trans-Resveratrol in Nutraceuticals: Issues in Retail Quality and Effectiveness
Abstract
:1. Introduction
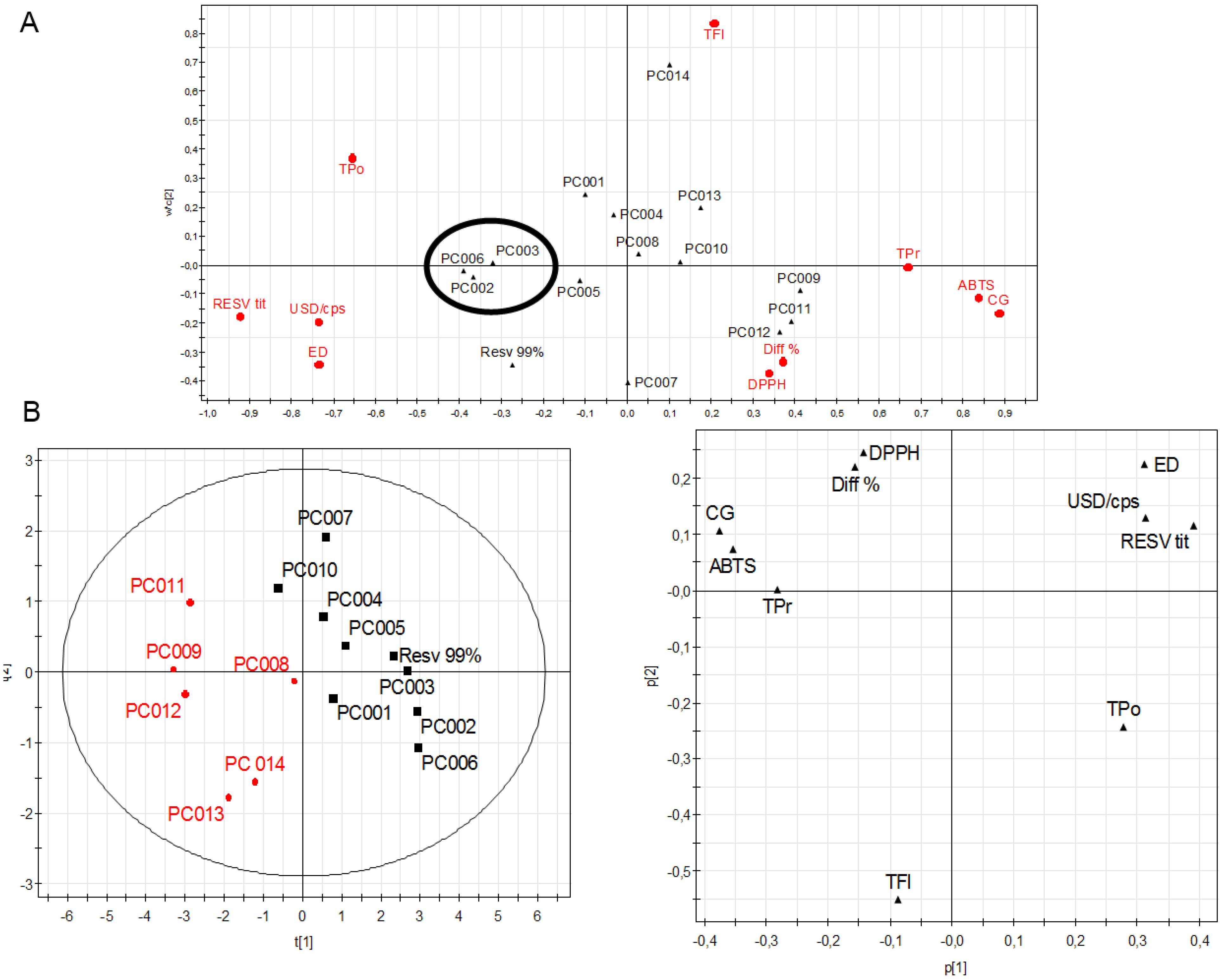
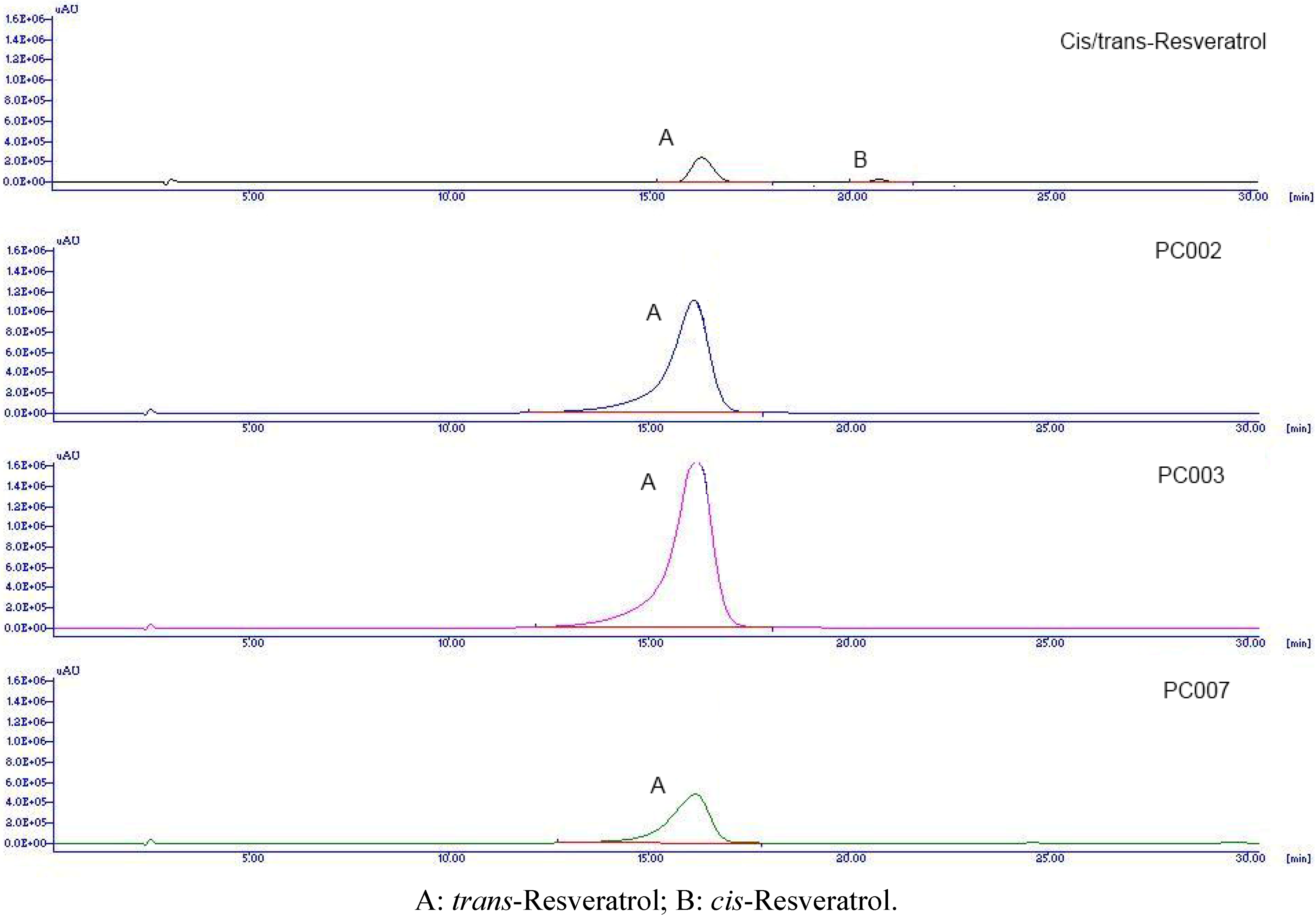
2. Results and Discussion
| Sample | Description | Weight per capsule (mg) | Claims | Declared quality | Form |
|---|---|---|---|---|---|
| PC001 | 500 mg of Polygonum cuspidatum extract standardized to 50% trans resveratol | 500 | Antioxidant, against age-related diseases | FDA Pharmaceutical GMP | P |
| PC002 | 500 mg of P. cuspidatum extract standardized to 99% trans resveratol | 500 | Antioxidant, against age-related diseases | FDA Pharmaceutical GMP | P |
| PC003 | 500 mg of P. cuspidatum extract standardized to 99% trans resveratol | 500 | Antioxidant | FDA certificated | P |
| PC004 | 600 mg of P. cuspidatum extract standardized to 50% trans resveratol | 600 | Antioxidant, against age-related diseases | n.a. | P |
| PC005 | 500 mg of P. cuspidatum extract standardized to 99% trans resveratol | 500 | Antioxidant, against age-related diseases | n.a. | P |
