The Effects of Davallic Acid from Davallia divaricata Blume on Apoptosis Induction in A549 Lung Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Structure Elucidation of Davallic Acid
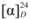 : +3.6° (c = 0.87, CHCl3). The LC-MS/MS analysis revealed a major peak at m/z 439.3 (M−H−) with very little fragmentation that confirmed the molecular formula to be C30H48O2 (MW 440). Further MS/MS analysis yielded very little fragment ions, which agrees with the triterpene compound nature. The compound was therefore identified as davallic acid (Figure 1) [11] based on its 1H- and 13C-NMR spectrum (Table 1) along with data comparisons with literature data.
: +3.6° (c = 0.87, CHCl3). The LC-MS/MS analysis revealed a major peak at m/z 439.3 (M−H−) with very little fragmentation that confirmed the molecular formula to be C30H48O2 (MW 440). Further MS/MS analysis yielded very little fragment ions, which agrees with the triterpene compound nature. The compound was therefore identified as davallic acid (Figure 1) [11] based on its 1H- and 13C-NMR spectrum (Table 1) along with data comparisons with literature data.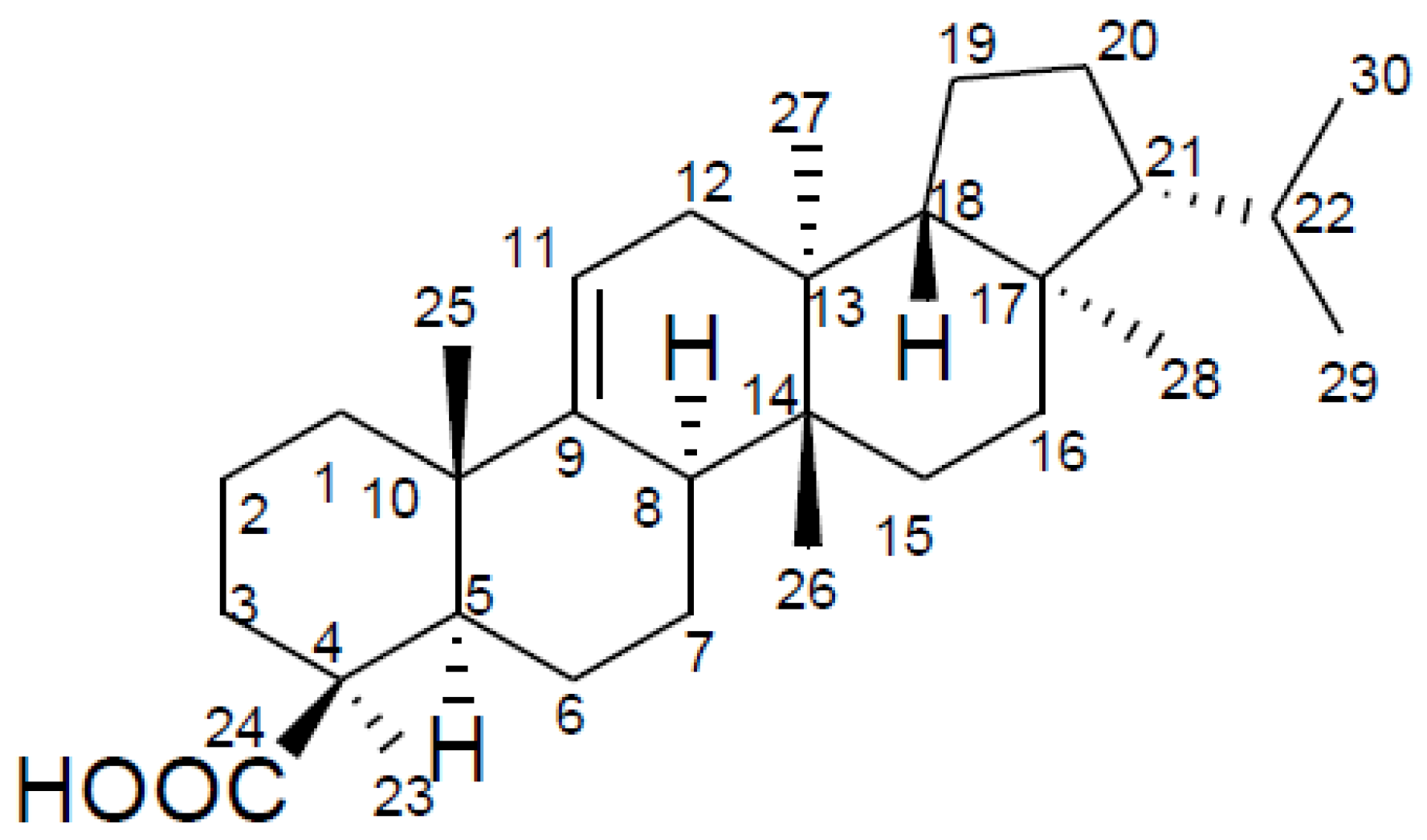
| Position | δH (J in Hz) | δC |
|---|---|---|
