Phenolic Content and Antioxidant Activity of Hibiscus cannabinus L. Seed Extracts after Sequential Solvent Extraction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Yield of Sequentially Prepared Extracts, Total Phenolic Content and Total Flavonoid Content
| Extract (according to polarity) | Yield (% w/dw sample) | Total Phenolic Content (mg GAE/g extract) | Total Flavonoids (mg RE/g extract) |
|---|---|---|---|
| Water | 6.65 ± 0.07 a | 18.78 ± 0.35 a | 2.49 ± 0.53 a,b |
| Methanol | 4.66 ± 0.20 b | 5.36 ± 0.88 b | 1.77 ± 0.28 a |
| Chloroform | 4.63 ± 0.72 b | 12.26 ± 1.27 c | 2.94 ± 0.10 b |
| Hexane | 16.60 ± 0.18 c | 2.16 ± 0.21 d | 1.64 ± 0.38 a |
2.2. Antioxidant Activity Tests
| Kenaf seed extracts | DPPH Radical scavenging activity (µg ATE/g sample) | Hydroxyl radical scavenging activity (g DMSOE/g sample) | β-Carotene Bleaching (mg ATE/g extract) | Metal Chelating (mg CAE/g extract) |
|---|---|---|---|---|
| Water | 2.30 a | 23.64 a | 7.15 a | 81.62 a |
| Methanol | 0.62 b | 20.30 b | 1.42 b | 43.27 bc |
| Chloroform | 0.56 b | 23.48 a | 1.46 b | 54.45 b |
| Hexane | 0.40 c | 3.33 c | 0.71 c | 29.86 c |
