By Improving Regional Cortical Blood Flow, Attenuating Mitochondrial Dysfunction and Sequential Apoptosis Galangin Acts as a Potential Neuroprotective Agent after Acute Ischemic Stroke
Abstract
:1. Introduction
2. Results and Discussion
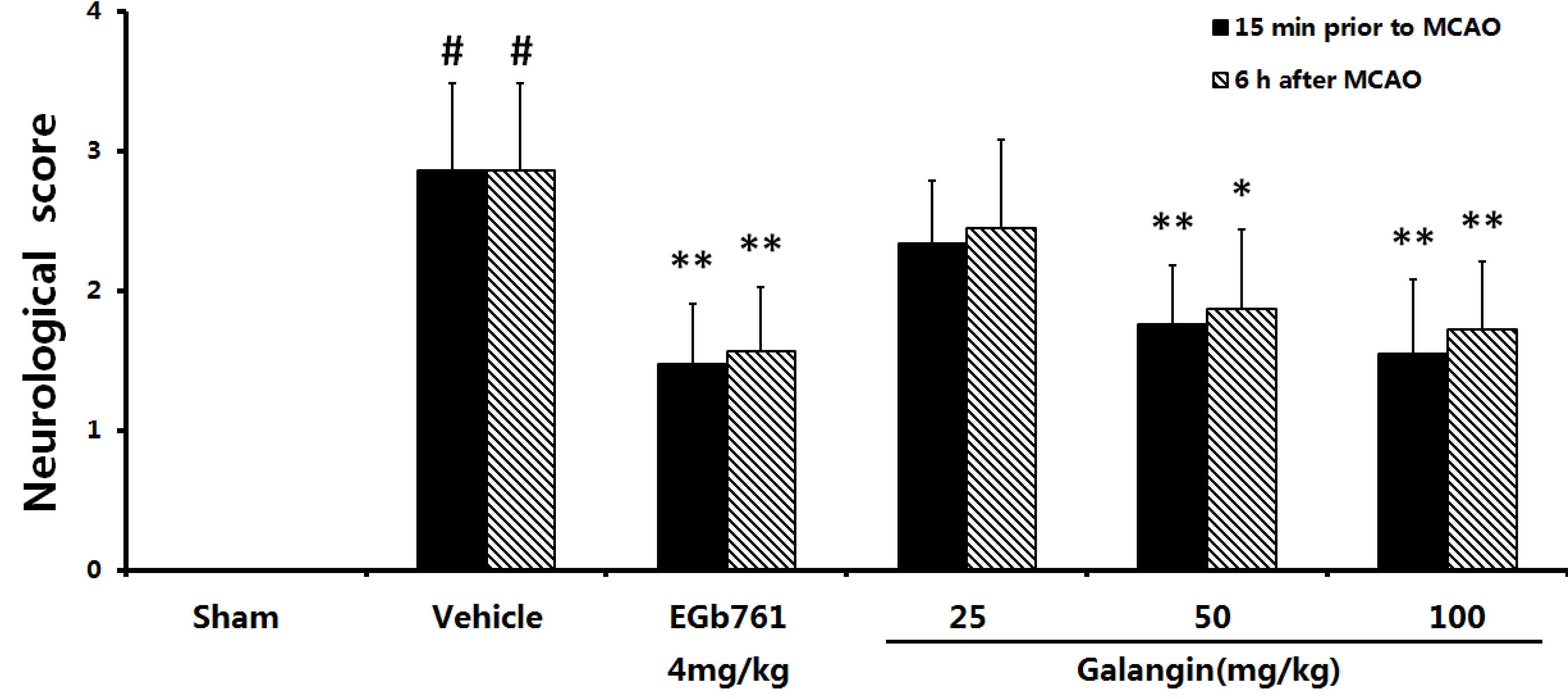
2.1. Neurological Defects
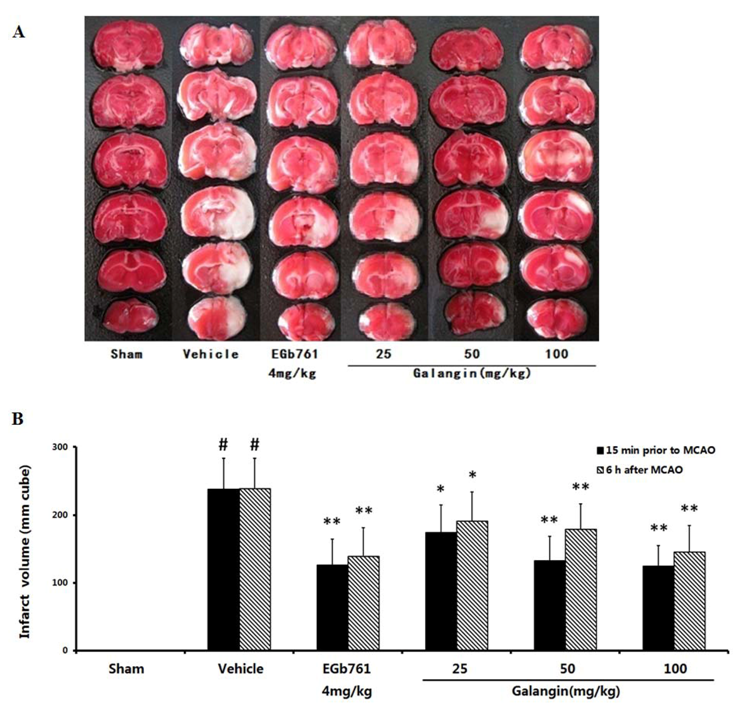
2.2. Cerebral Infarct Size
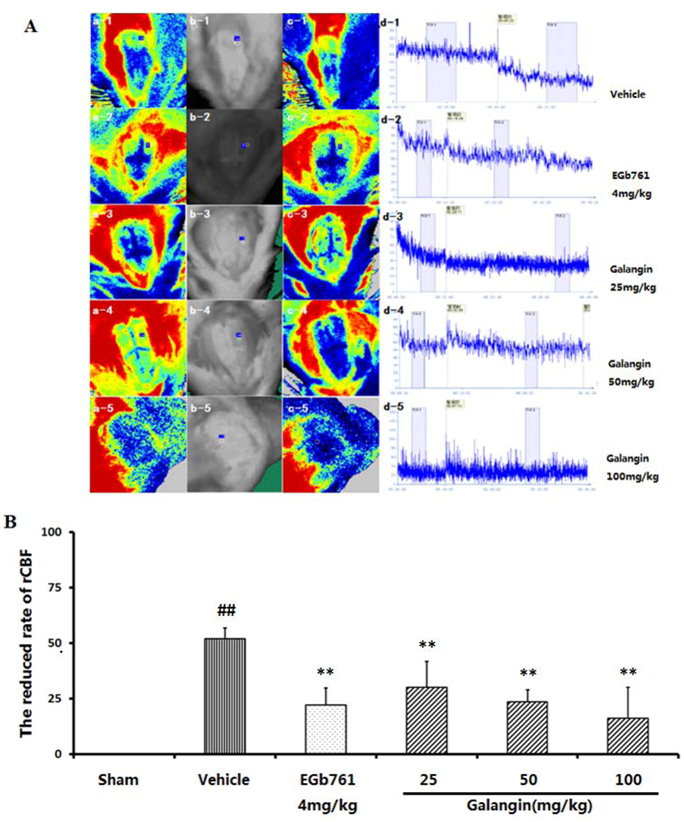
2.3. Regional Cortical Blood Perfusion
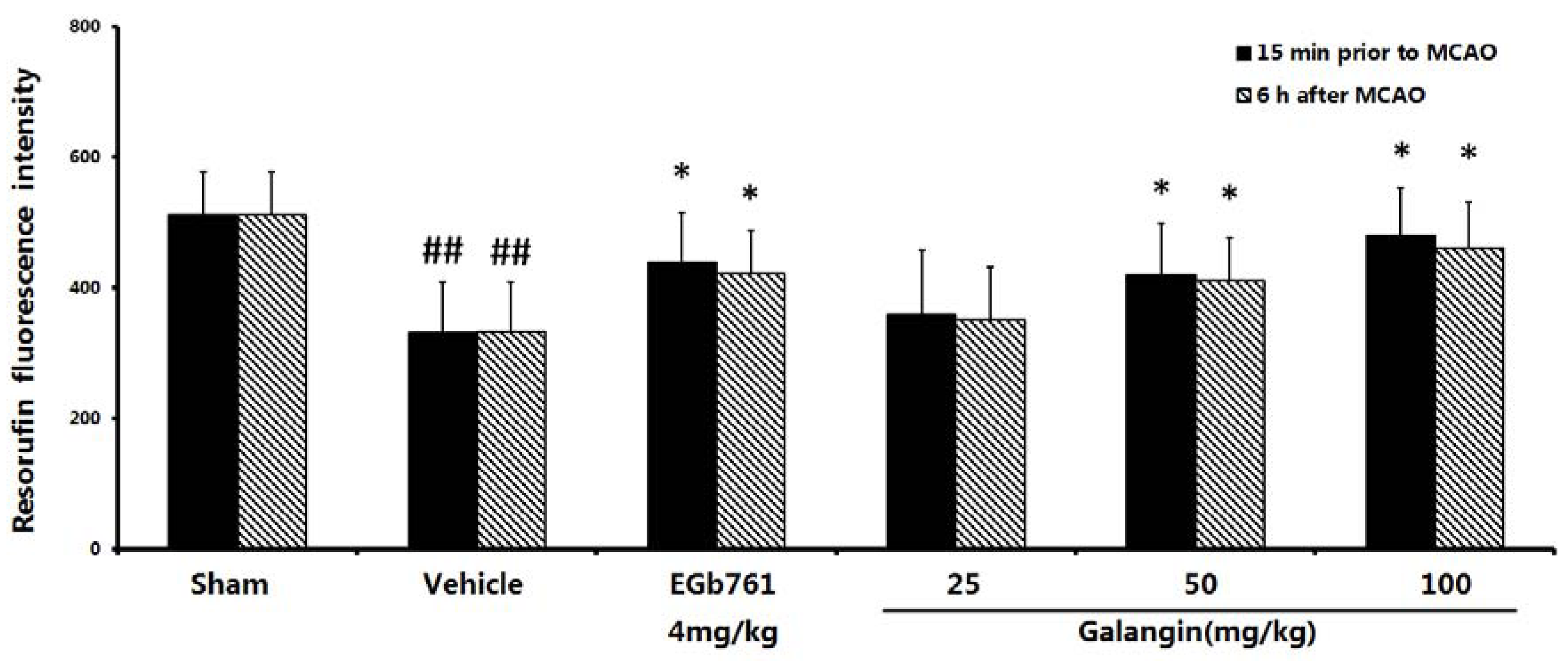
2.4. Measurement of Mitochondrial Function
2.5. Measurement of Mitochondrial Swelling
| Group | Dose (mg·kg−1) | n | Swelling/A520 (Slope absolute value) | |
|---|---|---|---|---|
| 15 min prior to MCAO | 6 h after MCAO | |||
| Sham | - | 10 | 0.053 ± 0.012 | 0.035 ± 0.067 |
| Vehicle | - | 10 | 0.402 ± 0.008 ## | 0.476 ± 0.011 ## |
| EGb761 | 4 | 10 | 0.093 ± 0.014 ** | 0.064 ± 0.007 ** |
| Galangin | 25 | 10 | 0.389 ± 0.021 ** | 0.355 ± 0.010 ** |
| Galangin | 50 | 10 | 0.187 ± 0.010 ** | 0.167 ± 0.008 ** |
| Galangin | 100 | 10 | 0.093 ± 0.008 ** | 0.089 ± 0.013 ** |
2.6. Mitochondrial Membrane Fluidity Measurement
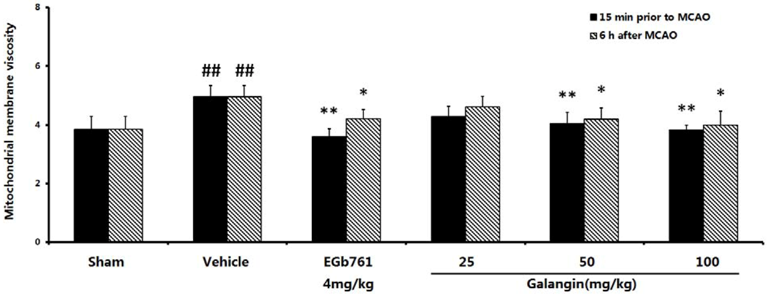
2.7. The Effect of Galangin on Mitochondria Transmembrane Potential after MCAO
2.7.1. Determination with Rhodamine 123
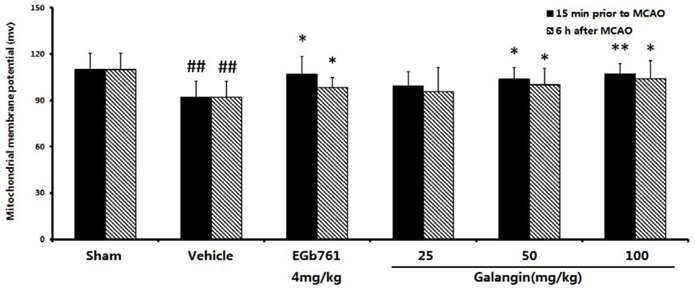
2.7.2. Determination by the JC-1 Method

2.8. Effects of Galangin on Mitochondrial ROS Levels
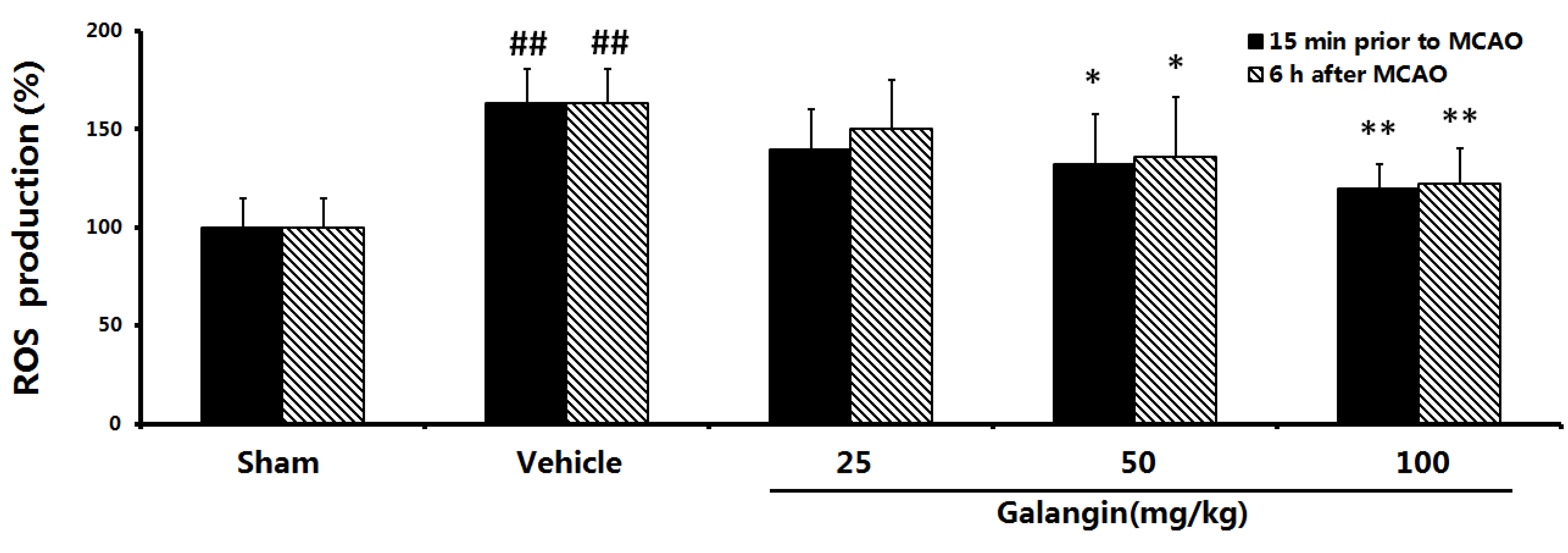
2.9. Evaluation of the Anti-Apoptotic Effect
2.9.1. Modulation of Bcl-2 and Bax Expression
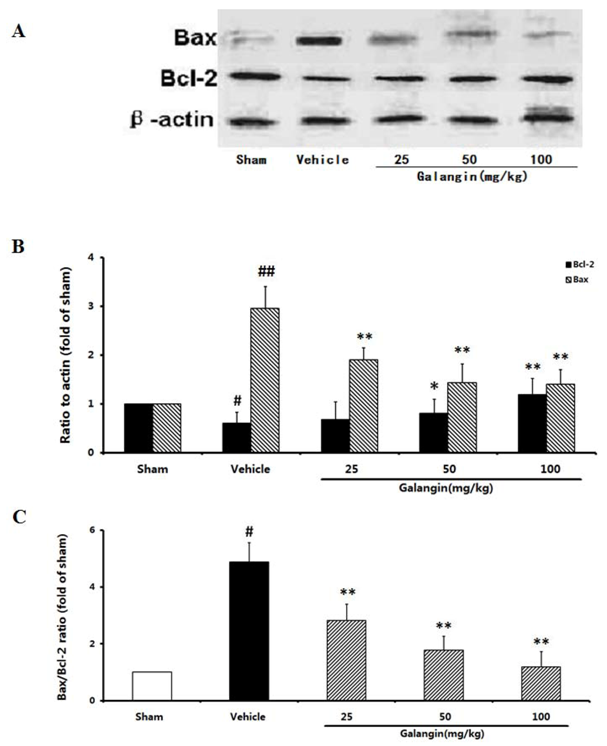
2.9.2. Modulation of Activated Caspase-3 and PARP Expression

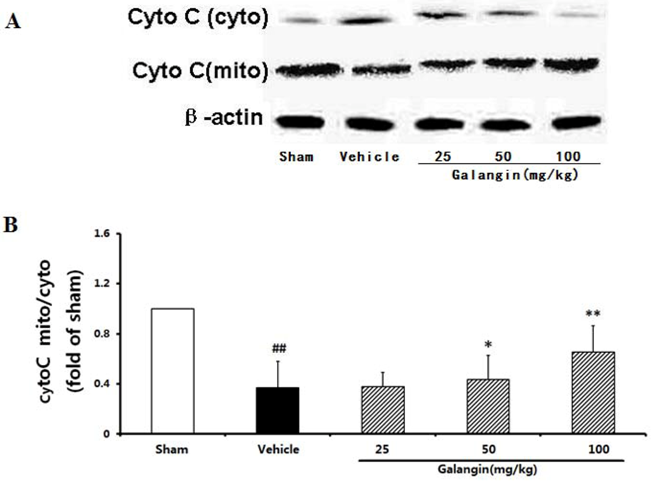
2.9.3. Modulation of Cytochome c Expression
