Abstract
By introducing long carbon-chain alkyl groups at the C-13 position of berberine and palmatine, 13-n-hexyl/13-n-octyl berberine and palmatine chloride analogues 4a–d were synthesized and examined by MTT assays for cytotoxic activity in seven human cancer cell lines (7701QGY, SMMC7721, HepG2, CEM, CEM/VCR, KIII, Lewis), yielding IC50 values of 0.02 ± 0.01–13.58 ± 2.84 μM. 13-n-Octyl palmatine (compound 4d) gave the most potent inhibitor activity, with an IC50 of 0.02 ± 0.01 μM for SMMC7721. In all cases, the 13-n-alkyl berberine and palmatine analogues 4a–d were more cytotoxic than berberine and palmatine. In addition, compounds 4a–d also exhibited more potent cytotoxicity than berberine and palmatine in mice with S180 sarcoma xenografted in vivo. The primary screening results indicated that the 13-n-hexyl/13-n-octyl berberine and palmatine analogues might be valuable source for new potent anticancer drug candidates.
1. Introduction
Berberine (1a, Figure 1), an isoquinoline alkaloid isolated from the roots and stem bark of Berberis species, is widely used as a traditional medicine for treating diarrhea [1] and gastrointestinal disorders [2].
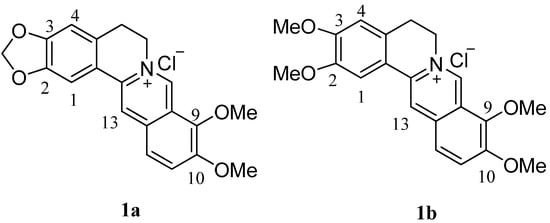
Figure 1.
Structures of the lead compounds 1a and 1b.
Berberine possesses various biological activities like antibacterial [3,4], antifungal [5], antimalarial [6], antileishmanial [7], anticancer [8,9,10,11,12], anti-Alzheimer’s disease [13,14], antiviral [15,16], cholesterol lowering effect [17] and hypoglycemic effect [18]. Different activities of a number of 8-, 9- and 13-substituted analogues of berberine have been reported. Various analogs using different chain lengths and terminal amino groups have been synthesized to study the DNA-binding affinity or as G-quadruplex stabilizing ligands [19,20,21,22,23,24,25,26].
In addition, palmatine (1b, Figure 1) is a close structural analog of berberine. The alkaloid has been used in the treatment of jaundice, dysentery, hypertension, inflammation, and liver-related diseases [27]. It has been reported that like berberine, some of the 8- and 13-alkyl analogues of palmatine also possess antimicrobial and antimalarial activities [28]. Palmatine also has significant antitumor activity against HL-60 leukemic cells [29].
In this study, berberine and palmatine analogues were first designed and synthesized by means of the introduction of long carbon-chain alkyl at carbon atom C-13 to improve the antiproliferative activity in vitro and in vivo.
2. Results and Discussion
2.1. Chemistry
The synthesis of the 13-n-alkyl substituted berberine and palmatine analogues involved two steps (Scheme 1). Treatment of commercially available berberine (1a) or palmatine (1b) with NaBH4in 5% NaOH at room temperature gave the key reduced intermediates 2a,b, the dihydrogenated products of 1a and 1b [30]. Then, compounds 2a,b were reacted with n-hexyl aldehyde (3a) and n-octyl aldehyde (3b), respectively, in a EtOH and HOAc solvent mixture, and then acidified with 2 mol/L HCl to yield the desired compounds 4a–d, which were fully characterized by 1H-NMR, LR-MS and elemental analysis.
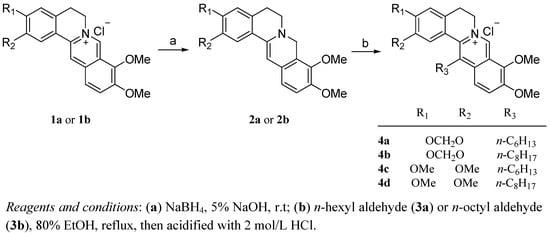
Scheme 1.
