Preparation of Novel meta- and para-Substituted N-Benzyl Protected Quinuclidine Esters and Their Resolution with Butyrylcholinesterase
Abstract
:1. Introduction
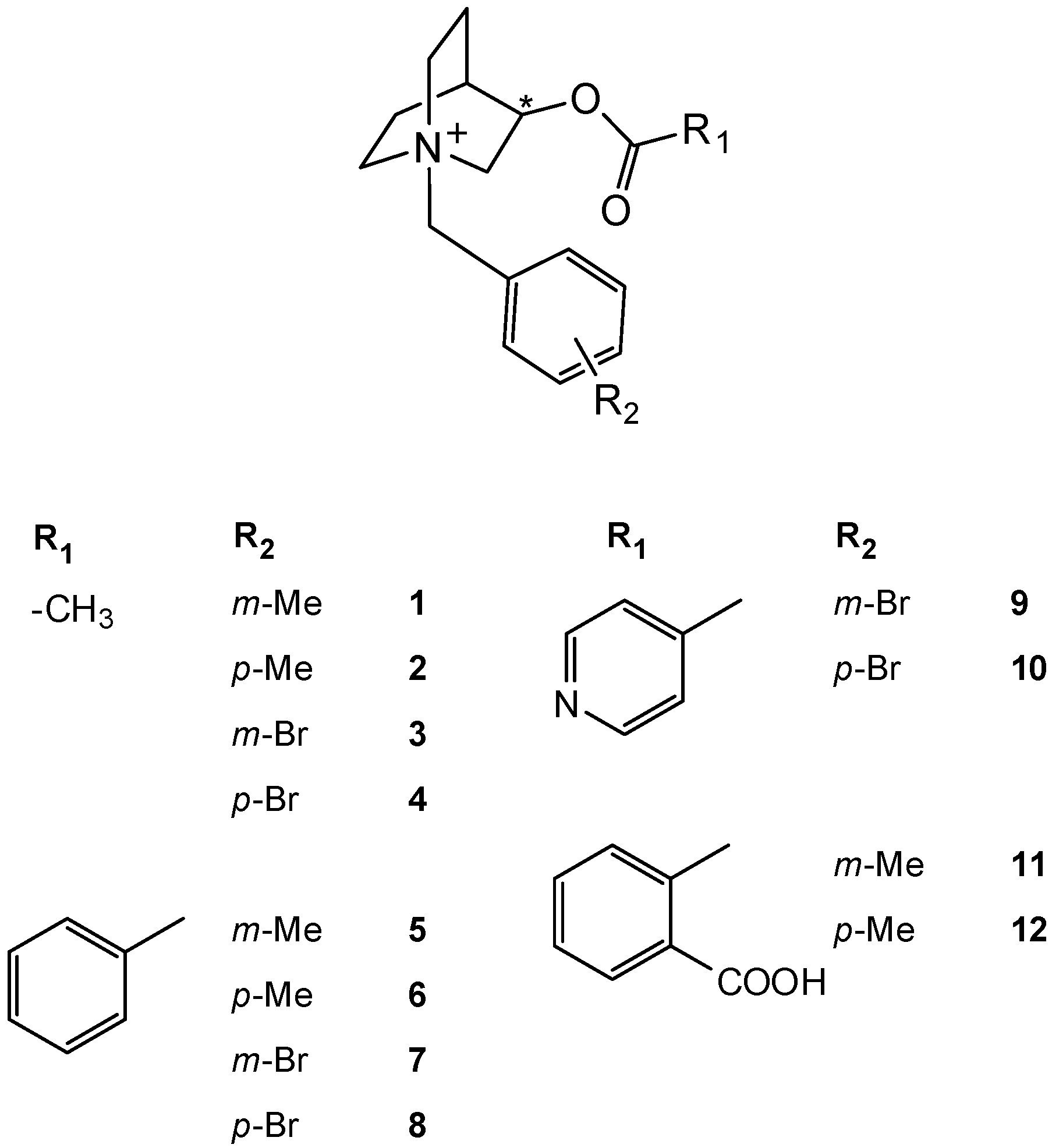

2. Results and Discussion
| Compound | Time (BChE hydrolysis) /min | [α]D26 ester | [α]D26 quinuclidin-3-ol | Optical purity (S)-ester /% | Optical purity (R)- quinuclidin-3-ol /% |
|---|---|---|---|---|---|
| 1 | 80 | −140 | −80 | 88 | 89 |
| 2 | 60 | −140 | −90 | 88 | 100 |
| 3 | 60 | −140 | −80 | 88 | 89 |
| 4 | 60 | −140 | −80 | 88 | 89 |
| 5 | 185 | −82.3 | −60 | 82 | 67 |
| 6 | 258 | −100 | −60 | 100 | 67 |
| 7 | 360 | −62 | −70 | 62 | 78 |
| 8 | 1320 | −100 | −70 | 100 | 78 |
| 9 | 60 | −200 | −70 | 95 | 78 |
| 10 | 45 | −200 | −70 | 95 | 78 |
| 11 | 170 | 110 | −70 | 85 | 78 |
| 12 | 75 | 100 | −80 | 77 | 89 |

3. Experimental
3.1. General
3.2. General Procedure for Quaternization of Esters
3.3. Kinetic Resolution with BChE
3.4. Catalytic Transfer Hydrogenation
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- Quinn, D.M. Acetylcholinesterase: Enzyme structure, reaction dynamics, and virtual transition states. Chem. Rev. 1987, 87, 955–979. [Google Scholar] [CrossRef]
- Sun, H.; El Yazal, J.; Lockridge, O.; Schopfer, L.M.; Brimijoin, S.; Pang, Y.-P. Predicted Michaelis-Menten complexes of cocaine-butyrylcholinesterase: Engineering effective butyrylcholinesterase mutants for cocaine detoxication. J. Biol. Chem. 2001, 276, 9330–9336. [Google Scholar]
- Doctor, B.P.; Saxena, A. Bioscavengers for the protection of humans against organophosphate toxicity. Chem. Biol. Interact. 2005, 157–158, 167–171. [Google Scholar] [CrossRef]
- Askar, K.A.; Kudi, A.C.; Moody, A.J. Comparison of two storage methods for the analysis of cholinesterase activities in food animals. Enzyme Res. 2010, 2010, 1–11. [Google Scholar] [PubMed]
- Askar, K.A.; Kudi, A.C.; Moody, A.J. Spontaneous reactivation and aging kinetics of acetylcholinesterase inhibited by dichlorvos and diazinon. J. Toxicol. Sci. 2011, 36, 237–241. [Google Scholar] [CrossRef]
- Schelhaas, M.; Glomsda, S.; Hänsler, M.; Jakubke, H.-D.; Waldmann, H. Enzymatic synthesis of peptides and ras lipopeptides employing choline ester as a solubilizing, protecting, and activating group. Angew. Chem. Int. Ed. 1996, 35, 106–109. [Google Scholar] [CrossRef]
- Primožič, I.; Hrenar, T.; Tomić, S.; Meić, Z. Interactions of chiral quinuclidin-3-yl benzoates with butyrylcholinesterase: Kinetic study and docking simulations. J. Phys. Org. Chem. 2002, 58, 608–614. [Google Scholar]