| PC006 | 500 mg of P. cuspidatum extract standardized to 99% trans resveratol | 500 | Antioxidant, against age-related diseases | n.a. | P |
| PC007 | 300 mg of trans-resveratol from P. cuspidatum standardized to 99% | 307 | Antioxidant | FDA certificated | M |
| PC008 | 100 mg of trans-resveratol from P. cuspidatum, with added quercetin, ferulic acid, Vitamin D3 | 365 | Antioxidant | n.a. | M |
| PC009 | 500 mg multi ingredient with extracts from Vitis vinifera seed, skins, wine, vitamin C. No standardization provided, but resveratrol mentioned in the ingredients | Antioxidant | n.a. | M | |
| PC010 | 40 mg of resveratrol (no cis/trans specifications) | 200 | Antioxidant, against age-related diseases | n.a. | P |
| PC011 | 75 mg of resveratrol (no cis/trans specifications) from P.cuspidatum, with added red wine and grape seed extracts . | 700 | Antioxidant | n.a. | M |
| PC012 | 16 mg of resveratrol (no cis/trans specifications), with vitamin C, green tea and Vitis vinifera peel and seed extracts | 1200 | Antioxidant, against age-related diseases | n.a. | M |
| PC013 | 200 mg of resveratrol (no cis/trans specifications), with added grape seed and red wine extracts, quercetin | 600 | Antioxidant, against age-related diseases | n.a. | M |
| PC014 | 150 mg of resveratrol 75% trans, with added silymarin, curcuma and astragalus extracts | 500 | Antioxidant, against age-related diseases | n.a. | M |
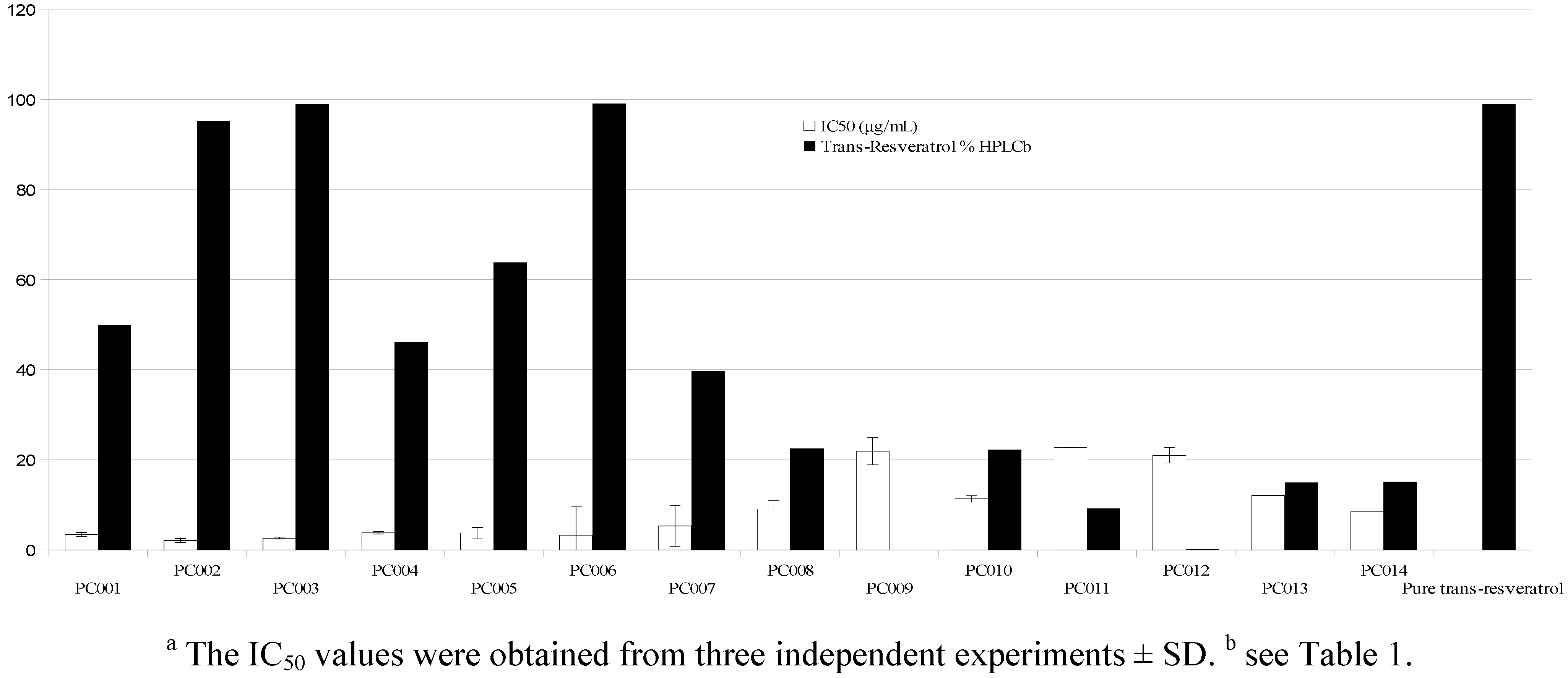
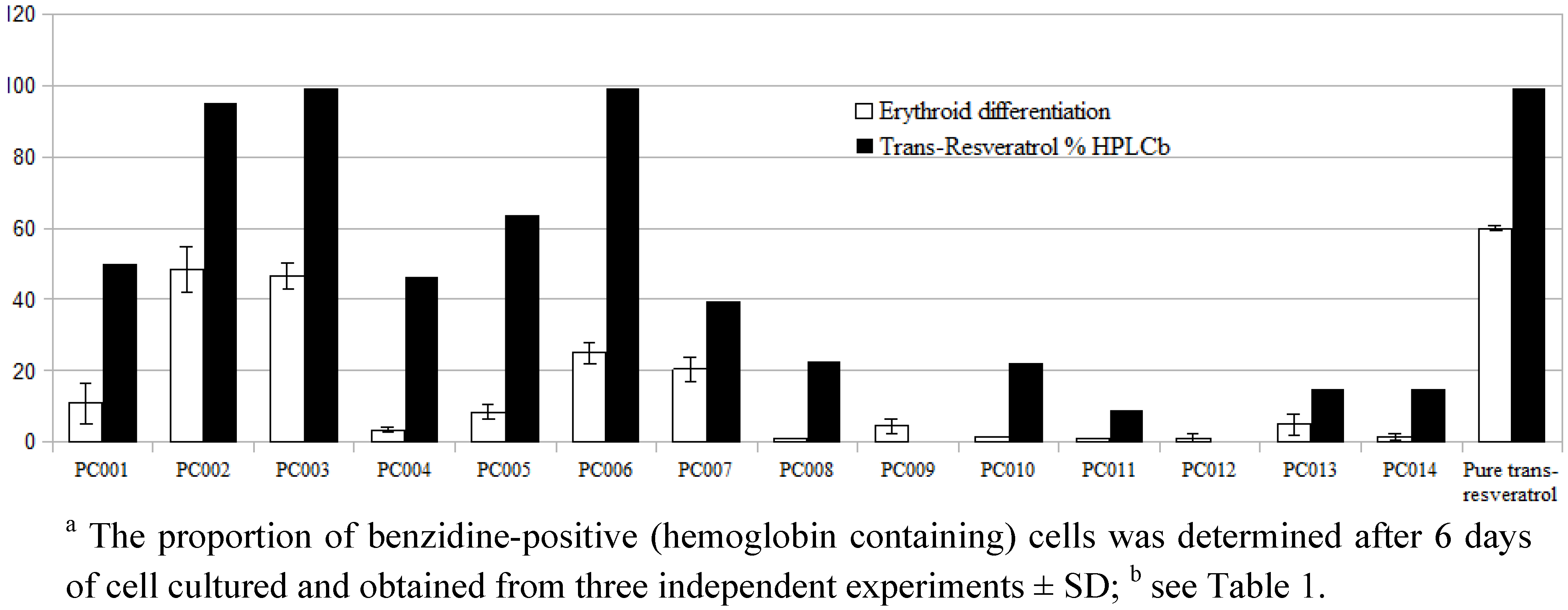
| Sample | Form a | Purity on-label | Trans-Resveratrol b | Deviation | Posology | Actual dosage c | Value per cps d |
|---|---|---|---|---|---|---|---|
| % | % | % | cps/die | mg | US Dollars | ||
| PC001 | P | 50 | 49.90 ± 1.94 | −0.2 | 1 | 249.5 | 0.77 (0.6) |
| PC002 | P | 99 | 95.18 ± 1.79 | −3.82 | 1 | 480.9 | 1.44 (0.58) |
| PC003 | P | 98 | 99.03 ± 1.59 | 1.03 | 1 | 506.5 | 0.73 (0.3) |
| PC004 | P | 50 | 46.21 ± 1.10 | −7.58 | 1 | 227.2 | 0.37 (0.32) |
| PC005 | P | 99 | 63.82 ± 0.35 | −35.53 | 1 | 322.5 | 0.93 (0.58) |
| PC006 | P | 99 | 99.07 ± 0.92 | 0.07 | 1 | 501 | 2.5 (1) |
| PC007 | M | 98 | 39.59 ± 0.81 | −60 | 1 | 120 | 1.1 (1.84) |
| PC008 | M | 27 | 22.48 ± 0.36 | −16.74 | 1 | 270.7 | 1.23 (0.9) |
