanation of allyl diazoacetates using a novel rhodium(II) catalyst. J. Am. Chem. Soc. 1991, 113, 1423–1424. [Google Scholar]
- Doyle, M.P.; Austin, R.E.; Bailey, A.S.; Dwyer, M.P.; Dyatkin, A.B.; Kalinin, A.V.; Kwan, M.M.Y.; Liras, S.; Oalmann, C.J.; Pieters, R.J.; et al. Enantioselective intramolecular cyclopropanations of allylic and homoallylic diazoacetates and diazoacetamides using chiral dirhodium(II) carboxamide catalysts. J. Am. Chem. Soc. 1995, 117, 5763–5775. [Google Scholar]
- Doyle, M.P.; Hu, W.; Chapman, B.; Marnett, A.B.; Peterson, C.S.; Vitale, J.P.; Stanley, S.A. Enantiocontrolled macrocycle formation by catalytic intramolecular cyclopropanation. J. Am. Chem. Soc. 2000, 122, 5718–5728. [Google Scholar]
- Che, C.M.; Huang, J.S.; Lee, F.W.; Li, Y.; Lai, T.S.; Kwong, H.L.; Teng, P.F.; Lee, W.S.; Lo, W.C.; Peng, S.M.; et al. Asymmetric inter- and intramolecular cyclopropanation of alkenes catalyzed by chiral ruthenium porphyrins. Synthesis and crystal structure of a chiral metalloporphyrin carbene complex. J. Am. Chem. Soc. 2001, 123, 4119–4129. [Google Scholar]
- Sabbioni, G.; Jones, J.B. Enzymes in organic synthesis. 39. Preparations of chiral cyclic acid-esters and bicyclic lactones via stereoselective pig liver esterase catalyzed hydrolyses of cyclic meso diesters. J. Org. Chem. 1987, 52, 4565–4570. [Google Scholar]
- Milewska, M.J.; Gdaniec, M.; Poloński, T. Synthesis, Stereochemistry, and chiroptical spectra of cyclopropyl lactones and thionolactones. Tetrahedron: Asymmetry 1996, 7, 3169–3180. [Google Scholar]
- Teng, P.F.; Lai, T.S.; Kwonga, H.L.; Cheb, C.M. Asymmetric inter- and intramolecular cyclopropanations of alkenes catalyzed by rhodium D4-porphyrin: A comparison of rhodium- and ruthenium-centered catalysts. Tetrahedron: Asymmetry. 2003, 14, 837–844. [Google Scholar]
- Sato, H.; Kim, Y.S.; Shibasaki, M. A catalytic asymmetric synthesis of a versatile intermediate for phorbol derivatives. TetrahedronLett. 1999, 40, 2973–2976. [Google Scholar]
- Bosone, E.; Caprara, G.; Corda, F.; Gozzo, F.; Menconi, A.; Piccardi, P.; Caprioli, V. Pyrethroids. U.S. Patent 4,599,358, 8 July 1986. [Google Scholar]
- Hirata, N.; Uemura, T. Process for producing 6,6-dimethyl-3-oxabicyclo[3.1.0]hexan-2-one. WO Patent Appl. 2007,052,383, 10 May 2007. [Google Scholar]
- Li, G.Y.; Zhang, J.; Chan, P.W.H.; Xu, Z.J.; Zhu, N.; Che, C.M. Enantioselective intramolecular cyclopropanation of cis-alkenes by chiral Ruthenium(II) Schiff base catalysts and crystal structures of (Schiff base)ruthenium complexes containing carbene, PPh3, and CO ligands. Organometallics 2006, 25, 1676–1688. [Google Scholar] [CrossRef]
- Botton, M.; Lorini, I.; Afonso, A.P.S. Ocorrência de Sitophilus zeamaisMots. (Coleoptera: Curculionidae) danificando a cultura da videira no Rio Grande do Sul (in Portuguese). Neotrop. Entomol. 2005, 34, 355–356. [Google Scholar]
- Chu, S.-S.; Liu, Z.-L.; Du, S.-S.; Deng, Z.-W. Chemical Composition and Insecticidal Activity Against Sitophilus zeamais of the Essential Oils Derived from Artemisia giraldii and Artemisia subdigitata. Molecules 2012, 17, 7255–7265. [Google Scholar] [CrossRef]
