Anticancer Activity of Chamaejasmine: Effect on Tubulin Protein
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cytotoxicity Assays
| Cell Line | IC50 (µM) | |
| chamaejasmine | taxol | |
| MCF-7 | 4.02 | 5.98 |
| A549 | 4.84 | 6.18 |
| SGC-7901 | 11.97 | 3.37 |
| HCT-8 | 12.45 | 5.06 |
| HO-4980 | 5.31 | 7.02 |
| Hela | 9.88 | 5.01 |
| Hep G2 | 14.36 | 6.17 |
| PC-3 | 2.28 | 3.98 |
| LNCap | 5.21 | 2.81 |
| Vero | 3.16 | 0.92 |
| MDCK | 4.57 | 0.83 |
| IC50 (µM) | 24 h | 48 h | 72 h |
|---|---|---|---|
| Chamaejasmine | 10.52 | 5.05 | 2.28 |
| Taxol | 16.24 | 6.71 | 3.98 |
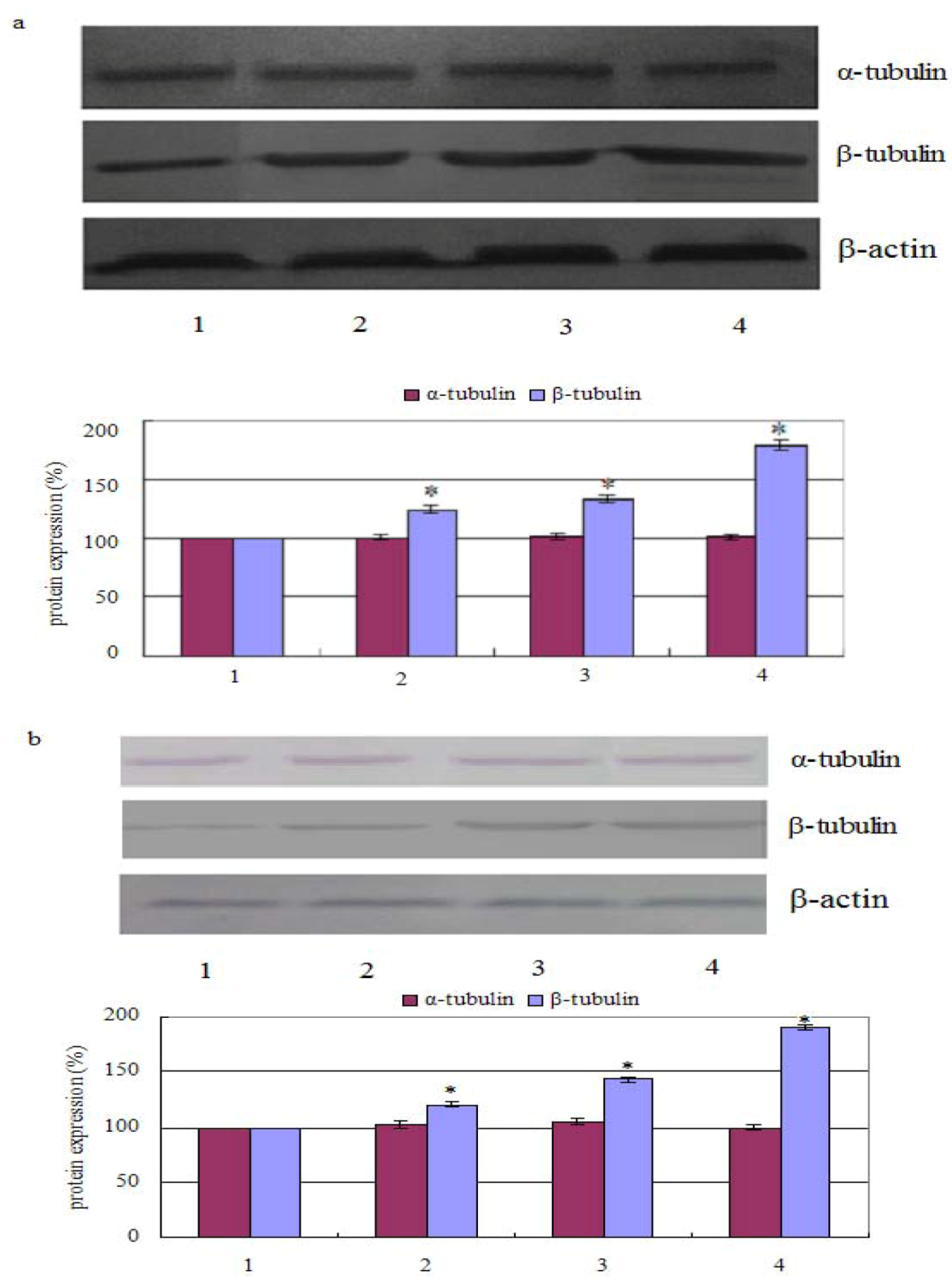
2.2. Measurement of Tubulin Polymerization
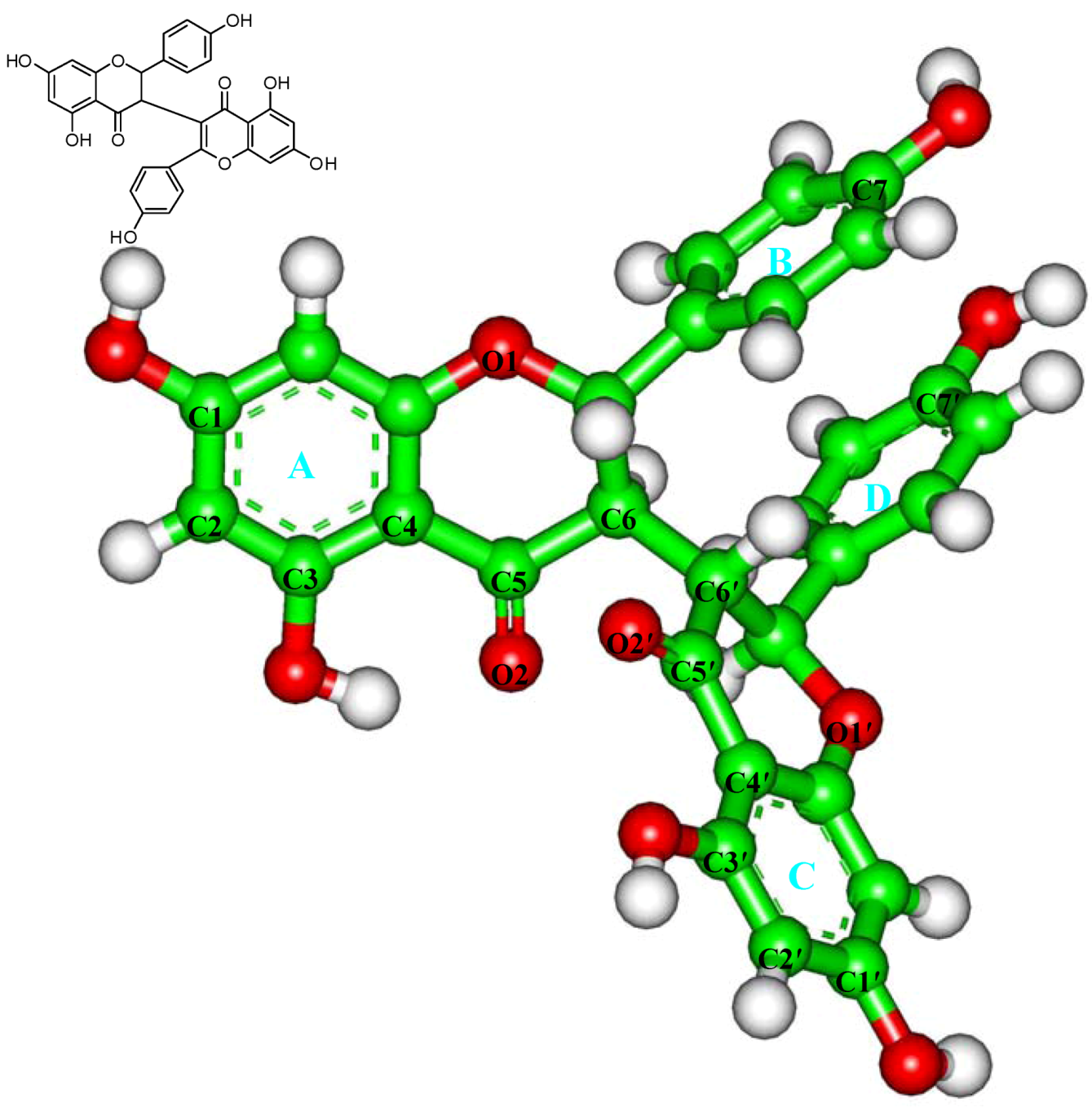
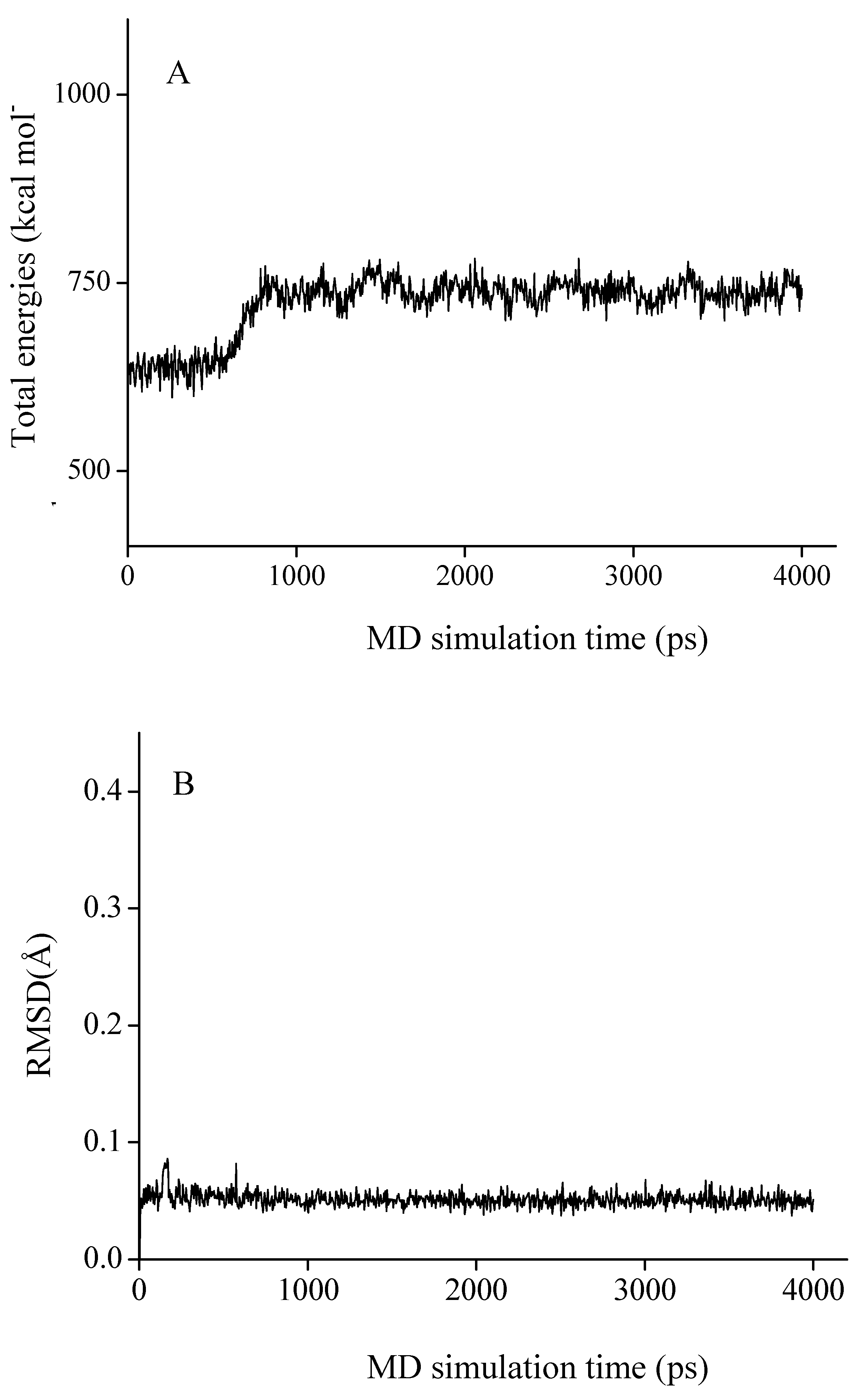
2.3. In Silico Inhibition Mechanism of Chamaejasmine
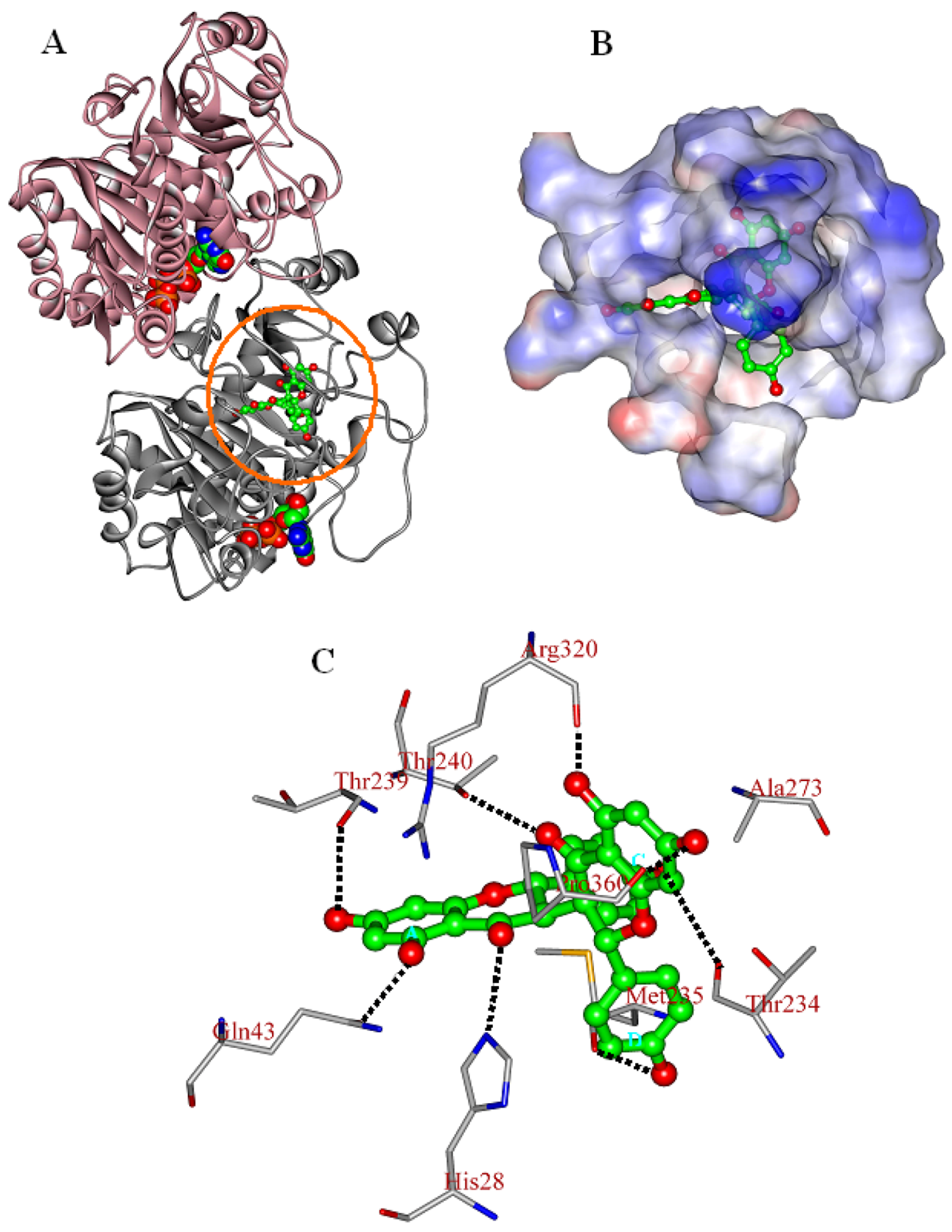
| Residue | EvdW | Eele | Esum |
|---|---|---|---|
| IleB24 | −2.40 | −0.60 | −3.00 |
| HisB28 | −5.91 | −3.13 | −9.04 |
| GlnB43 | −1.51 | −5.60 | −7.11 |
| ThrB234 | −4.98 | −3.95 | −8.93 |