- Primožič, I.; Hrenar, T.; Tomić, S.; Meić, Z. Stereoselective hydrolysis of quaternary quinuclidinium benzoates catalyzed by butyrylcholinesterase. Eur. J. Org. Chem. 2003, 295–301. [Google Scholar]
- Bosak, A.; Primožič, I.; Oršulić, M.; Tomić, S.; Simeon-Rudolf, V. Enantiomers of quinuclidin-3-ol derivatives: Resolution and interactions with human cholinesterases. Croat. Chem. Acta 2005, 78, 121–128. [Google Scholar]
- Mashkovsky, M.D.; Yakhontov, L.N.; Kaminka, M.E.; Mikhlina, E.E. Further development in research on the chemistry and pharmacology of synthetic quinuclidine derivatives. Prog. Drug Res. 1983, 27, 9–61. [Google Scholar]
- Primožič, I.; Tomić, S. Influence of the acyl moiety on the hydrolysis of quinuclidinium esters catalyzed by butyrylcholinesterase. Croat. Chem. Acta 2011, 84, 245–249. [Google Scholar] [CrossRef]
- Lieberman, H.; Elkin, S. Synthesis of several dibasic acid esters of 3-quinuclidinol as potential pharmacological agents. J. Pharm. Sci. 1968, 57, 684–688. [Google Scholar] [CrossRef]
- Ringdahl, B.; Jope, R.S.; Jenden, D.J. Inhibition of high affinity choline transport by stereoisomers of some 3-quinuclidinol derivatives. Biochem. Pharmacol. 1984, 33, 2819–2822. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Primožič, I.; Bolant, M.; Ramić, A.; Tomić, S. Preparation of Novel meta- and para-Substituted N-Benzyl Protected Quinuclidine Esters and Their Resolution with Butyrylcholinesterase. Molecules 2012, 17, 786-795. https://doi.org/10.3390/molecules17010786
Primožič I, Bolant M, Ramić A, Tomić S. Preparation of Novel meta- and para-Substituted N-Benzyl Protected Quinuclidine Esters and Their Resolution with Butyrylcholinesterase. Molecules. 2012; 17(1):786-795. https://doi.org/10.3390/molecules17010786
Chicago/Turabian StylePrimožič, Ines, Marijana Bolant, Alma Ramić, and Srđanka Tomić. 2012. "Preparation of Novel meta- and para-Substituted N-Benzyl Protected Quinuclidine Esters and Their Resolution with Butyrylcholinesterase" Molecules 17, no. 1: 786-795. https://doi.org/10.3390/molecules17010786
APA StylePrimožič, I., Bolant, M., Ramić, A., & Tomić, S. (2012). Preparation of Novel meta- and para-Substituted N-Benzyl Protected Quinuclidine Esters and Their Resolution with Butyrylcholinesterase. Molecules, 17(1), 786-795. https://doi.org/10.3390/molecules17010786




