2.1. Total Phenolics and Flavonoids Contents
Phenolic and flavonoid compounds, as important phytochemicals, are present in vegetables, fruits and cereal grains. These secondary metabolites are natural antioxidants that have multiple biological effects and play an important role in the defense against cardiovascular disease, aging and cancer [
17]. The total phenolics and flavonoids contents of leaves from the three varieties of
Labisia pumila Benth. extracts are presented in
Table 1. The results indicate that
L. pumila var.
pumila had a higher total flavonoids content (2.77 mg rutin equivalent/g DW) than var.
alata (2.49 mg rutin equivalent/g DW) and var
. lanceolata (2.29 mg rutin equivalent/g DW), but the leaves of var.
alata contained higher total phenolics (3.48 mg gallic acid equivalent/g DW) than var.
pumila (3.37 mg gallic acid equivalent/g DW) and var.
lanceolata (3.23 mg gallic acid equivalent/g DW). The HPLC analysis results also indicated that
L. pumila var.
pumila contained various types of flavonoids such as quercetin and daidzein not seen in
L. pumila var.
alata, while the phenolic pyrogallol was detected only in
L. pumila var.
alata.
Table 1.
Total phenolics and flavonoids content of the leaves of three varieties of Labisa pumila Benth.
Table 1.
Total phenolics and flavonoids content of the leaves of three varieties of Labisa pumila Benth.
| Variety | Phenolic Content 1 | Flavonoid Content 2 |
|---|
| Alata | 3.48 ± 0.01 a | 2.49 ± 0.13 b |
| Pumila | 3.37 ± 0.04 b | 2.77 ± 0.01 a |
| Lanceolata | 3.23 ± 0.02 c | 2.29 ± 0.02 c |
Total phenolics and flavonoids content of leaves in all varieties were significantly different from each other. An increase of total phenolic content in some plants upon heating could be due to the cleavage of esterified and glycosylated compounds [
18]. Guihua
et al. [
19] also found that the heating process increased the phenolics content due to the cleavage of bound (esterified and glycosylated) forms, thus leading to an increase in free forms.
2.3. Determination of Phenolics and Flavonoids Compounds by HPLC
In this study, reversed-phase (RP) HPLC was used to identify the flavonoid, isoflavonoid and phenolic compounds in the leaf extracts of all the varieties of
Labisia pumila Benth. The improvement of extraction efficiency by the microwave method is confirmed by RP-HPLC analysis of the plant extracts. From the obtained results, it is clearly shown that methanolic extracts from leaf part in all varieties exhibited variable patterns of flavonoids, isoflavonoids and phenolics compounds (
Table 5 and
Table 6). Apigenin, kaempferol, rutin and myricetin were the main flavonoid compounds present in all three varieties, with respective values of 94.72, 217.62, 116.85 and 103.21 µg/g dry sample in the leaves of var.
alata, 152, 541.78, 51.63, 147.79 µg/g dry sample of var.
pumila, and 53.92, 157.53, 28.93, 116.68 µg/g dry sample of var.
lanceolata. Quercitin and the isoflavonoid daidzein were only recorded in var.
pumila (210 and 142.65 µg/g dry sample, respectively) and
lanceolata (71.21 and 135.19 µg/g dry sample, respectively). Genistein as another isoflavonoid was only found in var.
lanceolata, with a value of 107.39 µg/g dry sample. This research also revealed that gallic acid and caffeic acid were the major phenolic compounds in the all extracts, whereas pyrogallol was only observed in
Labisia pumila var.
alata (1128.55 µg/g dry sample). The level of kaempferol in the leaves of var.
pumila was significantly higher than that seen in the
alata and
lanceolata varieties
. These values were lower than the amount of kaempferol found in Chinese tea leaves (1.56–3.31 mg/g dried leaves [
24]) but higher than strawberry, with a value 8 µg/g fresh weight [
25].
Table 5.
Concentration of different flavonoids and isoflavonoids in the leaves of three varieties of Labisia pumila Benth.
Table 5.