| MetB235 | −5.72 | −4.35 | −10.07 |
| SerB236 | −7.84 | −1.14 | −8.98 |
| GlyB237 | −5.51 | −0.65 | −6.16 |
| ThrB239 | −3.23 | −1.99 | −5.22 |
| ThrB240 | −6.64 | −6.11 | −12.75 |
| PheB244 | −3.79 | 0.53 | −3.26 |
| AlaB273 | −2.52 | −1.27 | −3.79 |
| ArgB320 | −4.84 | −19.36 | −24.20 |
| GlyB321 | −1.63 | −0.65 | −2.28 |
| ProB360 | −5.54 | −8.17 | −13.71 |
| ArgB369 | −3.00 | −0.23 | −3.23 |
| GlyB370 | −3.77 | 0.62 | −3.15 |
| LeuB371 | −3.11 | −0.33 | −3.44 |
| SerB374 | −8.22 | −1.07 | −9.29 |
| AlaB375 | −3.35 | −0.04 | −3.39 |
3. Experimental
3.1. Materials
3.2. Growth of Cells
3.3. Cytotoxicity Assays
3.4. Western Blot Assay
3.5. In Silico Simulation
3.5.1. System preparations
3.5.2. Docking
3.5.3. Molecular dynamics (MD)
3.6. Statistical Analysis
4. Conclusions
References and Notes
- Coxon, A.; Bush, T.; Saffran, D.; Kaufman, S.; Belmontes, B.; Rex, K.; Hughes, P.; Caenepeel, S.; Rottman, J.B.; Tasker, A.; et al. Broad antitumor activity in breast cancer xenografts by motesanib, a highly