2.10. Discussion
3. Experimental
3.1. Animals
3.2. Chemicals and Reagents
3.3. Animal Models and Experimental Protocol
3.4. Assessment of Neurological Defects
3.5. Cerebral Infarct Size
3.6. Regional Cortical Blood Perfusion
3.7. Preparation of Rat Brain Mitochondria
3.8. Measurement of Mitochondrial Viability
3.9. Measurement of Mitochondrial Swelling
3.10. Measurement of Mitochondrial Membrane fluidity
3.11. Measurement of Mitochondrial Transmembrane Potential (Δψm)
3.11.1. Rhodamine 123 Method
3.11.2. JC-1 Method
3.12. Measurement of Reactive Oxygen Species (ROS) Production in Mitochondria
3.13. Western Blot of Apoptosis Related Proteins
3.13.1. Cortex Proteins
3.13.2. Mitochondrial Proteins
3.14. Statistical Analyses
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the compounds galangin are available from the authors.
References
- Flynn, R.W.; MacWalter, R.S.; Doney, A.S. The cost of cerebral ischaemia. Neuropharmacology 2008, 55, 250–256. [Google Scholar] [CrossRef]
- Uzar, E.; Acar, A.; Evliyaoglu, O.; Firat, U.; Kamasak, K.; Gocmez, C.; Alp, H.; Tufek, A.; Tasdemir, N.; Ilhan, A. The anti-oxidant and anti-apoptotic effects of nebivolol and zofenopril in a model of cerebral ischemia/reperfusion in rats. Prog. Neuropsychopharmacol. Biol. Psychiat. 2012, 36, 22–28. [Google Scholar] [CrossRef]
- Kobayashi, T.; Mori, Y. Ca2+channel antagonists and neuroprotection from cerebral ischemia. Eur.J. Pharmacol. 1988, 363, 1–15. [Google Scholar] [CrossRef]
- Gardner, A.; Pagani, M.; Wibom, R.; Nennesmo, I.; Jacobsson, H.; Hällström, T. Alterations of rCBF and mitochondrial dysfunction in major depressive disorder: a case report. Acta Psychiatr. Scand. 2003, 107, 233–239. [Google Scholar] [CrossRef]