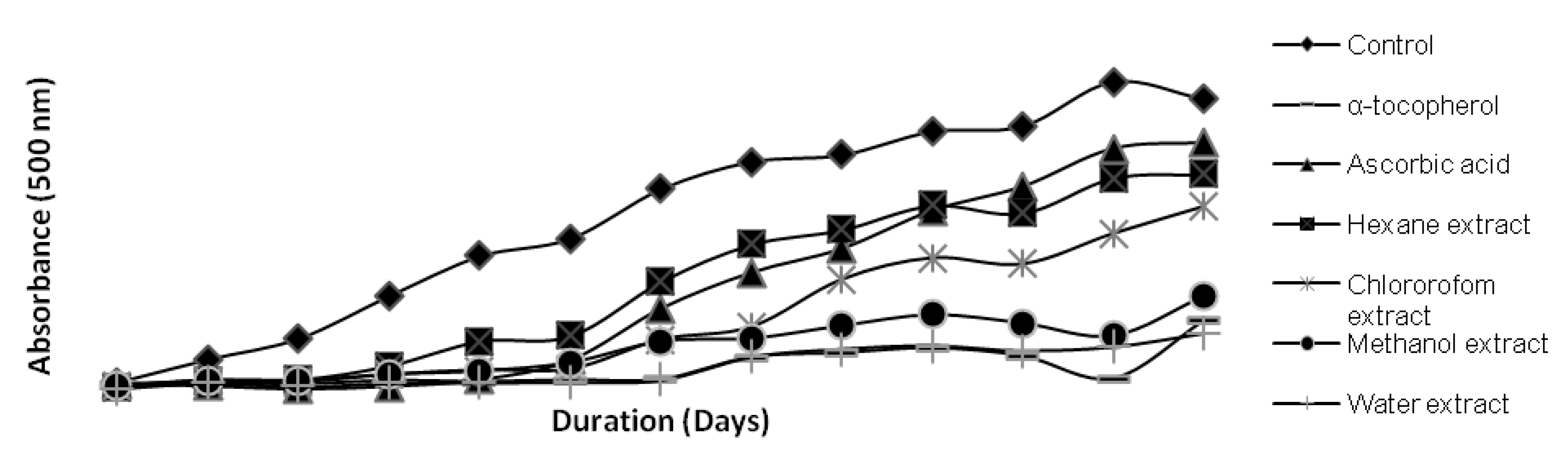
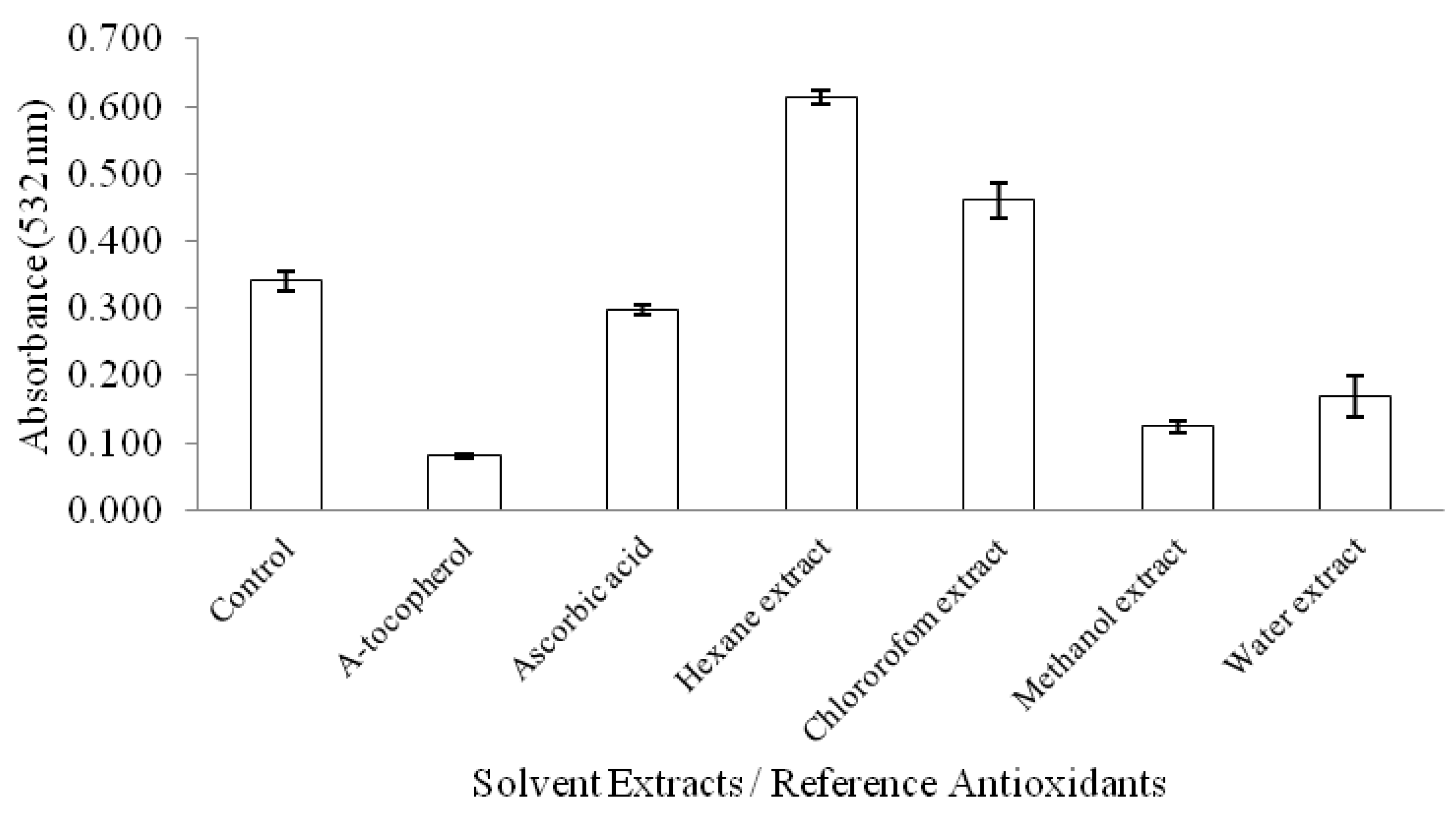
3. Experimental
3.1. Plant Material
3.2. Chemicals and Reagents
3.3. Preparation of Kenaf Seeds
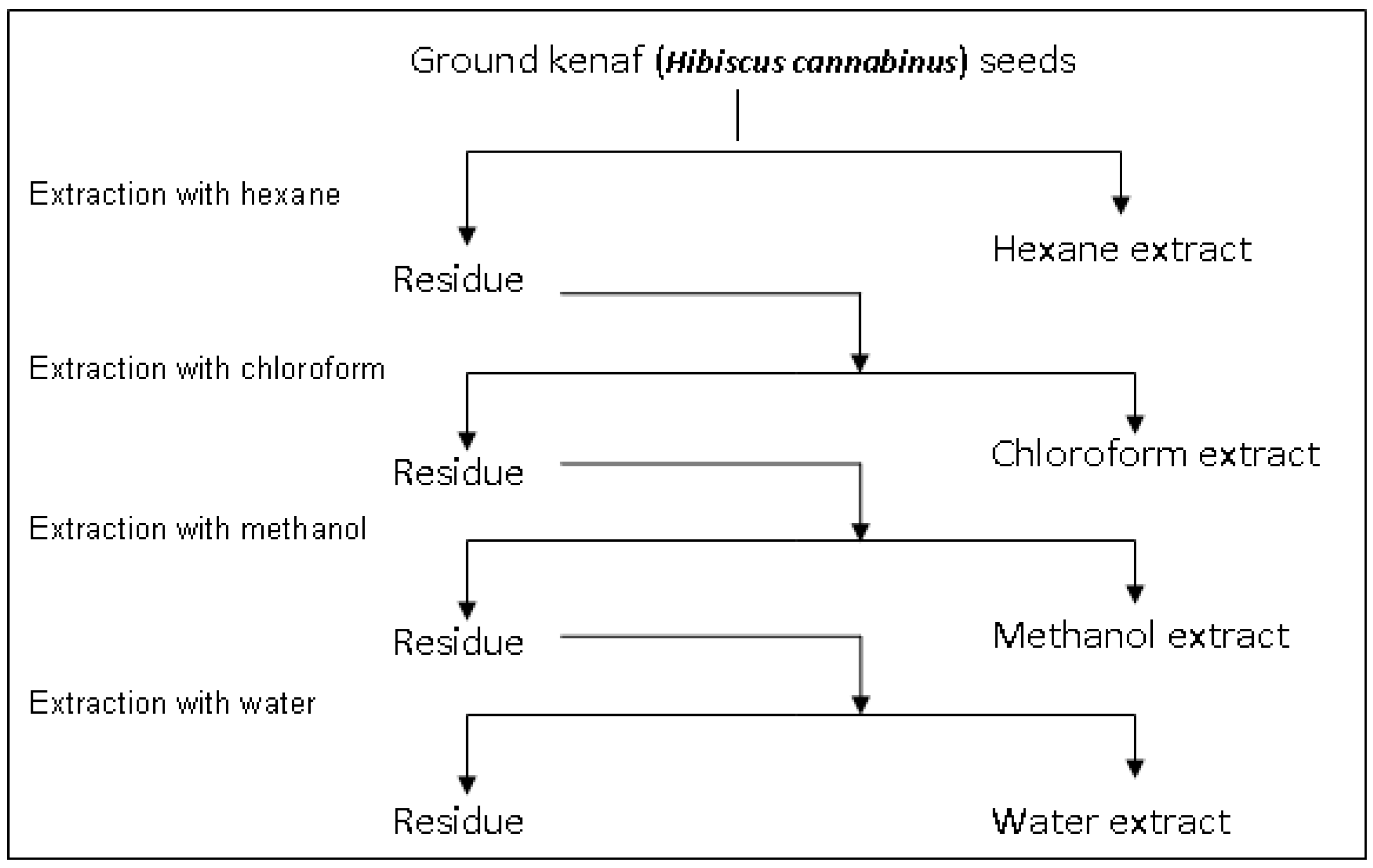
3.4. Sequential Solvent Extraction of Kenaf Seeds
3.5. Total Phenolic Content (TPC)
3.6. Total Flavonoid Content (TFC)
3.7. 2,2-Diphenyl-1-picrylhidrazyl (DPPH) Radical Scavenging Assay
3.8. Hydroxyl Radical Scavenging Assay
3.9. β-Carotene Bleaching Assay
- DR = Degradation rate; (a/b)/60
- a = Absorbance of sample at 470 nm before incubation
- b = Absorbance of sample at 470 nm after incubation
- DRc = Degradation rate of the control sample
- DRs = Degradation rate of tested sample
3.10. Metal Chelating Activity
3.11. Total Antioxidant Activity
3.11.1. Ferric Thiocyanate (FTC) Test
3.11.2. Thiobarbituric Acid Reactive Substances (TBARS) Assay
3.12. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Chan, K.W.; Iqbal, S.; Khong, N.M.H.; Babji, A.S. Preparation of deodorized antioxidant rich extracts from 15 selected spices through optimized aqueous extraction. J. Med. Plants Res. 2011, 5, 6067–6075. [Google Scholar]
