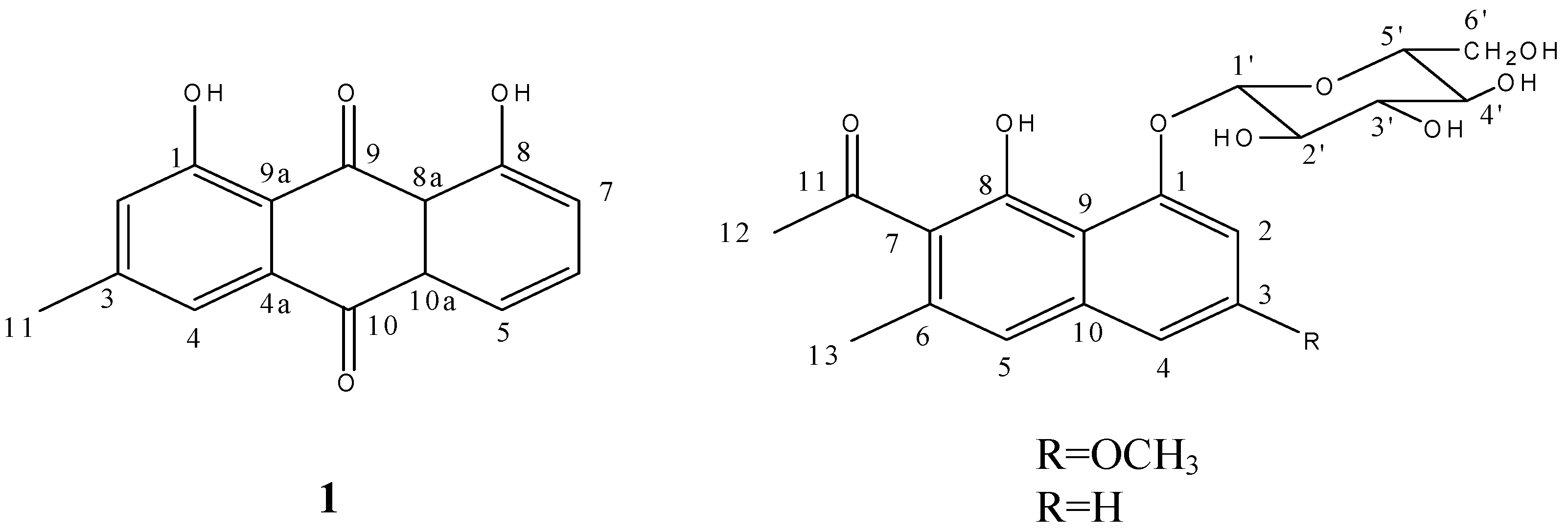
1. Introduction
Rumex, the second genera among the
Polygonaceae, comprises about 150 species widely distributed around the World. In China, it is represented by 26 species.
Rumex dentatus is one of them, which could be found almost everywhere in China [
1]. This traditional herb has been used as a medicine for many kinds of bacterial and fungal infection diseases, such as dysentery, enteritis, acariasis and eczema [
2]. Since
Rumex is in the same family with
Rheum, their chemical compositions have some common features, such as the presence of chrysophanol, emodin, aloe-emodin and physcion [
3,
4,
5,
6,
7,
8]. Besides anthraquinones, other main chemical constituents in
Rumex are flavonoids [
9,
10,
11,
12,
13], diphenylethenes [
10,
14] and naphthalenes [
15,
16]. Research on the chemical contents of
Rumex dentatus is rare. At this point, the chemical constituents from its root extract were scanned in our lab. Five compounds were obtained and three of them were identified by their physicochemical properties and spectroscopic analysis. Their antiproliferation activities were then tested with the MTT assay in four cell lines, including breast cancer MCF-7, gastric cancer 7901, melanoma A375 and oophoroma SKOV-3 and IC
50 values in each cell line were calculated.
2. Results and Discussion
The Feigl reaction of compound
1 was positive, which indicated this compound might be a quinone. The magnesium acetate reaction showed an orange color, indicating the presence of a β-OH or an α-OH located on the benzene ring or the two –OH that were not on the same ring. The molecular formula C
15H
10O
4 was assigned from its HRFABMS (
m/z 255.3316 [M+H]
+, calcd. 255.3399) and
1H,
13C-NMR data (
Table 1). By comparing the NMR data with reported ones [
17], this compound was identified as 3-methyl-1, 8-dihydroxy anthraquinone, that is to say, chrysophanol (
Figure 1).