selective, oral inhibitor of vascular endothelial growth factor, platelet-derived growth factor, and Kit receptors. Clin. Cancer Res. 2009, 15, 110–118. [Google Scholar]
- Munroe, M.E.; Arbiser, J.L.; Bishop, G.A. Honokiol, a natural plant product, inhibits inflammatory signals and alleviates inflammatory arthritis. J. Immunol. 2007, 179, 753–763. [Google Scholar]
- Kavallaris, M.; Verrills, N.M.; Hill, B.T. Anticancer therapy with novel tubulin-interacting drugs. Drug Resist. Updat. 2001, 4, 392–401. [Google Scholar] [CrossRef]
- Shi, Q.; Chen, K.; Morris-Natschke, S.L.; Lee, K.H. Recent progress in the development of tubulin inhibitors as antimitotic antitumor agents. Curr. Pharm. Des. 1998, 4, 219–248. [Google Scholar]
- Jordan, A.; Hadfield, J.A.; Lawrence, N.J.; McGown, A.T. Tubulin as a target for anticancer drugs: agents which interact with the mitotic spindle. Med. Res. Rev. 1998, 18, 259–296. [Google Scholar] [CrossRef]
- Islam, M.N.; Iskander, M.N. Microtubulin binding sites as target for developing anticancer agents. Mini Rev. Med. Chem. 2004, 4, 1077–1104. [Google Scholar] [CrossRef]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef]
- Teicher, B.A. Newer cytotoxic agents: Attacking cancer broadly. Clin. Cancer Res. 2008, 14, 1610–1617. [Google Scholar]
- Dumontet, C.; Sikic, B.I. Mechanisms of action of and resistance to antitubulin agents: microtubule dynamics, drug transport, and cell death. J. Clin. Oncol. 1999, 17, 1061–1070. [Google Scholar]
- Chang, J.Y.; Yang, M.F.; Chang, C.Y.; Chen, C.M.; Kuo, C.C.; Liou, J.P. 2-amino and 2'-aminocombretastatin derivatives as potent antimitotic agents. J. Med. Chem. 2006, 49, 6412–6415. [Google Scholar]
- Chang, J.Y.; Hsieh, H.P.; Chang, C.Y.; Hsu, K.S.; Chiang, Y.F.; Chen, C.M.; Kuo, C.C.; Liou, J.P. 7-Aroyl-aminoindoline-1-sulfonamides as a novel class of potent antitubulin agents. J. Med. Chem. 2006, 49, 6656–6659. [Google Scholar]