| PC009 | M | - | <0.4 | n.a. | 1 | n.a. | n.a. |
| PC010 | P | 20 | 22.18 ± 0.98 | 11 | 2 | 55.5 | 0.2 (0.72) |
| PC011 | M | 10.7 | 9.17 ± 0.11 | −14.3 | 2 | 128.5 | 0.22 (0.34) |
| PC012 | M | 1.3 | <0.4 | n.a. | 4 | n.a. | n.a. |
| PC013 | M | 33 | 14.92 ± 0.37 | −54.79 | 2 | 180.8 | 0.67 (0.74) |
| PC014 | M | 15 | 15.05 ± 0.53 | 0,05 | 2 | 226 | 0.41 (0.36) |
| Sample | Form a | Tot. Polyphenols b | Tot. Procyanidins c | Tot. Flavonoids d | IC50 DPPH | IC50 ABTS |
|---|---|---|---|---|---|---|
| g/100g | mg/mL | |||||
| PC001 | P | 75.60 ± 2.94 | 2.28 ± 0.16 | 11.61 ± 0.52 | 16.34 ± 1.39 | 1.15 ± 0.07 |
| PC002 | P | 93.72 ± 1.44 | 0.54 ± 0.08 | 0.00 | 13.15 ± 1.11 | 0.65 ± 0.05 |
| PC003 | P | 98.47 ± 1.01 | 0.57 ± 0.04 | 0.00 | 14.40 ± 1.53 | 0.81 ± 0.05 |
| PC004 | P | 77.86 ± 2.58 | 1.63 ± 0.15 | 9.81 ± 0.85 | 24.83 ± 1.79 | 1.37 ± 0.11 |
| PC005 | P | 64.31 ± 2.34 | 0.44 ± 0.02 | 0.00 | 18.81 ± 1.81 | 1.19 ± 0.20 |
| PC006 | P | 99.07 ± 1.05 | 0.39 ± 0.03 | 0.00 | 13.81 ± 0.99 | 0.77 ± 0.05 |
| PC007 | M | 36.32 ± 3.63 | 0.33 ± 0.04 | 0.00 | 38.65 ± 2.04 | 1.55 ± 0.03 |