Synthesis of the 13-n-alkyl substituted berberine and palmatine analogues 4a–d.
2.2. Biology
2.2.1. In Vitro Cytotoxic Effects
The cytotoxicity of berberine and palmatine and the herein presented analogues 4a–d were compared by means of a colorimetric microculture assay (MTT assay) in seven human cancer cell lines: 7701QGY, SMMC7721, HepG2 (human hepatoma), CEM (human acute lymphoblastic leukemia), CEM/VCR (vincristine-resistant CEM), K III (mice melanoma) and Lewis (mice lung carcinoma), yielding IC50 values mostly in the low micromolar or even submicromolar range (Table 1).

Table 1.
Cytotoxicity of berberine and palmatine analogues 4a–d compared with berberine and palmatine in various cancer cell lines.
| Comp. | IC50 (μM) a | ||||||
|---|---|---|---|---|---|---|---|
| 7701QGY | SMMC7721 | HepG2 | CEM | CEM/VCR | K III | Lewis | |
| 4a | 3.28 ± 0.27 | 0.37 ± 0.07 | 4.74 ± 0.48 | 3.64 ± 0.35 | 9.65 ± 2.37 | 25.47 ± 2.95 | 2.26 ± 0.49 |
| 4b | 1.79 ± 0.26 | 0.04 ± 0.02 | 3.16 ± 0.39 | 0.37 ± 0.03 | 5.19 ± 0.64 | 13.58 ± 2.84 | 0.86 ± 0.10 |
| 4c | 10.09 ± 1.98 | 0.68 ± 0.05 | 5.54 ± 0.24 | 1.97 ± 0.09 | 17.54 ± 1.98 | 30.58 ± 1.69 | 2.86 ± 0.54 |
| 4d | 1.08 ± 0.25 | 0.02 ± 0.01 | 2.28 ± 0.37 | 0.16 ± 0.11 | 4.80 ± 0.81 | 10.41 ± 2.58 | 0.34 ± 0.09 |
| berberine | 22.18 ± 1.12 | 2.09 ± 0.25 | 117.63 ± 3.13 | 45.04 ± 1.42 | 120.37 ± 3.84 | 84.29 ± 3.42 | 20.29 ± 4.42 |
| palmatine | ND | 23.19 ± 1.21 | ND | 5.68 ± 0.27 | 230.76 ± 5.21 | 74.13 ± 4.50 | 30.18 ± 2.76 |
ND = not determined. a 50% inhibitory concentrations in the MTT assay (72 h exposure). Values are means standard deviations obtained from at least two (mostly three) independent experiments.
13-n-Hexyl/13-n-octyl berberine and palmatine analogues 4a–d exhibited more potent cytotoxicity than berberine and palmatine in all seven cell lines. 13-n-octyl-berberine (4b) and 13-n-octyl-palmatine (4d) exerted the most potent antitumor activities, with IC50 values of 0.02 ± 0.01–13.58 ± 2.84 μM against various cancer cell lines, and 6-fold stronger antitumor activities than berberine and palmatine. Notably, compound 4d gave the most potent inhibitor activity, with an IC50 of 0.02 ± 0.01 μM for SMMC7721. Compared with the lead compounds, berberine analogues 4a,b showed similar activity to palmatine analogues 4c,d, which exhibited more potent activity against CEM and CEM/VCR cells (vincristine-resistant) lines. These results indicate that the alkylation of the natural products, berberine and palmatine, such as introduction of longer carbon-chain alkyl groups at the C-13-position, can remarkably enhance the antitumor activity. This modification is likely to make the compounds more lipophilic, which may increase the permeability of the cell membrane and improve the bioavailability of the lead compounds. Very recently, the new insights into 13-position substitution of berberine in enhancing the DNA binding had been reported by Kumar and colleagues [26], suggesting that 13-n-hexyl/13-n-octyl berberine and palmatine analogues may enhance the DNA binding action. The further investigation whether the long carbon-chain substituted berberines and palmatines could enhance the DNA binding action to generate more potent antitumor activity will be reported in due course.