| 1 | 41.8 | |
| 2 | 19.5 | |
| 3 | 42.7 | |
| 4 | 35.8 | |
| 5 | 46.9 | |
| 6 | 18.2 | |
| 7 | 19.8 | |
| 8 | 39.4 | |
| 9 | 149.7 | |
| 10 | 38.0 | |
| 11 | 5.36 (1H, m) | 116.7 |
| 12 | 36.5 | |
| 13 | 37.5 | |
| 14 | 38.3 | |
| 15 | 28.1 | |
| 16 | 36.4 | |
| 17 | 44.3 | |
| 18 | 51.7 | |
| 19 | 20.2 | |
| 20 | 28.0 | |
| 21 | 59.4 | |
| 22 | 29.0 | |
| 23 | 1.25 (3H, s) | 30.5 |
| 24 | 183.9 | |
| 25 | 1.02 (3H, s) | 23.1 |
| 26 | 0.75 (3H, s) | 15.4 |
| 27 | 0.83 (3H, s) | 15.3 |
| 28 | 0.78 (3H, s) | 13.7 |
| 29 | 0.85 (3H, d, J = 6.7) | 21.9 |
| 30 | 0.90 (3H, d, J = 6.7) | 22.7 |
2.2. The Effect of Davallic Acid on A549 Viability

2.3. The Effect of Davallic Acid on Cell Cycle
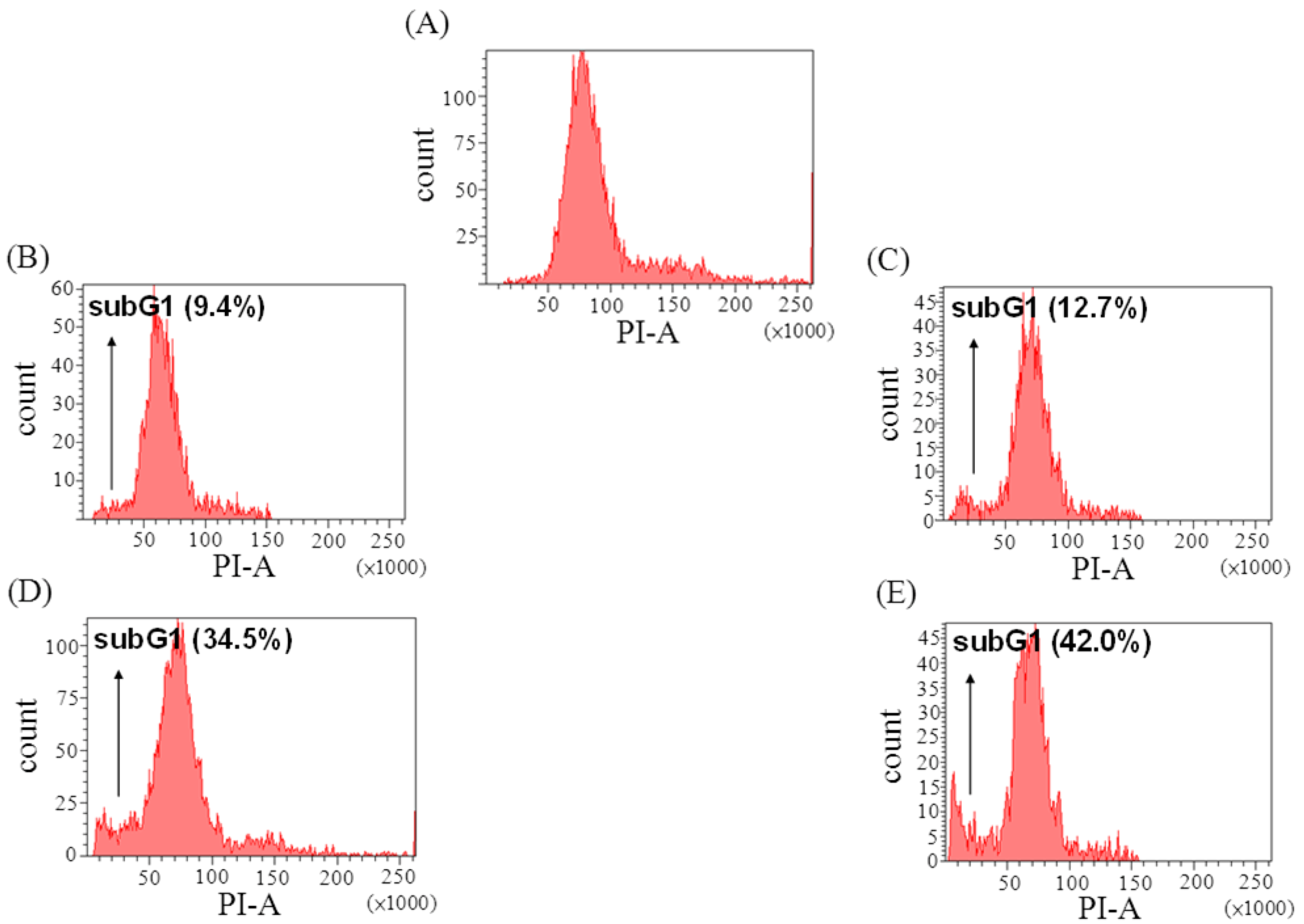
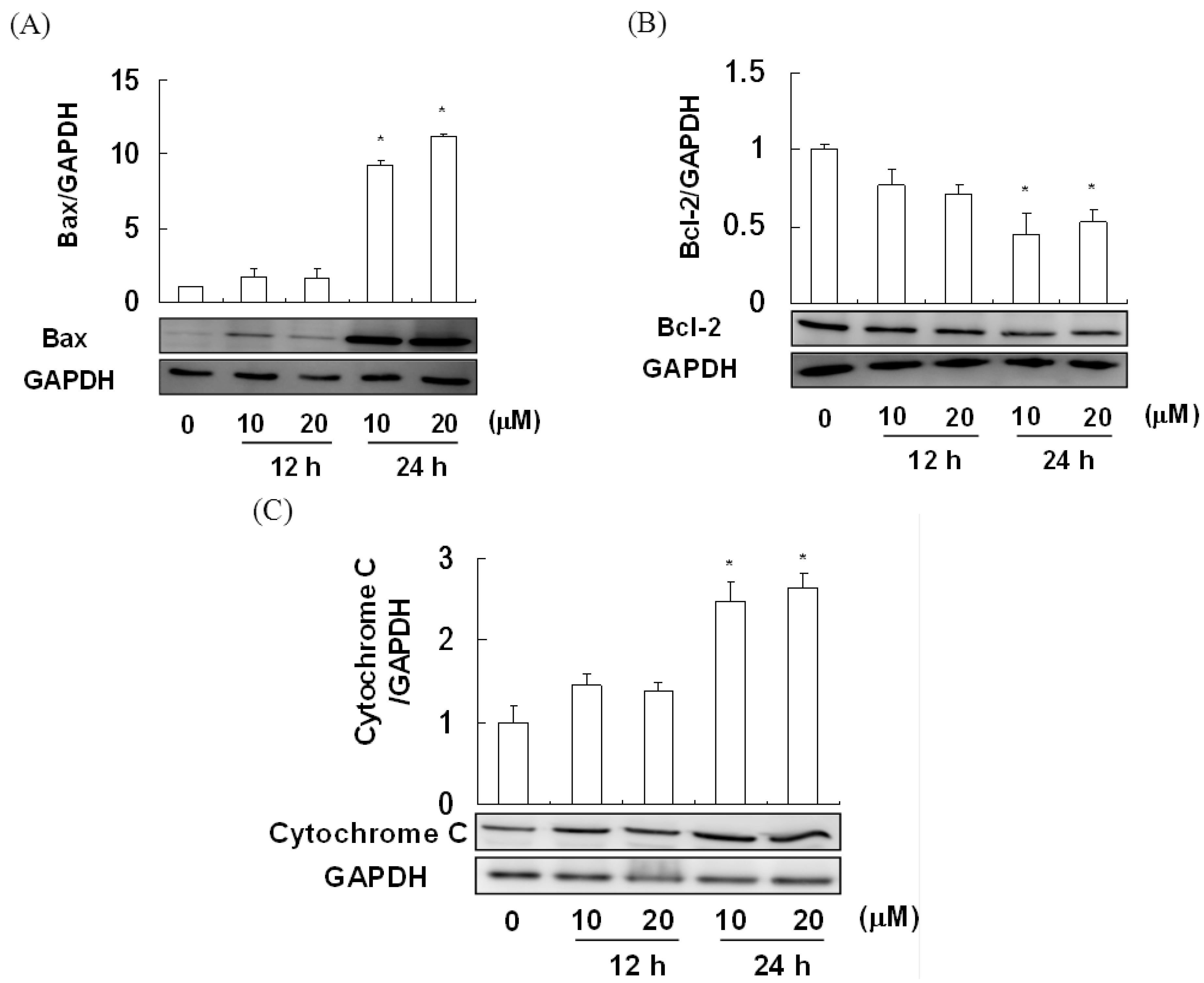
2.4. The Effect of Davallic Acid on Bax and Bcl-2 Proteins in A549 Cell
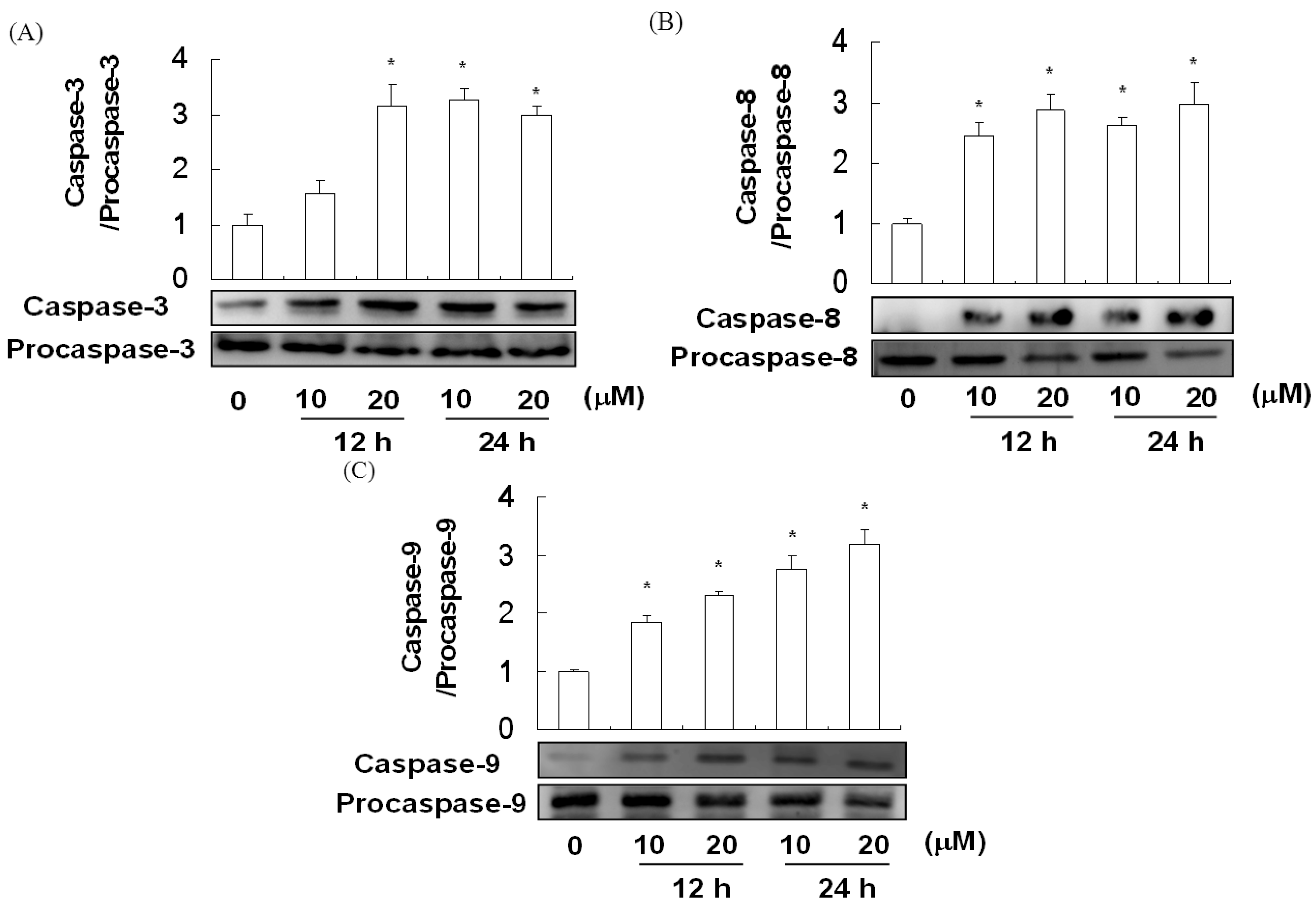
2.5. The Effect of Davallic Acid on Caspase Activity
3. Discussion
4. Experimental
4.1. General
4.2. Plant Material and Isolation
4.3. Cell Culture
4.4. Cell Viability
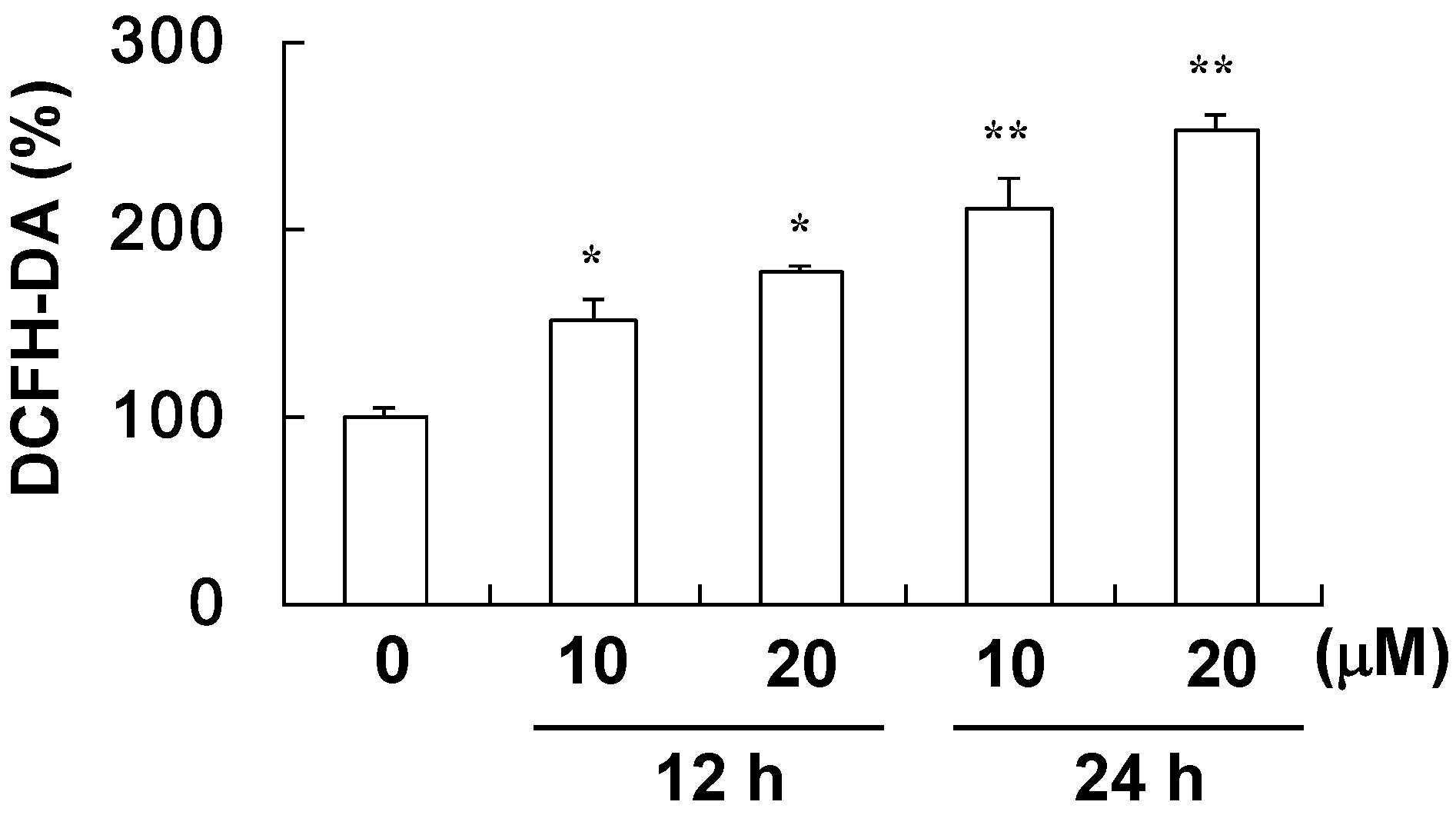
4.5. Assay for ROS Level
4.6. Cell Cycle Distribution
4.7. Immunoblot Analysis
4.8. Statistical Analysis
5. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- Yang, Y.T.; Weng, C.J.; Ho, C.T.; Yen, G.C. Resveratrol analog-3,5,4′-trimethoxy-trans-stilbene inhibits invasion of human lung adenocarcinoma cells by suppressing the MAPK pathway and decreasing matrix metalloproteinase-2 expression. Mol. Nutr. Food. Res. 2009, 53, 407–416. [Google Scholar] [CrossRef]
- Chang, H.C.; Huang, G.J.; Agrawal, D.C.; Kuo, C.L.; Wu, C.R.; Tsay, H.S. Antioxidant activities and polyphenol contents of six folk medicinal ferns used as “Gusuibu”. Bot. Stud. 2007, 48, 397–406. [Google Scholar]
- Sun, J.S.; Dong, G.C.; Lin, C.Y.; Sheu, S.Y.; Lin, F.H.; Chen, L.T.; Chang, W.H.; Wang, Y.J. The effect of Gu-Sui-Bu (Drynaria fortunei J. Sm) immobilized modified calcium hydrogenphosphate on bone cell activities. Biomaterials 2003, 24, 873–882. [Google Scholar] [CrossRef]
- Ko, Y.J.; Wu, J.B.; Ho, H.Y.; Lin, W.C. Antiosteoporotic activity of Davallia formosana. J. Ethnopharmacol. 2012, 139, 558–565. [Google Scholar] [CrossRef]
- Nakanishi, K.; Lin, Y.Y.; Kakisawa, H.; Hsu, H.Y.; Hsiu, H.C. Davallic acid, a triterpene with a novel akeleton. Tetrahedron Lett. 1963, 22, 1451–1457. [Google Scholar]
- Lin, Y.Y.; Kakisawa, H.; Shiobara, Y.; Nakanishi, K. The structure of Davallic acid. Chem. Pharm. Bull. 1965, 13, 986–995. [Google Scholar] [CrossRef]