| PC008 | M | 42.14 ± 2.84 | 0.37 ± 0.09 | 8.01 ± 0.36 | 20.23 ± 1.83 | 2.24 ± 0.07 |
| PC009 | M | 13.80 ± 0.96 | 5.05 ± 0.25 | 1.78 ± 0.12 | 18.45 ± 1.03 | 6.77 ± 0.24 |
| PC010 | P | 28.30 ± 1.94 | 0.79 ± 0.12 | 4.11 ± 0.21 | 25.65 ± 1.87 | 2.54 ± 0.19 |
| PC011 | M | 21.71 ± 1.25 | 7.86 ± 0.86 | 3.13 ± 0.18 | 32.65 ± 1.43 | 3.71 ± 0.06 |
| PC012 | M | 18.83 ± 1.14 | 3.33 ± 0.22 | 2.33 ± 0.16 | 16.93 ± 1.09 | 4.49 ± 0.03 |
| PC013 | M | 51.18 ± 0.94 | 8.13 ± 0.91 | 17.23 ± 0.55 | 10.94 ± 0.93 | 1.79 ± 0.06 |
| PC014 | M | 49.57 ± 1.84 | 0.58 ± 0.03 | 47.44 ± 1.56 | 18.51 ± 0.89 | 2.36 ± 0.08 |
| trans-Resveratrol | 17.9 ± 1.38 | 0.84 ± 0.05 | ||||
3. Experimental
3.1. Supplements
3.2. Reagents and Standards
3.3. Resveratrol Quantification
3.4. Total Procyanidins, Flavonoids and Polyphenols
3.5. Antioxidant Activity
3.6. K562 Cells and Cell Culture Conditions
3.7. Screening of Antiproliferative Activity
3.8. Screening of Erythroid-Differentiation Activity
3.9. Statistical Analysis
4. Conclusions
Acknowledgements
Conflict of Interest
- Sample Availability: Samples of trans resveratrol are available from the authors.
References
- Hooyenga, P.A.; Witkamp, R.F.; Groen, K. Herbal products: Marketing strategies and legislation. Int. J. Green Pharm. 2009, 3, 270–276. [Google Scholar] [CrossRef]
- Morris, C.A. Internet marketing of herbal products. JAMA 2003, 290, 1505–1509. [Google Scholar] [CrossRef]
- Bech-Larsen, T.; Grunert, K.G. The perceived healthiness of functional foods: A conjoint study of Danish, Finnish and American consumers perception of functional foods. Appetite 2003, 40, 9–14. [Google Scholar] [CrossRef]
- Bagchi, D. Nutraceuticals and functional foods regulations in the United States and around the world. Toxicology 2006, 221, 1–3. [Google Scholar] [CrossRef]
- Zhao, J. Nutraceuticals, Nutritional Therapy, Phytonutrients, and Phytotherapy for Improvement of Human Health: A Perspective on Plant Biotechnology Application. Rec. Pat. Biotechnol. 2007, 1, 75–97. [Google Scholar] [CrossRef]
- European Pharmacopoeia. Commission directive 2003/63/EC. In Off. J. Eur. Un.; 2003; pp. L159/46–L159/94.