- Kannan, K.; Holcombe, R.F.; Jain, S.K.; Alvarez-Hernandez, X.; Chervenak, R.; Wolf, R.E.; Glass, J. Evidence for the induction of apoptosis by endosulfan in a human T-cell leukemic line. Mol. Cell. Biochem. 2000, 205, 53–66. [Google Scholar] [CrossRef]
- Ciolino, H.P.; Yeh, G.C. The flavonoid galangin is an inhibitor of CYP1A1 activity and an agonist/antagonist of the aryl hydrocarbon receptor. Br. J. Cancer 1999, 79, 1340–1346. [Google Scholar] [CrossRef]
- Sivakumar, A.S.; Anuradha, C.V. Effect of galangin supplementation on oxidative damage and inflammatory changes in fructose-fed rat liver. Chem. Biol. Interact. 2011, 193, 141–148. [Google Scholar]
- Chen, J.C.; Ho, F.M.; Pei-Dawn, L.C.; Chen, C.P.; Jeng, K.C.; Hsu, H.B.; Lee, S.T.; Wen, T.W.; Lin, W.W. Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IκB kinase, Nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. Eur. J. Pharmacol. 2006, 521, 9–20. [Google Scholar]
- Blonska, M.; Bronikowska, J.; Pietsz, G.; Czuba, Z.P.; Scheller, S.; Krol, W. Effects of ethanol extract of propolis (EEP) and its flavones on inducible gene expression in J774A. 1 macrophages. J. Ethnopharmacol. 2004, 91, 25–30. [Google Scholar] [CrossRef]
- Morello, S.; Vellecco, V.; Alfieri, A.; Mascolo, N.; Cicala, C. Vasorelaxant effect of the flavonoid galangin on isolated rat thoracic aorta. Life Sci. 2006, 78, 825–830. [Google Scholar] [CrossRef]
- Khalil, M.L.; Sulaiman, S.A. The potential role of honey and its polyphenols in preventing heart diseases: a review. Afr. J. Tradit. Complement Altern. Med. 2010, 7, 315–321. [Google Scholar]