Concentration of different flavonoids and isoflavonoids in the leaves of three varieties of Labisia pumila Benth.
| Flavonoid and Isoflavonoid contents (µg/g dry sample) |
|---|
| Variety | Apigenin | Kaempferol | Myricetin | Naringin | Quercetin | Rutin | Daidzein | Genistein |
|---|
| Alata | 94.72 b | 217.62 c | 103.21 c | 310.91 a | ND | 116.85 a | ND | ND |
| Pumila | 152 a | 541.78 a | 147.79 a | 175.14 d | 210.01 a | 51.63 b | 142.65 a | ND |
| Lanceolata | 53.92 c | 157.53 b | 116.68 b | ND | 71.21 b | 28.93 c | 135.19 b | 107.39 |
Table 6.
Concentration of different phenolic compounds in the leaves of three varieties of Labisia pumila Benth.
Table 6.
Concentration of different phenolic compounds in the leaves of three varieties of Labisia pumila Benth.
| Phenolic contents (µg/g dry sample) |
|---|
| Variety | Gallic acid | Pyrogallol | Caffeic acid | Salicylic acid |
|---|
| Alata | 623.39 a | 1128.55 a | 62.13 c | ND |
| Pumila | 312.09 c | ND | 151.02 a | ND |
| Lanceolata | 508.81 b | ND | 147.78 b | ND |
Variety
pumila had demonstrated a significantly higher myricetin level than var.
lanceolata and
alata; and they were found to be higher than black current and blueberry, with respective values of 71 and 29 µg/g dry weight [
25]. Meanwhile rutin present in all three varieties (
alata,
pumila and
lanceolata) was found to be lower than citrus (3.26 mg/g fresh weight [
26] and
Amaranthus viridis (58.27 µg/mg dry weight) [
27]. Furthermore, quercetin present in
L. pumila var.
pumila was found to be higher compared to onion (201 µg/g DW) and lower than garlic (227 µg/g dry samples) which was analyzed by Crozier
et al. [
28].
The results from
Table 5 also show that the isoflavonoids levels (daidzein and genistein) in var.
pumila and var.
lanceolatae were lower than those found in soybeans with respective values of 341.47 ± 18.96 and 30.03 ± 7.17 mg/kg [
29]. As for the phenolic compounds (
Table 6), substantial (p < 0.05) amounts of gallic acid were recorded in var.
alata, followed by var.
lanceolata; the least came from var.
pumila. The pyrogallol contents detected in
Labisia pumila var.
alata was found to be lower compared to sea grass (
Posidonia aceanica) with a value of 1.3 mg/g dry weight [