Table 1.
1H and 13C-NMR data for chrysophanol (compound 1) (500 and 125 MHz, CDCl3, J in Hz and δ in ppm).
Table 1.
1H and 13C-NMR data for chrysophanol (compound 1) (500 and 125 MHz, CDCl3, J in Hz and δ in ppm).
| No. | δH | δC |
|---|
| 1 | / | 162.7 |
| 2 | 7.11 (1H, d,
J 1) | 124.5 |
| 3 | / | 149.3 |
| 4 | 7.66 (1H, d,
J 1) | 121.4 |
| 5 | 7.83 (1H, dd,
J 8.5, 1) | 119.9 |
| 6 | 7.67 (1H, t,
J 8.5) | 136.9 |
| 7 | 7.30 (1H, dd,
J 8.5, 1) | 124.6 |
| 8 | / | 162.4 |
| 9 | / | 192.6 |
| 10 | / | 182.0 |
| 11 | 2.47 (3H, s) | 22.2 |
| 4a | / | 115.9 |
| 8a | / | 115.5 |
| 9a | / | 108.2 |
| 10a | / | 135.7 |
| 1-OH | 12.03 | / |
| 8-OH | 12.13 | / |
Figure 1.
Structures of compounds 1–3.
Figure 1.
Structures of compounds 1–3.
Compound 2 gave a positive Molish reaction, which suggested this compound might be a glucoside. The molecular formula was assigned as C20H25O9 from its HRFABMS (m/z 410.5401 [M+H]+, calcd. 410.5410) and 1H, 13C-NMR data. There are two methyl signals at δ 19.92 and δ 32.64, and a methoxyl signal at δ 55.80 in the 13C-NMR. Considering the DEPT 135 spectrum evidence, signals at δ 61.25 (CH2), δ 70.43 (CH), δ 73.95 (CH), δ 76.77 (CH) and δ 78.28 (CH) should belong to a sugar. Three methenyl signals were observed at δ 101.75, δ 103.20 and δ 103.55. As one of them should be the terminal carbon of the sugar, the other two plus eight signals from δ 109.15 to δ 158.80 indicated the skeleton of this compound should be a naphthalene.
At the lowest field, there is a carbonyl signal at δ 204.86. Two methyl proton signals were also observed in the
1H-NMR at δ 2.23 (s, 3H) and δ 2.51 (s, 3H) and a methoxyl proton signal was found at δ 3.84 (s, 3H). Six methenyl proton signals from δ 3.19 to δ 3.84 should belong to the sugar. The signal at δ 5.06 (d,
J = 7.5 Hz, 1H) should be the terminal proton of the sugar. Aromatic proton signals were grouped according to their coupling and splitting. Signals at δ 6.91 (d,
J = 2.5 Hz, 1H) and δ 7.00 (d,
J = 2.5 Hz, 1H) were divided into group one and the signal at δ 7.09 (s, 1H) was in group two. The coupling constant of group one suggested these two protons should be in a
meta-position, and since group two has only one proton signal without splitting, it should located at the other benzene ring of the naphthalene. Emulsin hydrolysis and TLC detection indicated the sugar should be a D-sugar, but the absolute configuration of the carbohydrate couldn’t be determined since no optical rotation data could be provided. Acid hydrolysis and gas chromatography (GC) analysis showed the presence of glucose. With consideration of the chemical shifts of the carbon and proton, as well as the coupling constant of the terminal proton, the sugar was conjectured to be β-
D-glucose. The correlation from the terminal proton to the carbon signal at δ 103.20 in HSQC spectrum and the carbon signal at δ 155.87 in HMBC spectrum suggested which was the terminal carbon and where the glucose was located. The glycosidation position was labeled as position 1, then the correlations between C-1 and H-2 (δ 7.00), H-2 and C-3 (δ 158.80), C-3 and H-4 (δ 6.91) in HMBC (see
Figure 2) clarified the sequence of the A ring. The correlation from the methoxyl proton (δ 3.84) to C-3 indicated the location of the methoxyl. The
1H-NMR analysis showed an uncoupled aromatic proton (δ 7.09) on ring B, and the correlation from it to C-4 in HMBC told us this proton should be labeled as H-5, so the carbon at δ 119.26 which correlated with H-5 in HSQC is C-5. The methyl proton at δ 2.23 which correlated with C-5 in HMBC was determined to be located at C-6 (δ 134.13). The correlation from this methyl to the carbon at δ 123.72 suggested the chemical shift of C-7. Correlations from the other methyl group (δ 2.51) to C-7 and the carbonyl carbon (δ 204.86) indicated the substitution of an acetyl at C-7. Putting all the above atoms together, there’s a –OH missing when compared with the molecular formula. So the only position left (δ 151.52) should connect with a hydroxyl. In summary, compound
2 was identified as 6-methyl-7-acetyl-1,8-dihydroxy-3-methoxynaphthalene-1-
O-β-
D(
L)-glucoside (
Figure 1). All the proton and carbon signals assignments was established with HSQC,
1H-
1H COSY and HMBC (
Table 2).