- Guo, A.J.; Xie, H.Q.; Choi, R.C.; Zheng, K.Y.; Bi, C.W.; Xu, S.L.; Dong, T.T.; Tsim, K.W. Galangin, a flavonol derived from Rhizoma Alpiniae Officinarum, inhibits acetylcholinesterase activity in vitro. Chem. Biol. Interact. 2010, 187, 246–248. [Google Scholar] [CrossRef]
- Saponara, S.; Carosati, E.; Mugnai, P.; Sgaragli, G.; Fusi, F. The flavonoid scaffold as template for the design of modulators of the vascular Ca(v) 1.2 channels. Br. J. Pharmacol. 2011, 164, 1684–1697. [Google Scholar] [CrossRef]
- Macrae, I.M. Preclinical stroke research—Advantages and disadvantages of the most common rodent models of focal ischaemia. Br. J. Pharmacol. 2011, 164, 1062–1078. [Google Scholar] [CrossRef]
- Tan, C.B.; Gao, M.; Xu, W.R.; Yang, X.Y.; Zhu, X.M.; Du, G.H. Protective Effects of Salidroside on Endothelial Cell Apoptosis Induced by Cobalt Chloride. Biol. Pharm. Bull. 2009, 32, 1359–1363. [Google Scholar] [CrossRef]
- Iwanami, J.; Mogi, M.; Okamoto, S.; Gao, X.Y.; Li, J.M.; Min, L.J.; Ide, A.; Tsukuda, K.; Iwai, M.; Horiuchi, M. Pretreatment with eplerenone reduces stroke volume in mouse middle cerebral artery occlusion model. Eur. J. Pharmacol. 2007, 566, 153–159. [Google Scholar] [CrossRef]
- Rudzinski, W.; Swiat, M.; Tomaszewski, M.; Krejza, J. Cerebral hemodynamics and investigations of cerebral blood flow regulation. Nucl. Med. Rev. Cent. East. Eur. 2007, 10, 29–42. [Google Scholar]
- Della-Morte, D.; Rave, A.P.; Dave, K.R.; Lin, H.W.; Perez-Pinzon, M.A. Post-Ischemic Activation of protein kinase C epsilon protects the hippocampus from cerebral ischemic injury via alterations in cerebral blood flow. Neurosci. Lett. 2011, 487, 158–162. [Google Scholar] [CrossRef]
- Vosler, P.S.; Graham, S.H.; Wechsler, L.R.; Chen, J. Mitochondrial targets for stroke: Focusing basic science research toward development of clinically translatable therapeutics. Stroke 2009, 40, 3149–3155. [Google Scholar] [CrossRef]