30]. Meanwhile, the caffeic acid content in the leaves of all three varieties of
L. pumila, especially those of var.
pumila and
lanceolatea, was found to be higher than that of leaf extract of
Eucalyptus honey (4.9 mg/g) [
31] and apple (8.2 mg/g dry weight) [
32]. Secondary metabolites derived from plant such as essential oils, flavonoids and phenolics compounds exhibit biological activities. Compounds such as pyrogallol, gallic acid, naringin and quercetin have been reported to possess antioxidant properties as well as anti-inflammatory activities [
33,
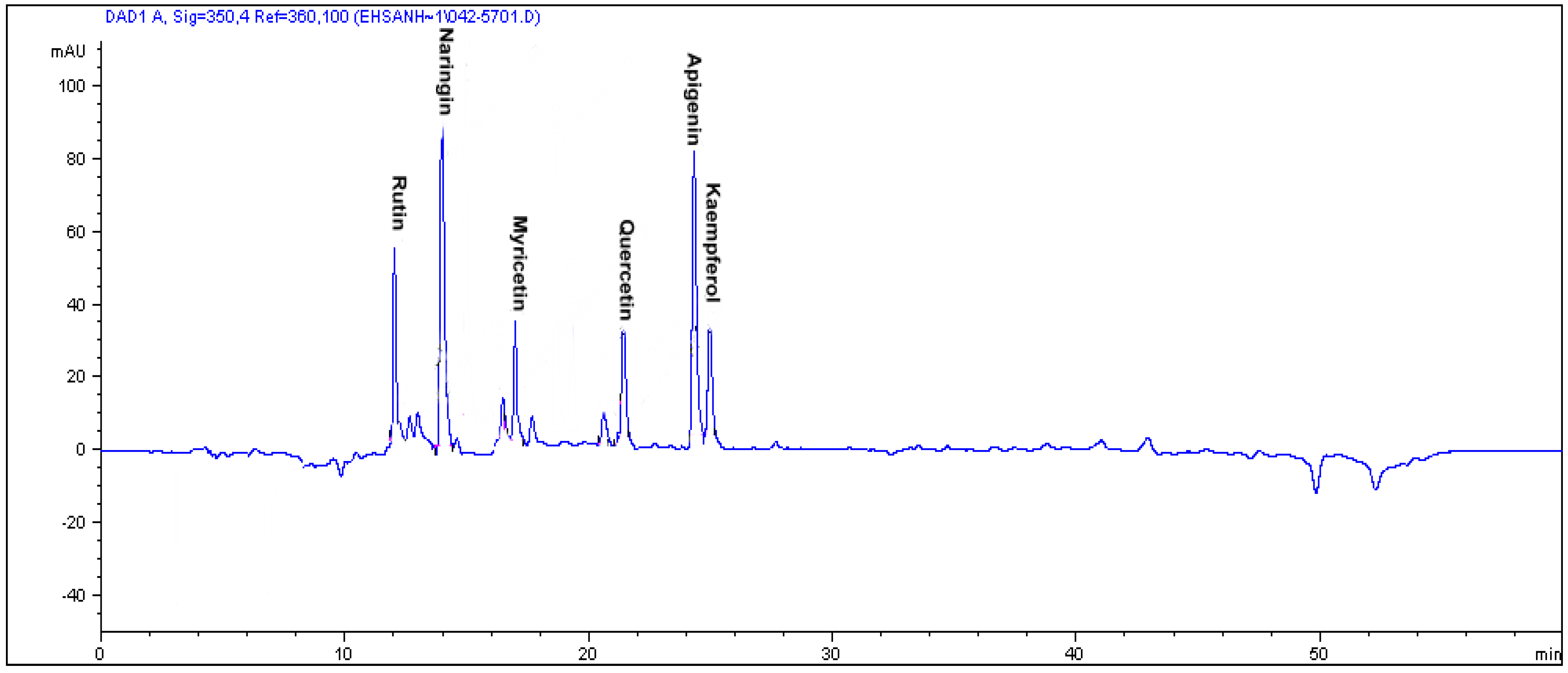
34]. The HPLC chromatogram in
Figure 1 shows the different flavonoids compounds in the leave of
Labisia pumila var.
pumila as an instance.
Figure 1.
The RP-HPLC chromatogram of flavonoid compounds in the leaves of Labisia pumila var. pumila. Identification of compounds: rutin, naringin, myricetin, quercetin, apigenin and kaempferol.
Figure 1.
The RP-HPLC chromatogram of flavonoid compounds in the leaves of Labisia pumila var. pumila. Identification of compounds: rutin, naringin, myricetin, quercetin, apigenin and kaempferol.
2.4. GC-MS Analysis of Three Varieties of Labisia pumila Benth.
Essential oils are volatile and natural complex mixtures of compounds characterized by strong odors and formed by aromatic plants as secondary metabolites [
35]. Volatile compounds in essential oils from medicinal and aromatic plants have been known since ancient times to possess many biological activities, especially antibacterial, antifungal and antioxidant properties [
36]. The constituents and the percentage values of composition of compounds present in the leaves of
Labisia pumila var. of
alata,
pumila and
lanceolata are shown in
Table 7,
Table 8 and
Table 9, respectively. The GC-MS analysis of methanolic crude extracts resulted in identification of more than 40 compounds in the leaves of
Labisia pumila Benth. The obtained results indicated that the main volatile compounds were heptadecanoic acid (20.39%), octadecanoic acid (16.24%) and 2,4,5-trimethyl-1,3-dioxolane (18.69%) in the leaf extracts of
Labisa pumila var. of
alata,
pumila and
lanceolata, respectively. Leaf of var.