2.2.2. In Vivo Anticancer Activity
Anticancer activity in vivo was investigated in the murine sarcoma S180 xenografted model on male Kunming mice using intraperitoneal (ip) injection using berberine, palmatine and cyclophosphamide (CP) as the positive control (Table 2).

Table 2.
Tumor inhibitory rate of berberine and palmatine and their analogues 4a–d in the murine sarcoma S180 xenografted model.
| Comp. | Dose mg/kg | Injection | Number of mice | Weight of mice (g) | Weight of tumor X±SD (g) | Tumor inhibitory rate (%) | ||
|---|---|---|---|---|---|---|---|---|
| Start | End | Start | End | |||||
| Control | - | iv | 10 | 10 | 19.48 ± 1.45 | 22.19 ± 2.20 | 2.20 ± 0.93 | - |
| CP | 30 | iv | 10 | 10 | 20.23 ± 1.25 | 20.81 ± 2.31 | 0.43 ± 0.28 ** | 80.61 |
| berberine | 30 | ip | 10 | 9 | 20.20 ± 1.26 | 17.66 ± 3.24 | 1.26 ± 0.54 ** | 42.99 |
| 4a | 1 | ip | 10 | 9 | 19.98 ± 1.33 | 17.86 ± 2.25 | 1.32 ± 0.63 ** | 40.00 |
| 2.5 | ip | 10 | 6 | 19.62 ± 1.52 | 18.04 ± 2.54 * | 1.01 ± 0.48 ** | 54.09 | |
| 4b | 1 | ip | 10 | 8 | 20.20 ± 2.22 | 19.17 ± 1.12 | 1.02 ± 0.25 ** | 53.52 |
| 2.5 | ip | 10 | 5 | 19.89 ± 1.54 | 17.11 ± 1.06 | 0.88 ± 0.28 ** | 59.86 | |
| palmatine | 30 | ip | 10 | 10 | 20.56 ± 2.03 | 20.95 ± 2.18 | 1.45 ± 0.31 ** | 34.09 |
| 4c | 5 | ip | 10 | 10 | 20.33 ± 1.28 | 20.45 ± 1.45 | 1.43 ± 0.44 ** | 34.88 |
| 10 | ip | 10 | 9 | 19.16 ± 1.21 | 18.88 ± 2.60 * | 1.16 ± 0.44 ** | 47.40 | |
| 4d | 5 | ip | 10 | 9 | 20.72 ± 1.45 | 19.15 ± 2.75 | 1.28 ± 0.38 ** | 42.05 |
| 10 | ip | 10 | 5 | 20.04 ± 1.57 | 18.63 ± 3.20 | 1.08 ± 0.42 ** | 50.96 | |
SD: standard deviation; * p < 0.05 vs. model group; ** p < 0.01 vs. model group.
As listed in Table 2, most of the 13-n-hexyl/13-n-octyl berberines and palmatines showed stronger antitumor activity and higher toxicity than their parent compounds, respectively. The 13-n-octyl isoquinoline alkaloid derivatives 4b,d exhibited more activity than the corresponding 13-n-hexyl derivatives 4a,c. Among them, compound 4b possessed the topmost antitumor activity, achieving a tumor inhibitory rate (TIR) of 59.86% at a dose of 2.5 mg/kg and showed better dose-efficacy relationship. Whereas compound 4a,b and d had perspicuously higher toxicity than berberine, palmatine and CP, and produced marked decreases in body weight and even the emergence of the phenomenon of death.