- Kelley, K.A.; Galeffi, F.; Gerich, F.J.; Turner, D.A.; Müller, M. Optical and pharmacological tools to investigate the role of mitochondria during oxidative stress and neurodegeneration. Prog. Neurobiol. 2006, 79, 136–171. [Google Scholar] [CrossRef]
- Koh, P.O. Gingko biloba extract (EGb 761) attenuates the focal cerebral ischemic injury-induced decrease in astrocytic phosphoprotein PEA-15 levels. Am. J. Chin. Med. 2011, 39, 971–979. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, D.; Zhu, H.; Wang, X. Experimental evidence of Ginkgo biloba extract EGB as a neuroprotective agent in ischemia stroke rats. Brain Res. Bull. 2012, 87, 193–198. [Google Scholar] [CrossRef]
- Shi, C.; Xiao, S.; Liu, J.; Guo, K.; Wu, F.; Yew, D.T.; Xu, J. Ginkgo biloba extract EGb761 protects against aging-associated mitochondrial dysfunction in platelets and hippocampi of SAMP8 mice. Platelets 2010, 21, 373–379. [Google Scholar] [CrossRef]
- Shen, J.; Lee, W.; Gu, Y.; Tong, Y.; Fung, P.C.; Tong, L. Ginkgo biloba extract (EGb761) inhibits mitochondria-dependent caspase pathway and prevents apoptosis in hypoxia-reoxygenated cardiomyocytes. Chin. Med. 2011, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Dave, K.R.; DeFazio, R.A.; Raval, A.P.; Torraco, A.; Saul, I.; Barrientos, A.; Perez-Pinzon, M.A. Ischemic preconditioning targets the respiration of synaptic mitochondria via protein kinase C epsilon. J. Neurosci. 2008, 28, 4172–4182. [Google Scholar] [CrossRef]
- Zheng, C.Y.; Zhang, H.Y.; Tang, X.C. Huperzine A attenuates mitochondrial dysfunction after middle cerebral artery occlusion in rats. J. Neurosci. Res. 2008, 86, 2432–2440. [Google Scholar] [CrossRef]