- Hwang, T.H.; Kashiwada, Y.; Nonaka, G.I.; Nishioka, I. Flavan-3-ol and proanthocyanidin allosides from Davallia divaricata. Phytochemistry 1989, 28, 891–896. [Google Scholar] [CrossRef]
- Hwang, T.H.; Kashiwada, Y.; Nonaka, G.I.; Nishioka, I. 4-Carboxymethyl flavan-3-ols and procyanidins from Davallia divaricata. Phytochemistry 1990, 29, 279–282. [Google Scholar]
- Umansky, V.; Ushmorov, A.; Ratter, F.; Chlichlia, K.; Bucur, M.; Lichtenauer, A.; Rocha, M. Nitric oxide-mediated apoptosis in human breast cancer cells requires changes in mitochondrial functions and is independent of CD95 (APO-1/Fas). Int. J. Oncol. 2000, 16, 109–117. [Google Scholar]
- Hsu, W.H.; Lee, B.H.; Pan, T.M. Red mold dioscorea-induced G2/M arrest and apoptosis in human oral cancer cells. J. Sci. Food Agric. 2010, 90, 2709–2715. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Rahman, A.U. Handbook of Natural Products Data, Volume 2: Pentacyclic Ttriterpenoids; Elsevier: London, UK, 1994; p. 1365. [Google Scholar]
- Shieh, D.F.; Chen, Y.Y.; Yen, M.H.; Chiang, L.C.; Lin, C.C. Emodin-induced apoptosis through p53-dependent pathway in human hepatoma cells. Life Sci. 2004, 74, 2279–2290. [Google Scholar] [CrossRef]