- Liou, J.P.; Wu, C.Y.; Hsieh, H.P.; Chang, C.Y.; Chen, C.M.; Kuo, C.C.; Chang, J.Y. 4- and 5-aroylindoles as novel classes of potent antitubulin agents. J. Med. Chem. 2007, 50, 4548–4552. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural products as sources of new drugs over the period 1981-2002. J. Nat. Prod. 2003, 66, 1022–1037. [Google Scholar]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef]
- Shi, Z.C. The Poisonous Plants in Chinese Pasture; China Agricultural Press: Beijing, China, 1997. [Google Scholar]
- Zhao, S.H.; Wang, S.Q. Progress of Investigations and Application of Insecticide Plants; Guangdong Agricultural Sciences: Guangdong, China, 1997; pp. 26–28. [Google Scholar]
- Feng, W.; Tetsuro, I.; Mitsuzi, Y. The antitumor activities of gnidimacrin isolated from Stellera chamaejasme L. Chin. J. Cancer Res. 1995, 17, 24–26. [Google Scholar]
- Tang, X.; Hou, T. Development of chamaejasmin microemulsion and its biological activity against Aphis craccivora and Culex pipiens pallens. Flavour. Fragr. J. 2008, 23, 258–262. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, G.G.; Ma, E.L.; Zhang, H.M.; Guan, J.; He, J. Amentoflavone and the extracts from Selaginella tamariscina and their anticancer activity. Asian J. Traditional Med. 2010, 5, 226–229. [Google Scholar]
- Downing, K.H. Structural basis for the interaction of tubulin with proteins and drugs that affect microtubule dynamics. Annu. Rev. Cell Dev. Biol. 2000, 16, 89–111. [Google Scholar] [CrossRef]
- Snyder, J.P.; Nettles, J.H.; Cornett, B.; Downing, K.H.; Nogales, E. The binding conformation of Taxol in beta-tubulin: A model based on electron crystallographic density. Proc. Natl. Acad. Sci. USA 2001, 98, 5312–5316. [Google Scholar]
- Fu, Y.; Li, S.; Zu, Y.; Yang, G.; Yang, Z.; Luo, M.; Jiang, S.; Wink, M.; Efferth, T. Medicinal chemistry of paclitaxel and its analogues. Curr. Med. Chem. 2009, 16, 3966–3985. [Google Scholar] [CrossRef]
- Lowe, J.; Li, H.; Downing, K.H.; Nogales, E. Refined structure of alpha beta-tubulin at 3.5 A resolution. J. Mol. Biol. 2001, 313, 1045–1057. [Google Scholar] [CrossRef]
- InisghtII Version 2005, 2005 ed.; Accelrys Inc.: San Diego, CA, USA, 2005.