Figure 2.
The key HMBC correlations of compound 2.
Figure 2.
The key HMBC correlations of compound 2.
Table 2.
1H and 13C-NMR data for compounds 2 and 3 (500 and 125 MHz, DMSO-d6, J in Hz and δ in ppm).
Table 2.
1H and 13C-NMR data for compounds 2 and 3 (500 and 125 MHz, DMSO-d6, J in Hz and δ in ppm).
| No | 2 | | 3 |
|---|
| δH | δC | | δH | δC |
|---|
| 1 | / | 155.9 | | / | 154.8 |
| 2 | 7.00 (1H, d,
J 2.5) | 103.5 | | 7.32 (1H, d,
J 8) | 111.2 |
| 3 | / | 158.8 | | 7.41 (1H, t,
J 8) | 128.0 |
| 4 | 6.91 (1H, d,
J 2.5) | 101.7 | | 7.48 (1H, d,
J 8) | 122.8 |
| 5 | 7.09 (1H, s) | 119.3 | | 7.23 (1H, s) | 120.0 |
| 6 | / | 134.1 | | / | 133.4 |
| 7 | / | 123.7 | | / | 125.8 |
| 8 | / | 151.5 | | / | 150.8 |
| 9 | / | 109.1 | | / | 113.7 |
| 10 | / | 137.3 | | / | 136.3 |
| 11 | / | 204.9 | | / | 205.2 |
| 12 | 2.51 (3H, s) | 32.6 | | 2.53 (3H, s) | 32.5 |
| 13 | 2.23 (3H, s) | 19.9 | | 2.26 (3H, s) | 19.7 |
| 1' | 5.06 (1H, d,
J 7.5) | 103.2 | | 5.07 (1H, d,
J 7.5) | 103.2 |
| 2' | 3.36 (1H, overlapping) | 73.9 | | 3.38 (1H, overlapping) | 73.9 |
| 3' | 3.34 (1H, overlapping) | 76.8 | | 3.37 (1H, overlapping) | 76.8 |
| 4' | 3.19 (1H, m) | 70.4 | | 3.21 (1H, dd,
J 8.5, 5.5) | 70.3 |
| 5' | 3.47 (1H, overlapping) | 78.3 | | 3.44 (1H, t,
J 9.5, 6) | 78.3 |
| 6' | 3.50 (1H, overlapping ) | 61.2 | | 3.51 (1H, ddd,
J 12, 6, 6) | 61.2 |
| 3.75 (1H, dd,
J
11, 5.5) | 3.77 (1H, dd,
J
10.5, 5.5) |
| 8-OH | 9.50 (1H, s) | / | | 9.60 (1H, s) | / |
| 3-OMe | 3.84 (3H, s) | 55.8 | | / | / |
Compound
3 also showed a positive Molish reaction. The molecular formula was assigned as C
19H
23O
8 from its HRFABMS (
m/z 379.5006 [M+H]
+, calc. for 379.5008) and
1H-,
13C-NMR data, which suggested this compound might have a similar skeleton as compound
2, except for a methoxyl group. Comparing the NMR spectra of these two compounds, the disappearances of the methoxyl carbon signal at δ 55.8 and the methoxyl proton signal at δ 3.84 also supported this deduction. Therefore, compound
3 was identified as 6-methyl-7-acetyl-1,8-dihydroxynaphthalene-1-
O-β-
D-glucoside (
Figure 1). The chemical shifts of all carbon atoms in these two compounds were compared to see the substituent chemical shift changing rules. As the chemical shifts of glucose and B ring are almost matched, the signal of C-3 shifted down-field from δ 128.0 to δ 158.8 and signals of C-2 and C-4 shifted up-field from δ 111.2 to δ 103.5 and from δ 122.8 to δ 101.7 due to the substituent of methoxyl at C-3. In addition as the
para-position of C-3, the signal of C-9 shifted up-field from δ 113.7 to δ 109.1. With all this proof, the previous hypothesis was confirmed. The assignment of all the proton and carbon signals was established with HSQC,
1H-
1H COSY and HMBC (
Table 2).