By reducing the dose, the toxic effect of the evaluated compounds can be decreased and their inhibition rate also dropped less. This preliminary in vivo test results showed compound 4c had good TIR and lower toxicity, indicating that it may have potential clinical value for treating cancer. Forthermore, Chen demonstrated the 13-hexyl-berberine gel could achieve effective anti-HSV (herpes simplex virus) concentrations in the dermis and it was safe to use [31]. The deep study on the administration routes and formulations of 13-n-alkyl berberine and palmatine analogues will be carried out.
3. Experimental
3.1. General
All melting points were measured on a Büchi melting point B-540 apparatus (Büchi Labortechnik, Flawil, Switzerland) and were uncorrected. Mass spectra (MS) were taken in ESI model on Agilent 1100 LC-MSD (Agilent, Palo Alto, CA, USA). 1H-NMR spectroscopy was performed using a Bruker ARX-300 instrument (Bruker, Fällanden, Switzerland), operating at 300 MHz with TMS as an internal standard. The chemical shifts were reported in ppm (δ) and coupling constants (J) values were given in Hertz (Hz). Signal multiplicities were represented by: s (singlet), d (doublet), dd (double doublet), t (triplet), dt (triple triplet), m (multiplet). Elemental analysis was performed by an Elementar Vario EL III instrument (Heraeus GmbH, Hanau, Germany) for C, H, and N and the results were within ±0.5% of the theoretical values. Unless noted otherwise, all solvents and reagents were commercially available and used without further purification.
3.2. Chemistry: General Procedure for the Synthesis of Compounds 4a–d
To a stirred solution of 1a or 1b (13.4 mmol) in 5% NaOH solution (100 mL), 5% NaOH (50 mL) solution containing NaBH4 (13.4 mmol) was added dropwise. The reaction mixture was stirred at room temperature for 3 h and the precipitated product was filtered, washed with 60% ethanol (20 mL), and then recrystallized from absolute ethanol to provide 2a or 2b as a brownlike solid. To a stirred solution of 2a or 2b (5.0 mmol) in 80% ethanol (8 mL) and HOAc (2 mL), aldehyde 3a or 3b (5.0 mmol) was added. The reaction mixture was heated to 85–95 °C for 5 h. The solvent was removed by evaporation, and the residue was acidified with 2 mol/L HCl (5 mL), then stirred at room temperature for 1 h. The solid was collected by filtration and then purified by flash chromatography over silica gel, affording the title compounds 4a–d as yellow powders.
2,3-Methylenedioxy-9,10-dimethoxy-13-n-hexylprotoberberine chloride (4a). Yield 65%; m.p. 192–193 °C; 1H-NMR (CDCl3): δ (ppm) 10.66 (s, 1H, Py-H), 7.87–7.94 (m, 2H, Ph-H), 7.12 (s, 1H, Ph-H), 6.90 (s, 1H, Ph-H), 6.11 (s, 2H, -OCH2-), 5.28–5.33 (m, 2H, -CH2-), 4.33 (s, 3H, -OCH3), 4.09 (s, 3H, -OCH3), 3.26–3.31 (m, 2H, -CH2-), 3.14–3.18 (m, 2H, -CH2-), 1.83–1.88 (m, 2H, -CH2-), 1.47–1.54 (m, 2H, -CH2-), 1.34–1.39 (m, 4H, -CH2-), 0.92 (t, 3H, J = 7.0 Hz, -CH3); ESI-MS m/z: 420.1 [M−Cl]+; Anal. calcd. for C26H30ClNO4 × 1/2H2O: C 67.16, H 6.72, N 3.01; found: C 67.21, H 6.68, N 3.01.