- Iqbal, S.; Younas, U.; Sirajuddin; Chan, K.W.; Sarfraz, R.A.; Uddin, M.K. Proximate composition and antioxidant potential of leaves from three varieties of mulberry (Morus spp.): A comparative study. Int. J. Mol. Sci. 2012, 13, 6651–6664. [Google Scholar] [CrossRef]
- Karpinska, M.; Borowski, J.; Danowska-Oziewicz, M. The use of natural antioxidants in ready-to-serve food. Food Chem. 2001, 72, 5–9. [Google Scholar] [CrossRef]
- Chan, K.W.; Khong, N.M.H.; Iqbal, S.; Ch’ng, S.E.; Babji, A.S. Preparation of clove buds deodorized aqueous extract (CDAE) and evaluation of its potential to improve oxidative stability of chicken meatballs in comparison to synthetic and natural food antioxidants. J. Food Qual. 2012, 35, 190–199. [Google Scholar] [CrossRef]
- Miraliakbari, H.; Shahidi, F. Antioxidant activity of minor components of tree nut oils. Food Chem. 2008, 111, 421–427. [Google Scholar] [CrossRef]
- Agricultural Research and Development Institute, MARDI. Proceedings of the third technical review meeting on the National Kenaf Research Project, Kuala Lumpur, Malaysia, 25–26 May 2004.