The compounds above were further evaluated for their antiproliferative activity using four cell lines including MCF-7 breast cancer cell line, gastric cancer 7901 cells, melanoma A375 cells and oophoroma SKOV-3 cells. Their antiproliferation activities were represented with IC
50 values (
Table 3). Chrysophanol (
1), was more active than the other two, especially in the oophoroma SKOV-3 cell line, where the IC
50 value was 5.62 μM. Compounds
2 and
3 showed no effects on the gastric cancer 7901 cells. The methoxyl group at C-3 in
2 seems to have a key influences of the antiproliferation activity since most of the IC
50 values of
2 were lower than those of
3.
Table 3.
IC50 values (μM) of compound 1–3 in four cell lines.
Table 3.
IC50 values (μM) of compound 1–3 in four cell lines.
| Compound | MCF-7 | 7901 | A375 | SKOV-3 |
|---|
| breast cancer | gastric cancer | melanoma | oophoroma |
|---|
| 1 | 20.4 ± 7.8 | 513 ± 265 | 83.1 ± 35.1 | 5.62 ± 1.58 |
| 2 | 269 ± 133 | - | 186 ± 57 | 40.7 ± 23.1 |
| 3 | 1580 ± 1860 | - | 275 ± 143 | 174 ± 114 |
3. Experimental
3.1. General
Melting points were determined on a MP-J3 microscope apparatus. UV were obtained on a SP-2102UV spectrophotometer. IR spectra (KBr) were recorded on a Jasco FTIR-4100. The NMR spectra were recorded on a Bruker Ultrashield Plus spectrometer (500 MHz for 1H-NMR and 125 MHz for 13C-NMR) with TMS as internal standard. The HR-ESI-MS were obtained with a Bruker APEX III spectrometer. GC were performed on a Shimadzu GC-QP2010. The OD values in MTT assay were measured by a POLARstar + OPTIMA Plate Reader (BMG Labtechnologies, Ortenberg, Germany). The purity of compounds were checked on a Waters 600 (Waters, Milford, MA, USA) HPLC system equipped with an Intersel C18 (5 μm, 4.6 × 250 mm) column. Column chromatography was performed with silica gel (200–300 mesh, Qingdao Haiyang Chemical Group Co. Ltd, Qingdao, China). TLC were detected on silica gel 60 F254 (Merck, Darmstadt, Germany) by spraying with 10% ethanolic H2SO4 reagent followed by heating.
3.2. Plant Material
Rumex dentatus L. roots were collected from the test herb field of Xi’an Jiaotong University, Xi’an, China, in December 2010. The plant was identified by Professor Junxian Wang at Xi’an Jiaotong University of Nature Products Chemistry and a voucher specimen has been deposited in Faculty of Pharmacy, School of Medicine, Xi’an Jiaotong University, Xi’an 710061, China.
3.3. Cell Culture
Cell lines were obtained from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China) and routinely cultured in RPMI-1640 (breast cancer MCF-7, melanoma A375 and oophoroma SKOV-3) or DMEM (gastric cancer 7901) medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin in a humidified incubator at 37 °C with 5% CO2.
3.4. Extraction and Isolation
One kilogram of Rumex dentatus L. roots were collected and air-dried. The meshed herb was then refluxed with methanol (×8) for 3 h to obtain the crude extract. The residue was dissolved in H2O (1 L) and then extracted successively with petroleum ether (1 L × 3), chloroform (1 L × 3), ethyl acetate (1 L × 3) and n-butanol (1 L × 3). The ethyl acetate part (13 g) was then subjected to silica gel column chromatography and eluted with chloroform-methanol system in the ratios of 500:1, 400:1, 300:1, 200:1, 150:1, 100:1, 80:1, 60:1, 50:1, 30:1, 10:1, 5:1 and 1:1 to give 350 fractions. The crystals in fractions 26–38 and fractions 67–75 were recrystallized to yield compounds 1 (12 mg) and 2 (30 mg). Fractions 80–100 were further subjected to silica gel CC with chloroform-acetone (10:3). Subfractions 51–57 afforded compound 3 (56 mg).