2,3-Methylenedioxy-9,10-dimethoxy-13-n-octylprotoberberine chloride (4b). Yield 56%; m.p. 188–189 °C; 1H-NMR (CDCl3): δ (ppm) 10.59 (s, 1H, Py-H), 7.79–7.86 (m, 2H, Ph-H), 7.04 (s, 1H, Ph-H), 6.83 (s, 1H, Ph-H), 6.04 (s, 2H, -OCH2-), 5.19–5.24 (m, 2H, -CH2-), 4.28 (s, 3H, -OCH3), 4.02 (s, 3H, -OCH3), 3.17–3.22 (m, 2H, -CH2-), 3.07–3.12 (m, 2H, -CH2-), 1.78–1.81 (m, 2H, -CH2-), 1.43–1.46 (m, 2H, -CH2-), 1.11–1.16 (m, 8H, -CH2-), 0.81(t, 3H, J = 7.0 Hz, -CH3); ESI-MS m/z: 448.1 [M−Cl]+; Anal. calcd. for C28H34ClNO4 × 3/4H2O: C 67.59, H 7.19, N 2.82; found: C 67.69, H 7.15, N 2.68.
2,3-Dimethoxy-9,10-dimethoxy-13-n-hexylprotoberberine chloride (4c). Yield 64%; m.p. 180–182 °C; 1H-NMR (CDCl3): δ (ppm) 10.89 (s, 1H, Py-H), 7.89–7.95 (m, 2H, Ph-H), 7.26 (s, 1H, Ph-H), 6.96 (s, 1H, Ph-H), 5.20–5.25 (m, 2H, -CH2-), 4.39 (s, 3H, -OCH3), 4.16 (s, 3H, -OCH3), 4.06 (s, 3H, -OCH3), 4.01 (s, 3H, -OCH3), 3.28–3.34 (m, 2H, -CH2-), 3.18–3.22 (m, 2H, -CH2-), 1.91–1.96 (m, 2H, -CH2-), 1.54–1.61 (m, 2H, -CH2-), 1.42–1.46 (m, 4H, -CH2-), 0.96 (s, 3H, J = 7.0 Hz, -CH3); ESI-MS m/z: 436.2 [M-Cl]+; Anal. calcd. for C27H34ClNO4 × 1/2H2O: C 67.42, H 7.33, N 2.91; found: C 67.71, H 7.45, N 2.99.
2,3-Dimethoxy-9,10-dimethoxy-13-n-octylprotoberberine chloride (4d). Yield 62%; m.p. 175–177 °C; 1H-NMR (CDCl3): δ (ppm) 10.83 (s, 1H, Py-H), 7.85–7.89 (m, 2H, Ph-H), 7.19 (s, 1H, Ph-H), 6.91 (s, 1H, Ph-H), 5.21–5.26 (m, 2H, -CH2-), 4.33 (s, 3H, -OCH3), 4.07 (s, 3H, -OCH3), 3.98 (s, 3H, -OCH3), 3.92 (s, 3H, -OCH3), 3.25–3.31 (m, 2H, -CH2-), 3.18 (s, 2H, -CH2-), 1.89–1.91 (m, 2H, -CH2-), 1.51–1.54 (m, 2H, -CH2-), 1.27–1.32 (m, 8H, -CH2-), 0.87 (s, 3H, J = 7.0 Hz, -CH3); ESI-MS m/z: 464.2 [M-Cl]+; Anal. calcd. for C29H38ClNO4 × H2O: C 67.23, H 7.78, N 2.57; found: C 67.54, H 7.88, N 2.70.
3.2. Pharmacology
3.2.1. MTT Assay
The MTT [32] cell proliferation assay was used to test the antitumor activity of berberine, palmatine and compounds 4a–d. The cells were seeded in RPMI-1640 medium (100 μL) in a 96-well plate at a concentration of 5000 cells per well. After culturing for 12 h at 37 °C and with 5% CO2, cells were incubated with scalar concentrations of the tested compounds for 24 h. MTT was added to the cultures at a final concentration of 5 μg/mL, and incubated for 4 h. The formazan crystals were formed and dissolved in DMSO (100 μL) each well. The optical density was measured at 570 nm with the reference wavelength 630 nm. All of the compounds were tested thrice independently using the same cell line. IC50 (concentration that inhibits 50% of cell growth) was calculated using the Bacus Laboratories Incorporated Slide Scanner (Bliss) software, and the data in this manuscript represent mean of two independent experiments.