lanceolata exhibited more volatile compounds (45 compounds) than var.
alata (31 compounds) and var.
pumila (24 compounds). The methanolic extracts of leaf from three varieties of
Labisia pumila contained bioactive compounds that may possess biological properties. Compounds such as acetic acid [
37], furfural [
38], fumaric acid, dimethyl ester [
24], eicosanoic acid and methyl ester [
39] have been reported to possess antimicrobial activity. Oleic acid is used as an excipient in pharmaceuticals and as an emulsifying or solubilising agent in aerosol products [
40]. The presence of these phytochemicals makes
Labisia pumila Benth. a potential source of bioactive compounds.
Table 7.
Chemical composition of methanolic extraction of L. pumila var. alata.
Table 7.
Chemical composition of methanolic extraction of L. pumila var. alata.
| No. | Composition (%) | Compound |
|---|
| 1 | 2.99 | Hydrazine, 1,2-dimethyl |
| 2 | 0.45 | Furfural |
| 3 | 0.39 | cis-3-Methyl-2-n-propylthiophane |
| 4 | 0.43 | 2-Furanmethanol |
| 5 | 0.45 | Benzyl Alcohol |
| 6 | 0.21 | Phenylethyl Alcohol |
| 7 | 0.19 | Ethanone |
| 8 | 0.48 | Octadecanal |
| 9 | 0.36 | 2,3 4a, 5,6,7-Hexahydro-1,4-benzodioxin |
| 10 | 0.32 | Menthyl acetate cyclohexanol,5-methyl-2-(1-methylethyl) |
| 11 | 0.29 | Diisobutoxybutane |
| 12 | 0.36 | Cyclododecanol |
| 13 | 0.25 | 3-(Hydroxymethyl)-6-(1-methylethyl)-2-cyclohexen-1-one |
| 14 | 2.31 | Hexadecanoic acid methyl ester |
| 15 | 9.14 | 2,4,5-Trimethyl-1,3-dioxolane, |
| 16 | 0.30 | Benzene-1,2,3,4-tetraol |
| 17 | 0.42 | N-Ethyl-N-nitrosoethanamine, |
| 18 | 0.25 | Isobenzofuran |
| 19 | 3.72 | 10-Octadecenoic acid methyl ester |
| 20 | 0.59 | Ethyl oleate |
| 21 | 9.36 | 9,12-Octadecadienoic acid methyl ester |
| 22 | 0.51 | 2-Propylthiophene, |
| 23 | 1.79 | Linoleic acid ethyl ester |
| 24 | 7.76 | 11,14,17-Eicosatrienoic acid |
| 25 | 1.61 | 9,12,15-Octadecatriene |
| 26 | 1.07 | Phytol |
| 27 | 20.39 | Heptadecanoic acid |
| 28 | 1.71 | Octadecanoic acid |
| 29 | 6.72 | 9-Hexadecenoic acid |
| 30 | 16.15 | 9,12-Octadecadienoic acid |
| 31 | 9.03 | 9,12, 15-Octadecatriene |
Table 8.
Chemical composition of methanolic extraction of L. pumila var. pumila.
Table 8.
Chemical composition of methanolic extraction of L. pumila var. pumila.