- Shen, H.; Kuo, C.C.; Chou, J.; Delvolve, A.; Jackson, S.N.; Post, J.; Woods, A.S.; Hoffer, B.J.; Wang, Y.; Harvey, B.K. Astaxanthin reduces ischemic brain injury in adult rats. FASEB J. 2009, 23, 1958–1968. [Google Scholar] [CrossRef]
- Everett, H.; Barry, M.; Sun, X.; Lee, S.F.; Frantz, C.; Berthiaume, L.G.; McFadden, G.; Bleackley, R.C. The myxoma poxvirus protein, M11L, Prevents apoptosis by direct interaction with the mitochondrial permeability transition pore. J. Exp. Med. 2002, 196, 1127–1140. [Google Scholar] [CrossRef]
- Clark, R.S.; Nathaniel, P.D.; Zhang, X.; Dixon, C.E.; Alber, S.M.; Watkins, S.C.; Melick, J.A.; Kochanek, P.M.; Graham, S.H. Boc-Aspartyl(OMe)-fluoromethyl ketone attenuates mitochondrial release of cytochrome c and delays brain tissue loss after traumatic brain injury in rats. J. Cereb. Blood Flow Metab. 2007, 27, 316–326. [Google Scholar] [CrossRef]
- Jiang, W.L.; Zhang, S.P.; Zhu, H.B.; Hou, J.; Tian, J.W. Cornin ameliorates cerebral infarction in rats by antioxidant action and stabilization of mitochondrial function. Phytother. Res. 2010, 24, 547–552. [Google Scholar]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef]
- Saraf, M.K.; Prabhakar, S.; Anand, A. Neuroprotective effect of Bacopamonniera on ischemia induced brain injury. Pharmacol. Biochem. Behav. 2010, 97, 192–197. [Google Scholar] [CrossRef]
- Boas, D.A.; Dunn, A.K. Laser speckle contrast imaging in biomedical optics. J. Biomed. 2010, 15. [Google Scholar] [CrossRef]
- Briers, J.D. Laser Doppler, speckle and related techniques for blood perfusion mapping and imaging. Physiol. Meas. 2001, 22, R35–R66. [Google Scholar] [CrossRef]