3.2.2. Anticancer Activity in Vivo
Cyclophosphamide was supplied by Shanghai Hualian Pharmaceutical Co. Ltd. (Shanghai, China); Kunming mice weighing 19–21 g were received from the Shanghai Laboratory Animal Center (Shanghai, China) and kept at five mice/cage at 22–28 °C on a 12 h light/dark cycle with food and water ad libitum. All animals were treated in accordance with the guidelines established by the Institutional Animal Care and Use Committee.
The S180 sarcoma tumor cells (supplied by the Pharmacology Laboratory of Shanghai Institute of Pharmaceutical Industry, Shanghai, China) were diluted with 0.9% normal saline solution to 1–2 × 107 cells/mL and transplanted s.c. via trocar into the left armpits by using an aseptic manipulation, 0.2 mL/mouse. Cyclophosphamide, berberine, palmatine and compounds 4a–d were rejected by the vein at various doses. Each mouse was weighed three times a week and at the end of the study; tumor was weighted by electron scales and tumor inhibition rate was calculated according to the following formula:
Tumor inhibition rate = (mean tumor weight of negative group − mean tumor weight of treated group)/mean tumor weight of negative group × 100%.
3.2.3. Statistical Analysis
All data are presented as mean ± standard deviations (S.D.). The confidence limits were set at p < 0.05. Statistical significance of the differences between groups was assessed by Student’s t-test.
4. Conclusions
The present study to investigate the effect of various long carbon-chain subinstituts at the C-13-position of berberines and palmatines on antitumor activity was successfully carried out. The primary screening results indicated that 13-n-octyl-palmatine (4d) displayed potent cytotoxic activity against seven cancer cells in vitro, but 13-n-hexyl-berberine (4c) exhibited better antitumor activity and less toxic effect in vivo. These studies may provide some guidances for the development of natural compounds as anticancer agents with potential clinical value. Further, structure-activity relation studies and mechanistic studies on this new class of berberine and palmatine compounds are currently in progress and will be reported in due course.
Acknowledgments
This study was supported by grant from National Natural Science Fund (No. 39870882) for financial assistance.
Sample Availability: Contact the first author.
References
- Rabbani, G.H.; Butler, T.; Knight, J.; Sanyal, S.C.; Alam, K. Randomized controlled trial of berberine sulfate therapy for diarrhea due to enterotoxigenic E. coli and Vibrio cholerae. J. Infect. Dis. 1987, 155, 979–984. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Mineshita, S. The Effect of Berberine Chloride on Experimental Colitis in Rats In Vivo and In Vitro. J. Pharmacol. Exp. Ther. 2000, 294, 822–829. [Google Scholar]
- Iwasa, K.; Kamigauchi, M.; Ueki, M.; Taniguchi, M. Antibacterial activity and tructure-activity relationships of berberine analogs. Eur. J. Med. Chem. 1996, 31, 469–478. [Google Scholar] [CrossRef]