- Srinivas, G.; Anto, R.J.; Srinivas, P.; Vidhyalakshmi, S.; Senan, V.P.; Karunagaran, D. Emodin induces apoptosis of human cervical cancer cells through poly (ADP-ribose) polymerase cleavage and activation of caspase-9. Eur. J. Pharmacol. 2003, 473, 117–125. [Google Scholar] [CrossRef]
- Hu, R.; Kong, A.H.I. Activation of MAP kinases, apoptosis and nutrigenomics of gene expression elicited by dietary cancer-prevention compounds. Nutrition 2004, 20, 83–88. [Google Scholar] [CrossRef]
- Burke, A.; Fitzerald, G.A. Oxidative stress and smoking-induced vascular injury. Prog. Cardiovas. Dis. 2003, 46, 79–90. [Google Scholar] [CrossRef]
- Nakao, L.S.; Jwai, L.K.; Kalil, J.; Augusto, O. Radical production from free and peptide-bound methionine sulfoxide oxidation by peroxynitrite and hydrogen peroxide/iron(II). FEBS Lett. 2003, 547, 87–91. [Google Scholar] [CrossRef]
- Pelicano, H.; Feng, L.; Zhou, Y.; Carew, J.S.; Feng, L.; Bhalla, K.N.; Keating, M.J.; Huang, P. Inhibition of mitochondrial respiration: A novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. J. Biol. Chem. 2003, 278, 37832–37839. [Google Scholar]
- Stohs, S.J.; Baghi, D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef]