- Draijer, M.; Hondebrink, E.; van Leeuwen, T.; Steenbergen, W. Review of laser speckle contrast techniques for visualizing tissue perfusion. Lasers. Med. Sci. 2009, 24, 639–651. [Google Scholar] [CrossRef]
- Xia, T.; Jiang, C.; Li, L.; Wu, C.; Chen, Q.; Liu, S.S. A study on permeability transition pore opening and cytochrome c release from mitochondria, induced by caspase-3 in vitro. FEBS Lett. 2002, 510, 62–66. [Google Scholar] [CrossRef]
- Hang, H.X.; Du, G.H.; Zhang, J.T. Assay of mitochondrial functions by resazurin in vitro. Acta Pharmacol. Sin. 2004, 25, 385–389. [Google Scholar]
- Tian, J.; Fu, F.; Geng, M.; Jiang, Y.; Yang, J.; Jiang, W.; Wang, C.; Liu, K. Neuroprotective effect of 20(S)-ginsenoside Rg3 on cerebral ischemia in rats. Neurosci. Lett. 2005, 374, 92–97. [Google Scholar] [CrossRef]
- Chen, L.M.; Zhou, X.M.; Cao, Y.L.; Hu, W.X. Neuroprotection of ginsenoside Re in cerebral ischemia-reperfusion injury in rats. J. Asian Nat. Prod. Res. 2008, 10, 439–445. [Google Scholar] [CrossRef]
- Hirano, K. Change in membrane fluidity of sand dollar egg cortices caused by Ca-induced exocytosis: microscopic analysis with fluorescence anisotropy. Dev. Growth. Differ. 1991, 33, 451–458. [Google Scholar]
- Zhang, H.X.; Du, G.H.; Zhang, J.T. Ischemic pre-conditioning preserves brain mitochondrial functions during the middle cerebral artery occlusion in rat. Neurol. Res. 2003, 25, 471–476. [Google Scholar] [CrossRef]
- He, X.L.; Wang, Y.H.; Gao, M.; Li, X.X.; Zhang, T.T.; Du, G.H. Baicalein protects rat brain mitochondria against chronic cerebral hypoperfusion-induced oxidative damage. Brain Res. 2008, 1249, 212–221. [Google Scholar]