- Samosorn, S.; Tanwirat, B.; Muhamad, N.; Casadei, G.; Tomkiewicz, D.; Lewis, K.; Suksamrarn, A.; Prammananan, T.; Gornall, K.C.; Beck, J.L.; et al. Antibacterial activity of berberine-NorA pump inhibitor hybrids with a methylene ether linking group. Bioorg. Med. Chem. 2009, 17, 3866–3872. [Google Scholar] [CrossRef]
- Park, K.D.; Lee, J.H.; Kim, S.H.; Kang, T.H.; Moon, J.S.; Kim, S.U. Synthesis of 13-(substituted benzyl) berberine and berberrubine derivatives as antifungal agents. Bioorg. Med. Chem. Lett. 2006, 16, 3913–3916. [Google Scholar] [CrossRef]
- Iwasa, K.; Kim, H.S.; Wataya, Y.; Lee, D.U. Antimalarial activity and structure-activity relationships of protoberberine alkaloids. Eur. J. Med. Chem. 1998, 13, 65–69. [Google Scholar]
- Vennerstrom, J.L.; Lovelace, J.K.; Waits, V.B.; Hanson, W.L.; Klayman, D.L. Berberine derivatives as antileishmanial drugs. Antimicrob. Agents Chemother. 1990, 34, 918–921. [Google Scholar] [CrossRef]
- Letasiová, S.; Jantová, S.; Cipák, L.; Múcková, M. Berberine-antiproliferative activity in vitro and induction of apoptosis/necrosis of the U937 and B16 cells. Cancer Lett. 2006, 239, 254–262. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Xun, K.L.; Wang, Y.T.; Chen, X.P. A systematic review of the anticancer properties of berberine, A natural product from Chinese herbs. Anticancer Drugs 2009, 20, 757–769. [Google Scholar] [CrossRef]
- Tang, J.; Feng, Y.B.; Tsao, S.; Wang, N.; Curtain, R.; Wang, Y.W. Berberine and Coptidis Rhizoma as novel antineoplastic agents: A review of traditional use and biomedical investigations. J. Ethnopharmacol. 2009, 126, 5–7. [Google Scholar] [CrossRef]
- Li, X.K.; Motwani, M.; Tong, W.; Bornmann, W.; Schwartz, G.K. Huanglian, A Chinese Herbal Extract, Inhibits Cell Growth by Suppressing the Expression of Cyclin B1 and Inhibiting CDC2 Kinase Activity in Human Cancer Cell. Mol. Pharmacol. 2000, 58, 1287–1293. [Google Scholar]
- Kim, S.; Choi, J.H.; Kim, J.B.; Nam, S.J.; Yang, J.H.; Kim, J.H.; Lee, J.E. Berberine Suppresses TNF-α-induced MMP-9 and Cell Invasion through Inhibition of AP-1 Activity in MDA-MB-231 Human Breast Cancer Cells. Molecules 2008, 13, 2975–2985. [Google Scholar] [CrossRef]
- Jiang, H.L.; Wang, X.; Huang, L.; Luo, Z.H.; Su, T.; Ding, K.; Li, X.S. Benzenediol-berberine hybrids: Multifunctional agents for Alzheimer’s disease. Bioorg. Med. Chem. 2011, 19, 7228–7235. [Google Scholar] [CrossRef]
- Ji, H.F.; Shen, L. Berberine: A Potential Multipotent Natural Product to Combat Alzheimer’s Disease. Molecules 2011, 16, 6732–6740. [Google Scholar] [CrossRef]
- Bodiwala, H.S.; Sabde, S.; Mitra, D.; Bhutani, K.K. Synthesis of 9-substituted derivatives of berberine as anti-HIV agents. Eur. J. Med. Chem. 2011, 46, 1045–1049. [Google Scholar] [CrossRef]
- Hayashi, K.; Minoda, K.; Nagaoka, Y.; Hayashi, T.; Uesatob, S. Antiviral activity of berberine and related compounds against human cytomegalovirus. Bioorg. Med. Chem. Lett. 2007, 17, 1562–1564. [Google Scholar] [CrossRef]
- Yang, P.; Song, D.Q.; Li, Y.H.; Kong, W.J.; Wang, Y.X.; Gao, L.M.; Liu, S.Y.; Cao, R.Q.; Jiang, J.D. Synthesis and structure-activity relationships of berberine analogues as a novel class of low-density-lipoprotein receptor up-regulators. Bioorg. Med. Chem. Lett. 2008, 18, 4675–4677. [Google Scholar]