- Robinson, M.W.; McFerran, N.; Trudgett, A.; Hoey, L.; Fairweather, I. A possible model of benzimidazole binding to beta-tubulin disclosed by invoking an inter-domain movement. J. Mol. Graph. Model 2004, 23, 275–284. [Google Scholar] [CrossRef]
- Geney, R.; Sun, L.; Pera, P.; Bernacki, R.J.; Xia, S.; Horwitz, S.B.; Simmerling, C.L.; Ojima, I. Use of the tubulin bound paclitaxel conformation for structure-based rational drug design. Chem. Biol. 2005, 12, 339–348. [Google Scholar] [CrossRef]
- Rodríguez-Menchaca, A.A.; Navarro-Polanco, R.A.; Ferrer-Villada, T.; Rupp, J.; Sachse, F.B.; Tristani-Firouzi, M.; Sánchez-Chapula, J.A. The molecular basis of chloroquine block of the inward rectifier Kir2.1 channel. Proc. Natl. Acad. Sci. USA 2008, 105, 1364–1368. [Google Scholar]
- Zhang, X.; Hu, Y.; Yuan, Z. Computational analyses of JAK1 kinase domain: subtle changes in the catalytic cleft influence inhibitor specificity. Biochem. Biophys. Res. Commun. 2008, 370, 72–76. [Google Scholar] [CrossRef]
- Beccati, D. Investigations of Prebiotics and of Inter- and Intra-Molecular Glycan-Protein Interactions; G. Ronzoni' Institute for Chemical and Biochemical Research: Milan, Italy, 2009. [Google Scholar]
- Yang, Z.W.; Yang, G.; Zu, Y.G.; Fu, Y.J.; Zhou, L.J. The conformational analysis and proton transfer of the neuraminidase inhibitors: A theoretical study. Phys. Chem. Chem. Phys. 2009, 11, 10035–10041. [Google Scholar]
- Yang, Z.; Nie, Y.; Yang, G.; Zu, Y.; Fu, Y.; Zhou, L. Synergistic effects in the designs of neuraminidase ligands: Analysis from docking and molecular dynamics studies. J. Theor. Biol. 2010, 267, 363–374. [Google Scholar]
- Wu, X.M.; Zu, Y.G.; Yang, Z.W.; Fu, Y.J.; Zhou, L.J.; Yang, G. Temperature-controlled molecular dynamics studies on the folding mechanism of the tubulin active peptides. Acta Phys. Chim. Sin. 2009, 4, 773–782. [Google Scholar]
- Yang, G.; Wu, X.M.; Zu, Y.G.; Yang, Z.W.; Fu, Y.J.; Zhou, L.J. Molecular dynamic simulations on the folding and conformational insights of the truncated peptides. J. Theo. Comput. Chem. 2009, 8, 317–331. [Google Scholar]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar]
- Mitra, A.; Sept, D. Taxol allosterically alters the dynamics of the tubulin dimer and increases the flexibility of microtubules. Biophys. J. 2008, 95, 3252–3258. [Google Scholar] [CrossRef]
- Affinity User Guide; Accelrys Inc.: San Diego, CA, USA, 2005.
- Adelman, S.A.; Doll, J.D. Generalized Langevin equation approach for atom/solid-surface scattering: General formulation for classical scattering off harmonic solids. J. Chem. Phys. 1976, 64, 2375–2388. [Google Scholar] [CrossRef]
- Mooberry, S.L.; Weiderhold, K.N.; Dakshanamurthy, S.; Hamel, E.; Banner, E.J.; Kharlamova, A.; Hempel, J.; Gupton, J.T.; Brown, M.L. Identification and characterization of a new tubulin-binding tetrasubstituted brominated pyrrole. Mol. Pharmacol. 2007, 72, 132–140. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fang, W.; Liu, S.; Nie, Y. Anticancer Activity of Chamaejasmine: Effect on Tubulin Protein. Molecules 2011, 16, 6243-6254. https://doi.org/10.3390/molecules16086243
Fang W, Liu S, Nie Y. Anticancer Activity of Chamaejasmine: Effect on Tubulin Protein. Molecules. 2011; 16(8):6243-6254. https://doi.org/10.3390/molecules16086243
Chicago/Turabian StyleFang, Wenlong, Songtao Liu, and Yingkun Nie. 2011. "Anticancer Activity of Chamaejasmine: Effect on Tubulin Protein" Molecules 16, no. 8: 6243-6254. https://doi.org/10.3390/molecules16086243
APA StyleFang, W., Liu, S., & Nie, Y. (2011). Anticancer Activity of Chamaejasmine: Effect on Tubulin Protein. Molecules, 16(8), 6243-6254. https://doi.org/10.3390/molecules16086243