- Ma, H.; Quan, F.; Chen, D.; Zhang, B.; Zhang, Y. Alterations in mitochondrial function and spermatozoal motility in goat spermatozoa following incubation with a human lysozyme plasmid. Anim. Reprod. Sci. 2010, 121, 106–114. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, S.; Wu, C.; Zhu, L.; Gao, J.; Fang, J.; Li, D.; Fu, M.; Liang, R.; Wang, L.; Cheng, M.; et al. By Improving Regional Cortical Blood Flow, Attenuating Mitochondrial Dysfunction and Sequential Apoptosis Galangin Acts as a Potential Neuroprotective Agent after Acute Ischemic Stroke. Molecules 2012, 17, 13403-13423. https://doi.org/10.3390/molecules171113403
Li S, Wu C, Zhu L, Gao J, Fang J, Li D, Fu M, Liang R, Wang L, Cheng M, et al. By Improving Regional Cortical Blood Flow, Attenuating Mitochondrial Dysfunction and Sequential Apoptosis Galangin Acts as a Potential Neuroprotective Agent after Acute Ischemic Stroke. Molecules. 2012; 17(11):13403-13423. https://doi.org/10.3390/molecules171113403
Chicago/Turabian StyleLi, Shaojing, Chuanhong Wu, Li Zhu, Jian Gao, Jing Fang, Defeng Li, Meihong Fu, Rixin Liang, Lan Wang, Ming Cheng, and et al. 2012. "By Improving Regional Cortical Blood Flow, Attenuating Mitochondrial Dysfunction and Sequential Apoptosis Galangin Acts as a Potential Neuroprotective Agent after Acute Ischemic Stroke" Molecules 17, no. 11: 13403-13423. https://doi.org/10.3390/molecules171113403
APA StyleLi, S., Wu, C., Zhu, L., Gao, J., Fang, J., Li, D., Fu, M., Liang, R., Wang, L., Cheng, M., & Yang, H. (2012). By Improving Regional Cortical Blood Flow, Attenuating Mitochondrial Dysfunction and Sequential Apoptosis Galangin Acts as a Potential Neuroprotective Agent after Acute Ischemic Stroke. Molecules, 17(11), 13403-13423. https://doi.org/10.3390/molecules171113403