- Bruce-Keller, A.J.; Begley, J.G.; Fu, W.; Butterfield, D.A.; Bredesen, D.E.; Hutchins, J.B.; Hensley, K.; Mattson, M.P. Bcl-2 protects isolated plasma and mitochondrial membranes against lipid peroxidation induced by hydrogen peroxide and amyloid beta-peptide. J. Neurochem. 1998, 70, 31–39. [Google Scholar]
- Desagher, S.; Martinou, J.C. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000, 10, 369–377. [Google Scholar] [CrossRef]
- Hsu, Y.T.; Wolter, K.G.; Youle, R.J. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc. Natl. Acad. Sci. USA 1997, 94, 3668–3672. [Google Scholar] [CrossRef]
- Hockenbery, D.M.; Oltvai, Z.N.; Yin, X.M.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 1993, 75, 241–251. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Shimizu, S. Bcl-2 family: Life-or-death switch. FEBS Lett. 2000, 466, 6–10. [Google Scholar] [CrossRef]
- Kawakami, M.; Inagawa, R.; Hosokawa, T.; Saito, T.; Kurasaki, M. Mechanism of apoptosis induced by copper in PC12 cells. Food Chem.Toxicol. 2008, 46, 2157–2164. [Google Scholar] [CrossRef]
- Neuzil, J.; Wang, X.F.; Dong, L.F. Molecular mechanism of ‘mitocan’-induced apoptosis in cancer cells epitomizes the multiple roles of reactive oxygen species and Bcl-2 family proteins. FEBS Lett. 2006, 580, 5125–5129. [Google Scholar] [CrossRef]