Compound
1, orange needles (methanol); mp 145–147 °С; [α]
D25 +21.3 (c 0.55, acetone); UV λ
max (MeOH): 258.50, 289.00, 433.00; IR bands (KBr): 3451, 3024,2957, 1673, 1622, 1561, 1382, 1262, 1196, 1053, 989, 870 cm
−1;
1H (500 MHz, CDCl
3) and
13C-NMR (125 MHz, CDCl
3) data: see
Table 1; HRFABMS (positive ion mode)
m/z: 255.3316 [M+H]
+. Calc. for C
15H
11O
4 255.3399.
Compound
2, white needles(acetone); mp 190–192 °С; [α]
D25 +53.5 (c 0.50, MeOH); UV λ
max (MeOH): 225.78, 312.54; IR bands (KBr): 3431, 2948, 1725, 1576, 1567, 1557, 1409, 1383, 1266, 1180, 1054, 871 cm
−1;
1H (500 MHz, DMSO-
d6) and
13C-NMR (125 MHz, DMSO-
d6) data: see
Table 2; HRFABMS (positive ion mode)
m/z: 410.5401 [M+H]
+. Calc. for C
20H
26O
9 410.5410.
Compound
3, white needles (acetone); mp 193–195 °С; [α]
D25 +58.2 (c 0.55, MeOH); UV λ
max (MeOH): 224.60, 310.12; IR bands (KBr): 3478, 2928, 1742, 1557, 1526, 1384, 1256, 1184, 1054, 872 cm
−1;
1H (500 MHz, DMSO-
d6) and
13C-NMR (125 MHz, DMSO-
d6) data: see
Table 2; HRESIMS
m/z: 379.5006 [M+H]
+. Calc. for C
19H
23O
8 379.5008.
3.5. Hydrolysis
Compound 2 (2 mg) was dissolved in PBS (2 mL) and emulsin (20 μL, 80 u/μL) was added. Then the mixture was incubated in a shaker bath at 50 °С and 75 rpm for 5 h. The hydrolysis product was extracted with chloroform and concentrated. Then TLC was employed to determine if the indican was hydrolyzed to an aglycone.
Acid hydrolysis was performed by a reported method [
18]. Briefly, compound
2 (10 mg) was dissolved in 12% HCl (5 mL) and heated at 90 °С for 2 h. The aglycone was extracted with chloroform, and the pH value of aqueous residue was adjusted to 7.0 with 12% NaOH. NaBH
4 (40 mg) was added and the mixture was acidified with dilute CH
3COOH. Excess boric acid was removed by distilling with methanol. Pyridine (1 mL) was added to dissolve the reduced sugar and acetic anhydride (1 mL) was then added. The whole mixture was heated at the room temperature for 12 h to afford the corresponding alditol acetates. The product was then analyzed by GC-QP2010 (Shimadzu), which was equipped with an FID and N
2 was used as the carrier gas with flow speed at 1 mL/min. An HP-1 (30 m × 0.32 mm) capillary column was employed for the analysis and injector temperature was 280 °C. The temperature program was started at 180 °C, then increased to 280 °C at the speed of 10 °C/min after 10 min, and kept at 280 °C for 5 min. The standard sugar was treated the same way and analyzed under the same condition (
D-glucose, t
R, 16.8 min).
3.6. MTT Assay
To perform the antiproliferation assay, cells were plated in 96-well microtiter plates at the concentration of 1 × 106/well in appropriate growth media (DMEM for gastric cancer and RPMI-1640 for others). The DMSO solution (1 mL) of test compounds were added 24 h later. Negative controls received normal growth media and the same amount of DMSO. Plates were incubated for 2 days after treatment, then 20 mL MTT solution (5 g/L) was added into each well and incubated for another 4 h. Supernatants were removed and formazan crystals were dissolved in 200 mL dimethylsulfoxide. A plate reader was used to measure the staining intensity of each well at 490 nm. The IC50 values were calculated with the SPSS software using five drug concentrations (104–1 μM) and the assays were performed in triplicate.