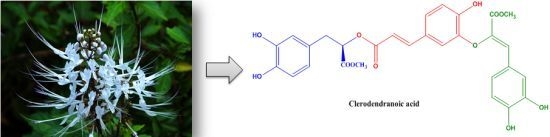
Clerodendranoic Acid, a New Phenolic Acid from Clerodendranthus spicatus
Abstract
:1. Introduction
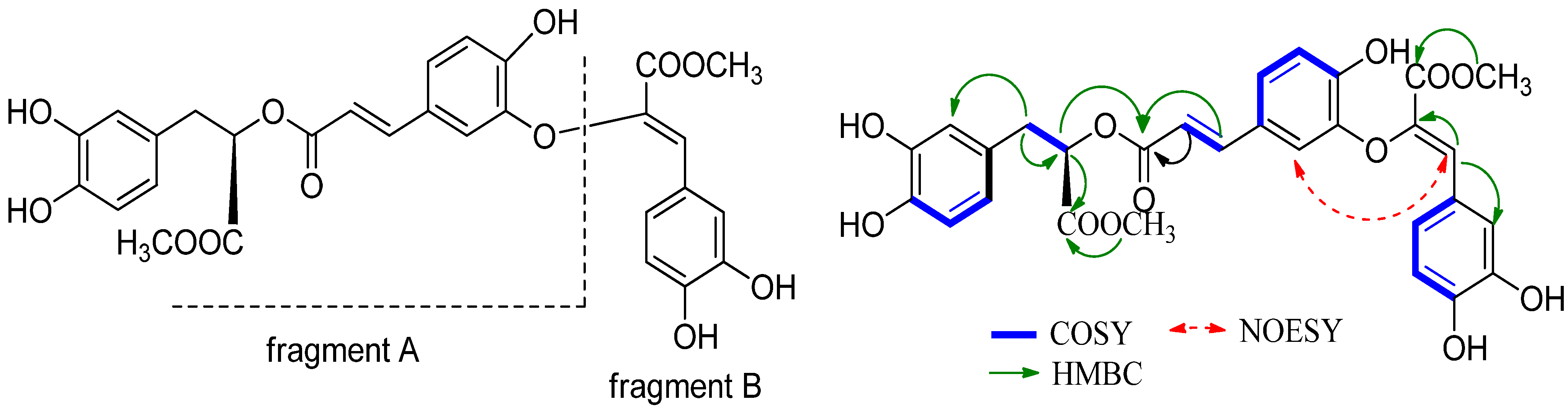
2. Results and Discussion
| Position | δC | δH | Position | δC | δH |
|---|---|---|---|---|---|
| 1 | 128.9 | - | 6′ | 125.9 | 7.20 (br d, 7.8) |
| 2 | 117.7 | 6.68 (br s) | 7′ | 147.3 | 7.50 (d, 14.2) |
| 3 | 146.3 | - | 8′ | 115.2 | 6.17 (d, 14.2) |
| 4 | 145.6 | - | 9′ | 168.2 | - |
| 5 | 116.7 | 6.67 (d, 7.8) | 1′′ | 126.1 | - |
| 6 | 122.0 | 6.54 (br d, 7.8) | 2′′ | 118.2 | 7.31 (br s) |
| 7 | 38.1 | 2.99 (dd, 8.4, 14.4) | 3′′ | 146.7 | - |
| 3.03 (dd, 4.8, 14.4) | |||||
| 8 | 74.9 | 5.16 (dd, 4.8, 8.4) | 4′′ | 149.2 | - |
| 9 | 173.4 | - | 5′′ | 116.5 | 6.76 (br d, 7.8) |
| 1′ | 127.7 | - | 6′′ | 125.4 | 7.11 (br d, 7.8) |
| 2′ | 115.1 | 6.93 (br s) | 7′′ | 129.9 | 7.35 (s) |
| 3′ | 146.4 | - | 8′′ | 138.8 | - |
| 4′ | 151.4 | - | 9′′ | 165.9 | - |
| 5′ | 118.4 | 6.94 (d, 7.8) | |||
| 9-OCH3 | 52.9 | 3.67 (s) | 9′′-OCH3 | 52.9 | 3.74 (s) |

3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectral Data
4. Conclusions
Acknowledgments
References
- Xie, Z.Y. National Herbal Compendium, 2nd; Kuang, L.J., Ed.; People’s Medical Publishing House: Beijing, China, 1996; p. 572. [Google Scholar]
- Jia, M.R.; Li, X.W. Chinese Ethnic Materia Medica, 1st; Zhao, Y.X., Ed.; China Medical Science and Technology Press: Beijing, China, 2005; p. 166. [Google Scholar]
- Zhao, A.H.; Zhao, Q.S.; Li, R.T.; Sun, H.D. Chemical constituents from Clerodendranthus spicatus. Acta Bot. Yunnanica 2004, 26, 563–568. [Google Scholar]
- Tezuka, Y.; Stampoulis, P.; Banskota, A.H.; Awale, S.; Tran, K.Q.; Saiki, I.; Kadota, S. Constituents of the Vietnamese medicinal plant Orthosiphon stmineus. Chem. Pharm. Bull. 2000, 48, 1711–1719. [Google Scholar] [CrossRef]
- Chen, Y.L.; Tan, C.H.; Tan, J.J.; Zhao, X.M.; Jiang, S.H.; Zhu, D.Y. Two new diterpenoid glucosides from Clerodendranthus spicatus. Helv. Chim. Acta 2009, 92, 2802–2807. [Google Scholar] [CrossRef]
- Awale, S.; Tezukam, Y.; Banskota, A.H.; Kadota, S. Inhibition of NO production by highly-oxygenated diterpenes of Orthosiphon stmineus and their structure-activity relationship. Biol. Pharm. Bull. 2003, 26, 468–473. [Google Scholar] [CrossRef]
- Nguyen, M.T.T.; Awale, S.; Tezuka, Y.; Hsuing, C.C.; Kadota, S. Staminane- and Isopimarane-type diterpenes from Orthosiphon stmineus of Taiwan and their nitric oxide inhibitory activity. J. Nat. Prod. 2004, 67, 654–658. [Google Scholar] [CrossRef]
