Isostreptazolin and Sannaphenol, Two New Metabolites from Streptomyces sannanensis
Abstract
:1. Introduction
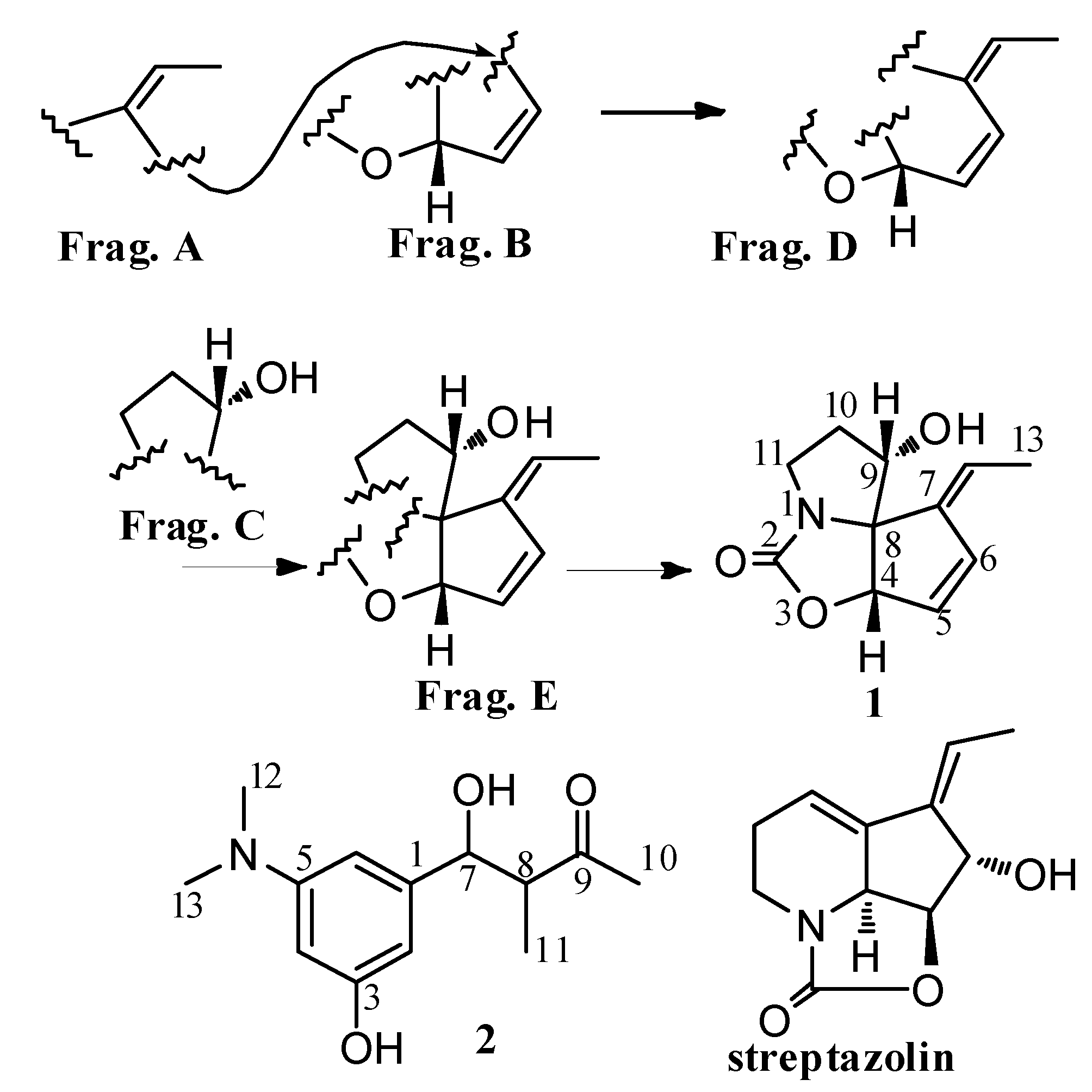
2. Results and Discussion
2.1. Chemistry
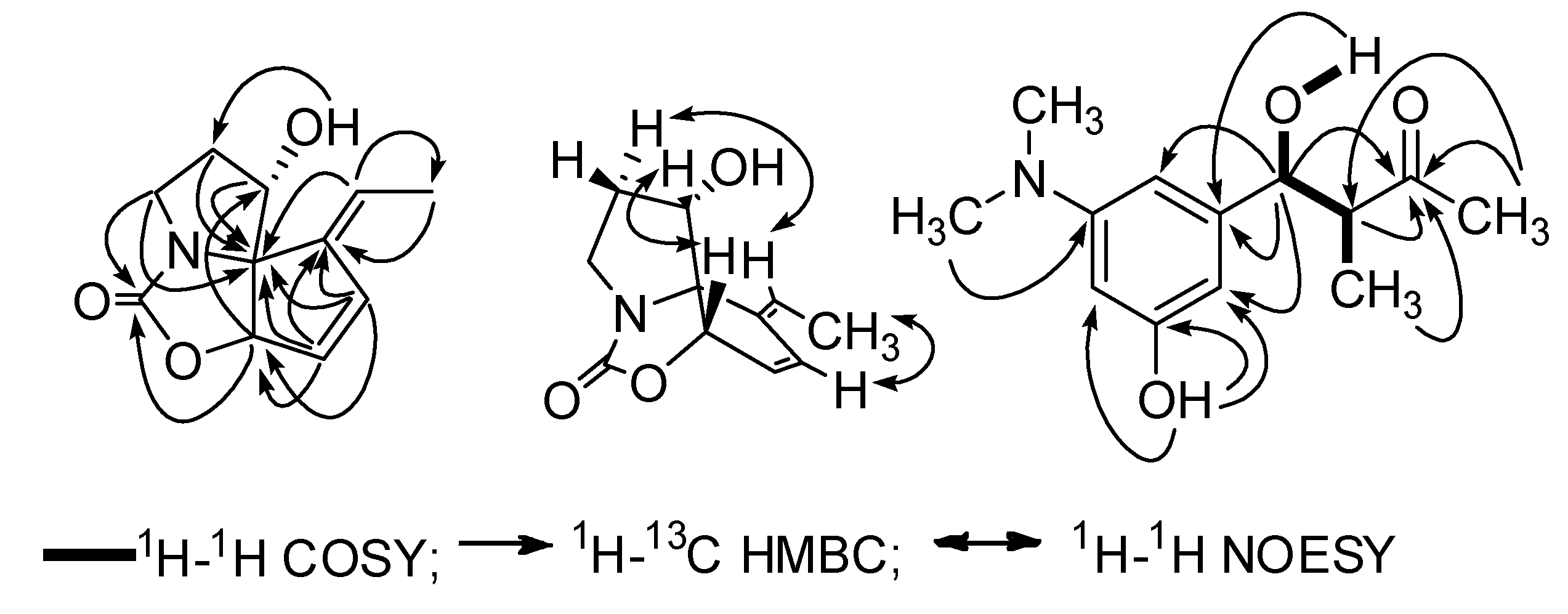
| No. | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH | δC | δH | |
| 1 | - | - | 145.7 | - |
| 2 | 160.1 | - | 101.7 | 6.14 1H, brs |
| 3 | - | - | 151.4 | - |
| 4 | 82.6 | 5.50 1H, d, J = 1.8 | 98.2 | 5.98 1H, brt, J = 1.8 |
| 5 | 131.8 | 6.21 1H, brd, J = 5.8 | 157.7 | - |
| 6 | 134.1 | 6.80 1H, d, J = 5.8 | 101.8 | 6.09 1H, brs |
| 7 | 144.1 | - | 73.2 | 4.75 1H, t, J = 4.8 |
| 8 | 79.0 | - | 53.9 | 2.72 1H, m |
| 9 | 72.8 | 4.11 1H, brt, J = 4.0 | 210.2 | - |
| 10 | 35.6 | 2.48 1H, m | 28.9 | 2.05 3H, s |
| 2.05 1H, m | ||||
| 11 | 42.9 | 3.58 1H, ddd, J = 11.0, 9.2, 7.3 | 10.5 | 0.88 3H, d, J = 6.6 |
| 3.11 1H, ddd, J = 11.0, 9.9, 4.0 | ||||
| 12 | 118.9 | 5.44 1H, q, J = 7.2 | 40.0 | 2.83 3H, s |
| 13 | 14.6 | 1.75 3H, d, J = 7.2 | 40.0 | 2.83 3H, s |
| 3-OH | 8.97 1H, brs | |||
| 7-OH | 5.19 1H, d, J = 4.8 | |||
| 9-OH | 5.47 1H, d, J = 4.0 | |||
2.2. Biological Activity
3. Experimental
3.1. General
3.2. Producing Organism
3.3. Extraction and Isolation
3.4. Compound Characterization
3.5. Cytotoxic Assay
4. Conclusions
Acknowledgements
- Samples Availability: Samples of the compounds 1 and 2 are available from the authors.