- Wolter, K.G.; Hsu, Y.T.; Smith, C.L.; Nechushtan, A.; Xi, X.G.; Youle, R.J. Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 1997, 139, 1281–1292. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Kamendulis, L.M.; Hocevar, B.A. Oxidative stress and oxidative damage in carcinogenesis. Toxicol. Pathol. 2010, 38, 96–109. [Google Scholar] [CrossRef]
- Jacob, J.K.; Hakimuddin, F.; Paliyath, G.; Fisher, H. Antioxidant and antiproliferative activity of polyphenols in novel high-polyphenol grape lines. Food Res. Int. 2008, 41, 419–428. [Google Scholar] [CrossRef]
- Cui, C.B.; Tezuka, Y.; Kikuchi, T.; Tamaoki, T.; Park, J.H. Constituents of fern, Davallia mariesii Moore. I. Isolution and structures of Davallialactone and a new flavanone glucuronide. Chem. Pharm. Bull. 1990, 38, 3218–3225. [Google Scholar] [CrossRef]
- Hsieh, Y.S. The study of Chinese medicinal herbs inhibitis cancer cell migration/invasion. Yearbook Chin. Med. Pharm. 2006, 24, 257–284. [Google Scholar]
- SPSS, Version 17.0; a computer program used for survey authoring and deployment, data mining, text analytics, statistical analysis, and collaboration and deployment; SPSS Inc.: Chicago, IL, USA, 2008.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cheng, A.-S.; Chang, W.-C.; Cheng, Y.-H.; Chen, K.-Y.; Chen, K.-H.; Chang, T.-L. The Effects of Davallic Acid from Davallia divaricata Blume on Apoptosis Induction in A549 Lung Cancer Cells. Molecules 2012, 17, 12938-12949. https://doi.org/10.3390/molecules171112938
Cheng A-S, Chang W-C, Cheng Y-H, Chen K-Y, Chen K-H, Chang T-L. The Effects of Davallic Acid from Davallia divaricata Blume on Apoptosis Induction in A549 Lung Cancer Cells. Molecules. 2012; 17(11):12938-12949. https://doi.org/10.3390/molecules171112938
Chicago/Turabian StyleCheng, An-Sheng, We-Chang Chang, Yu-Hsiang Cheng, Kai-Yu Chen, Kai-Hsien Chen, and Tsu-Liang Chang. 2012. "The Effects of Davallic Acid from Davallia divaricata Blume on Apoptosis Induction in A549 Lung Cancer Cells" Molecules 17, no. 11: 12938-12949. https://doi.org/10.3390/molecules171112938
APA StyleCheng, A.-S., Chang, W.-C., Cheng, Y.-H., Chen, K.-Y., Chen, K.-H., & Chang, T.-L. (2012). The Effects of Davallic Acid from Davallia divaricata Blume on Apoptosis Induction in A549 Lung Cancer Cells. Molecules, 17(11), 12938-12949. https://doi.org/10.3390/molecules171112938