- Tang, L.Q.; Wei, W.; Chen, L.M.; Liu, S. Effects of berberine on diabetes induced by alloxan and a high-fat/high-cholesterol diet in rats. J. Ethnopharmacol. 2006, 108, 109–115. [Google Scholar] [CrossRef]
- Chen, W.H.; Pang, J.Y.; Qin, Y.; Peng, Q.; Cai, Z.; Jiang, Z.H. Synthesis of linked berberine dimers and their remarkably enhanced DNA-binding affinities. Bioorg. Med. Chem. Lett. 2005, 15, 2689–2692. [Google Scholar] [CrossRef]
- Franceschin, M.; Rossetti, L.; D’Ambrosio, A.; Schirripa, S.; Bianco, A.; Ortaggi, G.; Savino, M.; Schultes, C.; Neidle, S. Natural and synthetic G-quadruplex interactive berberine derivatives. Bioorg. Med. Chem. Lett. 2006, 16, 1707–1711. [Google Scholar]
- Zhang, W.J.; Ou, T.M.; Lu, Y.J.; Huang, Y.Y.; Wu, W.B.; Huang, Z.S.; Zhou, J.L.; Wong, K.Y.; Gu, L.Q. 9-Substituted berberine derivatives as G-quadruplex stabilizing ligands in telomeric DNA. Bioorg. Med. Chem. 2007, 15, 5493–5501. [Google Scholar]
- Bbadra, K.; Kumar, G.S. Therapeutic Potential of Nucleic Acid-Binding Isoquinoline Alkaloids: Binding Aspects and Implications for Drug Design. Med. Res. Rev. 2011, 31, 821–862. [Google Scholar] [CrossRef]
- Islam, M.M.; Basu, A.; Hossain, M.; Sureshkumar, G.; Hotha, S.; Kumar, G.S. Enhanced DNA Binding of 9-ω-Amino Alkyl Ether Analogs from the Plant Alkaloid Berberine. DNA Cell Biol. 2011, 30, 123–133. [Google Scholar] [CrossRef]
- Islam, M.M.; Basu, A.; Kumar, G.S. Binding of 9-O-(ω-amino) alkyl ether analogues of the plant alkaloid berberine to poly(A): Insights into self-structure induction. Med. Chem. Commun. 2011, 2, 631–637. [Google Scholar] [CrossRef]
- Basu, A.; Jaisankar, P.; Kumar, G.S. Synthesis of novel 9-O-N-aryl/aryl-alkyl amino carbonyl methyl substituted berberine analogs and evaluation of DNA binding aspects. Bioorg. Med. Chem. 2012, 20, 2498–2505. [Google Scholar] [CrossRef]
- Bhowmik, D.; Hossain, M.; Buzzetti, F.; D’Auria, R.; Lombardi, P.; Kumar, G.S. Biophysical Studies on the Effect of the 13 Position Substitution of the Anticancer Alkaloid Berberine on Its DNA Binding. J. Phys. Chem. 2012, 116, 2314–2324. [Google Scholar]
- Chang, Y.L.; Usami, S.; Hsieh, M.T.; Jiang, M.J. Effects of palmatine on isometric force and intracellular calcium levels of arterial smooth muscle. Life Sci. 1999, 64, 597–606. [Google Scholar] [CrossRef]
- Vennerstrom, J.L.; Klayman, D.L. Protoberberine Alkaloids as Antimalarials. J. Med. Chem. 1988, 31, 1084–1087. [Google Scholar] [CrossRef]
- Kuo, C.L.; Chou, C.C.; Yung, B.Y. Berberine complexes with DNA in the berberine-induced apoptosis in human leukemic HL-60 cells. Cancer Lett. 1995, 93, 193–200. [Google Scholar] [CrossRef]
- Iwasa, K.; Kamigauchi, M.; Sugiura, M.; Nanba, H. Antimicrobial Activity of Some 13-Alkyl Substituted Protoberberinium Salts. Planta Med. 1997, 63, 196–198. [Google Scholar] [CrossRef]
- Wei, H.L.; Wang, S.Q.; Xu, F.; Xu, L.F.; Zheng, J.R.; Chen, Y. Evaluation of a 13-hexyl-berberine hydrochloride topical gel formulation. Drug Dev. Ind. Pharm. 2012, 38, 1–6. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J. Natl. Cancer. Inst. 1990, 82, 1107–1112. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).