- Boniglia, C.; Carratù, B.; Gargiulo, R.; Giammarioli, S.; Mosca, M.; Sanzini, E. Content of phytoestrogens in soy-based dietary supplements. Food Chem. 2009, 115, 1389–1392. [Google Scholar] [CrossRef]
- Cassinese, C.; De Combarieu, E.; Falzoni, M.; Fuzzati, N.; Pace, R.; Sardone, N. New liquid chromatography method with ultraviolet detection for analysis of anthocyanins and anthocyanidins in Vaccinium myrtillus fruit dry extracts and commercial preparations. J. AOAC Int. 2007, 90, 911–919. [Google Scholar]
- Lockwood, G.B. The quality of commercially available nutraceutical supplements and food sources. J. Pharm. Pharmacol. 2011, 63, 3–10. [Google Scholar] [CrossRef]
- Renaud, S.; de Lorgeril, M. Wine, Alcohol, Platelets, And the French paradox for coronary heart disease. Lancet 8808, 1523–1526. [Google Scholar]
- Jerkovic, V.; Collin, S. Occurrence of resveratrol and piceid in American and European hop cones. J. Agric. Food Chem. 2007, 55, 8754–8758. [Google Scholar] [CrossRef]
- Alarcon De la Lastra, C.A.; Villegas, I. Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Mol. Nutr. Food Res. 2005, 49, 405–430. [Google Scholar] [CrossRef]
- Baur, J.; Sinclair, D. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Disc. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Scott, E.; Steward, W.P.; Gescher, A.J.; Brown, K. Resveratrol in human cancer chemoprevention—Choosing the “right” dose. Mol. Nutr. Food Res. 2012, 56, 7–13. [Google Scholar] [CrossRef]
- Signorelli, P.; Ghidoni, R. Resveratrol as an anticancer nutrient: Molecular basis, Open questions and promises. J. Nutr. Biochem. 2005, 16, 449–466. [Google Scholar] [CrossRef]
- Smoliga, J.M.; Baur, J.; Hausenblas, H. Resveratrol and health—A comprehensive review of human clinical trials. Mol. Nutr. Food Res. 2011, 55, 1129–1141. [Google Scholar] [CrossRef]
- Szkudelska, K.; Szkudelski, T. Resveratrol, Obesity and diabetes. Eur. J. Pharmacol. 2010, 635, 1–8. [Google Scholar] [CrossRef]
- Vang, O.; Ahmad, N.; Baile, C.; Baur, J.; Brown, K.; Csiszar, A.; Das, D.K.; Delmas, D.; Gottfried, C.; Lin, H.; et al. What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS One 2011, 6, e19881. [Google Scholar]
- Juan, M.E.; Alfaras, I.; Planas, J.M. Colorectal cancer chemoprevention by trans-resveratrol. Pharm. Res. 2012, 65, 584–591. [Google Scholar] [CrossRef]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef]
- Trela, B.C.; Waterhouse, A.L. Resveratrol: Isomeric molar absorptivities and stability. J. Agric. Food Chem. 1996, 44, 1253–1257. [Google Scholar] [CrossRef]
- Bertelli, A.A.; Giovannini, L.; Bernini, W.; Migliori, M.; Fregoni, M.; Bavaresco, L.; Bertelli, A. Antiplatelet activity of cis-resveratrol. Drugs Exp. Clin. Res. 1996, 22, 61–63. [Google Scholar]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Athar, M.; Back, J.H.; Tang, X.; Kim, K.H.; Kopelovich, L.; Bickers, R.; Kim, A.L. Resveratrol: A review of pre-clinical studies for human cancer prevention. Toxicol. Appl. Pharmacol. 2007, 224, 274–283. [Google Scholar] [CrossRef]
- Rodriguez, C.M.; Arous, N.; Bachir, D.; Smith-Ravin, J.; Romeo, P.H.; Galacteros, F.; Garel, M.C. Resveratrol, A natural dietary phytoalexin, Possesses similar properties to hydroxyurea towards erythroid differentiation. Br. J. Haematol. 2001, 113, 500–507. [Google Scholar] [CrossRef]