- Liu, T.X. Biology and Life History of Ascia monuste monuste (Lepidoptera: Pieridae), a Potential Pest of Cruciferous Vegetables. Ann. Entomol. Soc. Am. 2005, 98, 726–731. [Google Scholar] [CrossRef]
- Näsman, J.H. 3-Methyl-2(5H)-furanone-(2(5H)-furanone, 3-methyl). Org. Synt. 1998, 68, 162–174. [Google Scholar]
- Ohga, K.; Matsuo, T. Photoinduced addition of isopropyl alcohol to α, β-unsaturated lactones. J. Org. Chem. 1974, 39, 106–108. [Google Scholar]
- Sanseverino, A.M.; Mattos, M.C.S. Hydrobromination of alkenes with PBr3/SiO2: a simple and efficient regiospecific preparation of alkyl bromides. J. Braz. Chem. Soc. 2001, 12, 685–687. [Google Scholar] [CrossRef]
- Ahmed, A.S.E.; Bashir, N.H.H.; Assad, Y.O.H. Susceptibility of Periplaneta americana L. (Orthoptera: Blattidae) population from Wad Medani (Sudan Gezira) to three public health insecticides. Resist. Pest Manag. Newsl. 2010, 19, 8–14. [Google Scholar]
- Pereira, C.J.; Pereira, E.J.G.; Cordeiro, E.M.G.; Della Lucia, T.M.C.; Totola, M.R.; Guedes, R.N.C. Oganophosphate resistance in the maize weevil Sitophilus zeamais: magnitude and behavior. Crop Prot. 2009, 28, 168–173. [Google Scholar]
- Silva, G.A.; Picanço, M.C.; Bacci, L.; Crespo, A.L.B.; Rosado, J.F.; Guedes, R.N.C. Control failure likelihood and spatial dependence of insecticide resistance in the tomato pinworm, Tuta absoluta. Pest Manag. Sci. 2011, 67, 913–920. [Google Scholar] [CrossRef]
- Gomez-Dantes, H.; San Martin, J.L.; Danis-Lozano, R.; Manrique-Saide, P. Estrategias de prevencion y control de salud publica en Mesoamerica: un enfoque basado en evidencia. Salud Publica Mex. 2011, 53, 349–357. [Google Scholar]
- Perrin, D.D.; Armarego, W.L.F. Purification of Laboratory Chemicals, 3rd ed; Butterworths-Heinemann: Oxford, UK, 1997. [Google Scholar]
- Sample Availability: Samples are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Alvarenga, E.S.d.; Carneiro, V.M.T.; Resende, G.C.; Picanço, M.C.; Farias, E.D.S.; Lopes, M.C. Synthesis and Insecticidal Activity of an Oxabicyclolactone and Novel Pyrethroids. Molecules 2012, 17, 13989-14001. https://doi.org/10.3390/molecules171213989
Alvarenga ESd, Carneiro VMT, Resende GC, Picanço MC, Farias EDS, Lopes MC. Synthesis and Insecticidal Activity of an Oxabicyclolactone and Novel Pyrethroids. Molecules. 2012; 17(12):13989-14001. https://doi.org/10.3390/molecules171213989
Chicago/Turabian StyleAlvarenga, Elson S. de, Vânia M. T. Carneiro, Gabriela C. Resende, Marcelo C. Picanço, Elizeu De Sá Farias, and Mayara Cristina Lopes. 2012. "Synthesis and Insecticidal Activity of an Oxabicyclolactone and Novel Pyrethroids" Molecules 17, no. 12: 13989-14001. https://doi.org/10.3390/molecules171213989
APA StyleAlvarenga, E. S. d., Carneiro, V. M. T., Resende, G. C., Picanço, M. C., Farias, E. D. S., & Lopes, M. C. (2012). Synthesis and Insecticidal Activity of an Oxabicyclolactone and Novel Pyrethroids. Molecules, 17(12), 13989-14001. https://doi.org/10.3390/molecules171213989