References and Notes
- Li, Y.; Zheng, D.; Li, J.; Han, L.; Cui, X.; Lang, L.; Li, M.; Wang, Z.; Zhao, J.; Huang, X. Sannanine, a new cytotoxic alkaloid from Streptomyces sannanensis. J. Antibiot. 2009, 62, 647–648. [Google Scholar] [CrossRef]
- Drautz, H.; Zähner, H.; Kupfer, E.; Keller-Schierlein, W. Stoffwechselprodukte von mikroorganismen 205. mitteilung† isolierung und struktur von streptazolin. Helv. Chim. Acta 1981, 64, 1752–1765. [Google Scholar]
- Karrer, A.; Dobler, M. Stoffwechselprodukte von mikroorganismen 217. mitteilung† röntgenstrukturanalyse von O-acetyldihydrostreptazolin. Helv. Chim. Acta 1982, 65, 1432–1435. [Google Scholar]
- Puder, C.; Loya, S.; Hizi, A.; Zeek, A. New co-metabolites of the streptazolin pathway. J. Nat. Prod. 2001, 64, 42–45. [Google Scholar] [CrossRef]
- Nomura, I.; Mukai, C. Total synthesis of (±)-8α-hydroxystreptazolone. Org. Lett. 2002, 4, 4301–4304. [Google Scholar] [CrossRef]
- Mayer, M.; Thiericke, R. Biosynthesis of streptazolin. J. Org. Chem. 1993, 58, 3486–3489. [Google Scholar] [CrossRef]
- Li, F.; Warshakoon, N.C.; Miller, M.J. Synthetic application of acylnitroso Diels-Alder derived aminocyclopentenols: total synthesis of (+)-streptazolin. J. Org. Chem. 2004, 69, 8836–8841. [Google Scholar] [CrossRef]
- Grabley, S.; Hammann, P.; Kluge, H.; Wink, J.; Kricke, P.; Zeeck, A. Secondary metabolites by chemical screening 4†, detection, isolation and biological activities of chiral synthons from Streptomyces. J. Antibiot. 1991, 44, 797–800. [Google Scholar] [CrossRef]
- Puder, C.; Krastel, P.; Zeeck, A. Streptazones A, B1, B2, C, and D: New piperidine alkaloids from streptomycetes. J. Nat. Prod. 2000, 63, 1258–1260. [Google Scholar] [CrossRef]
- Grabley, S.; Kluge, H.; Hoppe, H.U. New lead structures by Diels-Alder reactions of streptazolin with naphthoquinones. Angew.Chem. Int. Ed. Engl. 1987, 26, 664–665. [Google Scholar] [CrossRef]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazoliumbasedsemiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zheng, D.; Han, L.; Li, Y.; Li, J.; Rong, H.; Leng, Q.; Jiang, Y.; Zhao, L.; Huang, X. Isostreptazolin and Sannaphenol, Two New Metabolites from Streptomyces sannanensis. Molecules 2012, 17, 836-842. https://doi.org/10.3390/molecules17010836
Zheng D, Han L, Li Y, Li J, Rong H, Leng Q, Jiang Y, Zhao L, Huang X. Isostreptazolin and Sannaphenol, Two New Metabolites from Streptomyces sannanensis. Molecules. 2012; 17(1):836-842. https://doi.org/10.3390/molecules17010836
Chicago/Turabian StyleZheng, Dan, Li Han, Yiqing Li, Jun Li, He Rong, Qiao Leng, Yi Jiang, Lixing Zhao, and Xueshi Huang. 2012. "Isostreptazolin and Sannaphenol, Two New Metabolites from Streptomyces sannanensis" Molecules 17, no. 1: 836-842. https://doi.org/10.3390/molecules17010836
APA StyleZheng, D., Han, L., Li, Y., Li, J., Rong, H., Leng, Q., Jiang, Y., Zhao, L., & Huang, X. (2012). Isostreptazolin and Sannaphenol, Two New Metabolites from Streptomyces sannanensis. Molecules, 17(1), 836-842. https://doi.org/10.3390/molecules17010836