| No. | Composition (%) | Compound |
|---|
| 1 | 0.31 | Guanidine |
| 2 | 2.73 | Methyl formate |
| 3 | 0.31 | Methoxypyrazine |
| 4 | 0.33 | 2-Furanmethanol |
| 5 | 1.22 | Benzyl alcohol |
| 6 | 0.49 | Tetradecyloxirane |
| 7 | 0.45 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol |
| 8 | 0.54 | Eicosenoic acid methyl ester |
| 9 | 2.36 | Hexadecanoic acid |
| 10 | 12.68 | 2,4,5-Trimethyl-1,3-dioxolane, |
| 11 | 0.39 | 5-Hydroxy-2-methylthiopyrimidine |
| 12 | 0.66 | 2,3-Dihydrobenzofuran |
| 13 | 3.75 | 10-Octadecenoic acid methyl ester |
| 14 | 11.18 | 9,12-Octadecadienoic acid methyl ester |
| 15 | 11.07 | 9,12,15-octadecatriene |
| 16 | 0.44 | 2,4-Dimethylphenol |
| 17 | 0.96 | Phytol |
| 18 | 0.51 | D-Tyrosine |
| 19 | 0.87 | Anthracene |
| 20 | 16.24 | Octadecanoic acid |
| 21 | 0.65 | n-Hexadecanoic acid |
| 22 | 5.97 | 9 Hexadecenoic acid |
| 23 | 13.40 | 9,12-Octadecadienoic acid |
| 24 | 12.47 | 9,12,15-Octadecatrien-1- ol , (Z,Z,Z) |
Table 9.
Chemical composition of methanolic extraction of L. pumila var. lanceolata.
Table 9.
Chemical composition of methanolic extraction of L. pumila var. lanceolata.
| No. | Composition (%) | Compound |
|---|
| 1 | 2.27 | Methyl formate |
| 2 | 0.34 | Propanedioic acid |
| 3 | 0.29 | Z-β-Terpineol |
| 4 | 0.70 | Furanone |
| 5 | 1.37 | Hexanoic acid |
| 6 | 3.79 | Benzyl alcohol |
| 7 | 0.34 | Phenylethyl alcohol |
| 8 | 0.93 | Hexanoic acid |
| 9 | 1.54 | Tetradecyloxirane |
| 10 | 0.62 | Triethylenediamine |
| 11 | 0.48 | Dodecyloxirane, |
| 12 | 0.46 | 2,5-Dimethyl-4-hydroxy-3(2H)-furanone |
| 13 | 0.38 | Dihydro-3-hydroxy-4,4-dimethyl-2(3H)-furanone |
| 14 | 1.18 | Cyclododecanol |
| 15 | 0.78 | 1,6,10-Dodecatrien-3-ol |
| 16 | 0.54 | 3-Methylphenol, |
| 17 | 2.88 | Pentadecanoic acid |
| 18 | 18.69 | 2,4,5-Trimethyl-1,3-dioxolane |
| 19 | 2 | D-Gluconic acid |
| 20 | 1.53 | Benzene-1,2,3,4-tetraol |
| 21 | 0.44 | 2,5-bis(1,1-Dimethylethyl)phenol, |
| 22 | 0.70 | 1-Methoxy-9-octadecene |
| 23 | 0.36 | 8-Methoxy-1,6-octadiene |
| 24 | 0.89 | 2,3-Dihydrobenzofuran |
| 25 | 0.71 | Benzoic acid |
| 26 | 1.95 | 9-Octadecenoic acid |
| 27 | 0.34 | Lauric anhydride |
| 28 | 3.87 | 11,14-Ecosadienic acid |
| 29 | 0.95 | Cyclopropaneoctanoic acid |
| 30 | 3.29 | 9,12,15 Octadecatrien-1-ol |
| 31 | 0.39 | 3-tert-Butyl-4-hydroxyanisole |
| 32 | 4.21 | Phytol |
| 33 | 0.55 | Heptadecanoic acid |
| 34 | 1.23 | D-Tyrosine |
| 35 | 11.96 | n-Hexadecanoic acid |
| 36 | 0.48 | Undecanentrile |
| 37 | 0.48 | 4-Hydroxy-3,5-dimethoxybenzoic acid , |
| 38 | 0.50 | 2,6,10,14,18-Pentamethyl-2,6,10,14,18-eicosapentaene |
| 39 | 1.02 | Octadecanoic acid |
| 40 | 5.45 | 9-Hexadecenoic acid |
| 41 | 1.57 | Di-n-octyl phthalate (DNOP) |
| 42 | 7.37 | 9,12-Octadecadienoic acid |
| 43 | 5.36 | 11,14,17-Ecosatrienoic acid |
| 44 | 1.65 | 2-(But-2-enylideneamino)-propionitrile |
| 45 | 3.14 | N-aminoacetyltyramine, |