- Chan, K.W.; Ismail, M. Supercritical carbon dioxide fluid extraction of Hibiscus cannabinus L. seed oil: A potential solvent-free and high antioxidative edible oil. Food Chem. 2009, 114, 970–975. [Google Scholar] [CrossRef]
- Mohamed, A.; Bhardwaj, H.; Hamama, A.; Webber, C. Chemical composition of kenaf (Hibiscus cannabinus L.) seed oil. Ind. Crops Prod. 1995, 4, 157–165. [Google Scholar] [CrossRef]
- Conkerton, E.; Wan, P.; Richard, O. Hexane and heptane as extraction solvents for cottonseed: A laboratory-scale study. J. Am. Oil Chem. Soc. 1995, 72, 963–965. [Google Scholar] [CrossRef]
- Nyam, K.L.; Tan, C.P.; Lai, O.M.; Long, K.; Che Man, Y. Physicochemical properties and bioactive compounds of selected seed oils. LWT-Food Sci. Technol. 2009, 42, 1396–1403. [Google Scholar]
- Matthaous, B. Antioxidant activity of extracts obtained from residues of different oilseeds. J. Agric. Food Chem. 2002, 50, 3444–3452. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Kroh, L.W.; Maprsel, J.T. Radical scavenging activity of black cumin (Nigella sativa L.), coriander (Coriandrum sativum L.), and niger (Guizotia abyssinica Cass.) crude seed oils and oil fractions. J. Agric. Food Chem. 2003, 51, 6961–6969. [Google Scholar] [CrossRef]
- Iqbal, S.; Bhanger, M.; Anwar, F. Antioxidant properties and components of some commercially available varieties of rice bran in Pakistan. Food Chem. 2005, 93, 265–272. [Google Scholar] [CrossRef]
- Iqbal, S.; Bhanger, M.; Anwar, F. Antioxidant properties and components of bran extracts from selected wheat varieties commercially available in Pakistan. LWT-Food Sci. Technol. 2007, 40, 361–367. [Google Scholar] [CrossRef]
- Wettasinghe, M.; Shahidi, F. Antioxidant activity of preformed cooked cured-meat pigment in a [beta]-carotene/linoleate model system. Food Chem. 1997, 58, 203–207. [Google Scholar]
- Zhang, H.; Chen, F.; Wang, X.; Yao, H.Y. Evaluation of antioxidant activity of parsley (Petroselinum crispum) essential oil and identification of its antioxidant constituents. Food Res. Int. 2006, 39, 833–839. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Nakatani, N. Antioxidant effects of some ginger constituents. J. Food Sci. 1993, 58, 1407–1410. [Google Scholar]
- Sample Availability: Samples are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yusri, N.M.; Chan, K.W.; Iqbal, S.; Ismail, M. Phenolic Content and Antioxidant Activity of Hibiscus cannabinus L. Seed Extracts after Sequential Solvent Extraction. Molecules 2012, 17, 12612-12621. https://doi.org/10.3390/molecules171112612
Yusri NM, Chan KW, Iqbal S, Ismail M. Phenolic Content and Antioxidant Activity of Hibiscus cannabinus L. Seed Extracts after Sequential Solvent Extraction. Molecules. 2012; 17(11):12612-12621. https://doi.org/10.3390/molecules171112612
Chicago/Turabian StyleYusri, Noordin Mohd, Kim Wei Chan, Shahid Iqbal, and Maznah Ismail. 2012. "Phenolic Content and Antioxidant Activity of Hibiscus cannabinus L. Seed Extracts after Sequential Solvent Extraction" Molecules 17, no. 11: 12612-12621. https://doi.org/10.3390/molecules171112612
APA StyleYusri, N. M., Chan, K. W., Iqbal, S., & Ismail, M. (2012). Phenolic Content and Antioxidant Activity of Hibiscus cannabinus L. Seed Extracts after Sequential Solvent Extraction. Molecules, 17(11), 12612-12621. https://doi.org/10.3390/molecules171112612