- Shibuya, H.; Bohgaki, T.; Matsubara, T.; Watarai, M.; Ohashi, K.; Kitagawa, I. Indonesian Medicinal Plants. XXII. Chemical structures of two new isopimarane-type diterpenes, orthosiphonones A and B, and a new benzochromene, orthochromene A from the leaves of Orthosiphon aristatus (Lamiaceae). Chem. Pharm. Bull. 1999, 47, 695–698. [Google Scholar] [CrossRef]
- Sumaryono, W.; Proksch, P.; Wray, V.; Hartmann, T. Qualitative and quantitative analysis of the phenolic constituents from Orthosiphon aristatus. Planta Med. 1991, 57, 176–180. [Google Scholar] [CrossRef]
- Wang, M.; Liang, J.Y.; Chen, X.Y. Water-soluble constituents of Clerodendranthus spicatus. Chin. J. Nat. Med. 2007, 5, 27–30. [Google Scholar]
- Zou, J.; Zhu, Y.D.; Zhao, W.M. Two new alkyl glycosides from Clerodendranthus spicatus. J. Asian Nat. Prod. Res. 2008, 10, 602–606. [Google Scholar] [CrossRef]
- Tanaka, T.; Nishimura, A.; Kouno, I.; Nonaka, G.; Young, T.J. Isolation and characterization of yunnaneic acids A–D, four novel caffeic acid metabolites from Salvia yunnanensis. J. Nat. Prod. 1996, 59, 843–849. [Google Scholar] [CrossRef]
- Dapkevicius, A.; van Beek, T.A.; van Veldhuizen, A.; de Groot, A.; Linssen, J.P.; Venskutonis, R. Isolation and structure elucidation of radical scavengers from Thymus vulgaris leaves. J. Nat. Prod. 2002, 65, 892–896. [Google Scholar] [CrossRef]
- Agata, I.; Kusakabe, H.; Hatano, T.; Nishibe, S.; Okuda, T. Melitric acid A and B, new trimeric caffeic acid derivatives from Melissa officinalis. Chem. Pharm. Bull. 1993, 41, 1608–1611. [Google Scholar] [CrossRef]
- Sample Availability: Samples of all the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zheng, Q.; Sun, Z.; Zhang, X.; Yuan, J.; Wu, H.; Yang, J.; Xu, X. Clerodendranoic Acid, a New Phenolic Acid from Clerodendranthus spicatus. Molecules 2012, 17, 13656-13661. https://doi.org/10.3390/molecules171113656
Zheng Q, Sun Z, Zhang X, Yuan J, Wu H, Yang J, Xu X. Clerodendranoic Acid, a New Phenolic Acid from Clerodendranthus spicatus. Molecules. 2012; 17(11):13656-13661. https://doi.org/10.3390/molecules171113656
Chicago/Turabian StyleZheng, Qingxia, Zhaocui Sun, Xiaopo Zhang, Jinquan Yuan, Haifeng Wu, Junshan Yang, and Xudong Xu. 2012. "Clerodendranoic Acid, a New Phenolic Acid from Clerodendranthus spicatus" Molecules 17, no. 11: 13656-13661. https://doi.org/10.3390/molecules171113656
APA StyleZheng, Q., Sun, Z., Zhang, X., Yuan, J., Wu, H., Yang, J., & Xu, X. (2012). Clerodendranoic Acid, a New Phenolic Acid from Clerodendranthus spicatus. Molecules, 17(11), 13656-13661. https://doi.org/10.3390/molecules171113656