- Liu, B.Q.; Gao, Y.Y.; Niu, X.F.; Xie, J.S.; Meng, X.; Guan, Y.; Wang, H.Q. Implication of unfolded protein response in resveratrol-induced inhibition of K562 cell proliferation. Biochem. Biophys. Res. Comm. 2010, 391, 778–782. [Google Scholar] [CrossRef]
- Fibach, E.; Prus, E.; Bianchi, N.; Zuccato, C.; Breveglieri, G.; Salvatori, F.; Finotti, A.; Lipucci di Paola, M.; Brognara, E.; Lampronti, I.; et al. Resveratrol: Antioxidant activity and induction of fetal hemoglobin in erythroid cells from normal donors and β-thalassemia patients. Int. J. Mol. Med. 2012, 29, 974–982. [Google Scholar]
- Lopez Hernandez, J.; Paseiro Losada, P.; Sanches-Silva, A.T.; Lage-Yusty, M.A. Study of the changes of trans-resveratrol caused by ultraviolet light and determination of trans- and cis-resveratrol in Spanish white wines. Eur. Food Res. Technol. 2007, 225, 789–796. [Google Scholar] [CrossRef]
- Chaovanalikit, A.; Wrolstad, R.E. Total antocyanins and total phenolics of fresh and processed cherries and their antioxidant properties. Food Chem. Toxicol. 2004, 69, 67–72. [Google Scholar]
- Scartezzini, P.; Antognoni, F.; Poli, F.; Sabbioni, C. Vitamin C content and antioxidant activity of the fruit and of the ayurvedic preparation of Emblica officinalis Gaertn. J. Ethnopharmacol. 2005, 104, 113–118. [Google Scholar]
- Porter, L.J.; Hrstich, L.N.; Chan, B.C. The conversion of procyanidins and prodelphinidins to cyanidins and delphinidins. Phytochemistry 1986, 25, 225–330. [Google Scholar]
- Bruni, R.; Rossi, D.; Muzzoli, M.; Romagnoli, C.; Paganetto, G.; Besco, E.; Choquecillo, F.; Peralta, K.; Lora, W.S.; Sacchetti, G. Antimutagenic, Antioxidant and antimicrobial properties of Maytenus krukovii bark. Fitoterapia 2006, 77, 538–545. [Google Scholar] [CrossRef]
- Luximon-Ramma, A.; Bahorun, T.; Soobrattee, M.A.; Aruoma, O.I. Antioxidant activities of phenolic, proanthocyanidin, and flavonoid components in extracts of Cassia fistula. J. Agric. Food Chem. 2002, 50, 5042–5047. [Google Scholar]
- Bianchi, N.; Chiarabelli, C.; Borgatti, M.; Mischiati, C.; Fibach, E.; Gambari, R. Accumulation of γ-globin mRNA and induction erythroid differentiation after treatment of human leukaemic K562 cells with tallimustine. Br. J. Haematol. 2001, 113, 951–961. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rossi, D.; Guerrini, A.; Bruni, R.; Brognara, E.; Borgatti, M.; Gambari, R.; Maietti, S.; Sacchetti, G. trans-Resveratrol in Nutraceuticals: Issues in Retail Quality and Effectiveness. Molecules 2012, 17, 12393-12405. https://doi.org/10.3390/molecules171012393
Rossi D, Guerrini A, Bruni R, Brognara E, Borgatti M, Gambari R, Maietti S, Sacchetti G. trans-Resveratrol in Nutraceuticals: Issues in Retail Quality and Effectiveness. Molecules. 2012; 17(10):12393-12405. https://doi.org/10.3390/molecules171012393
Chicago/Turabian StyleRossi, Damiano, Alessandra Guerrini, Renato Bruni, Eleonora Brognara, Monica Borgatti, Roberto Gambari, Silvia Maietti, and Gianni Sacchetti. 2012. "trans-Resveratrol in Nutraceuticals: Issues in Retail Quality and Effectiveness" Molecules 17, no. 10: 12393-12405. https://doi.org/10.3390/molecules171012393
APA StyleRossi, D., Guerrini, A., Bruni, R., Brognara, E., Borgatti, M., Gambari, R., Maietti, S., & Sacchetti, G. (2012). trans-Resveratrol in Nutraceuticals: Issues in Retail Quality and Effectiveness. Molecules, 17(10), 12393-12405. https://doi.org/10.3390/molecules171012393