An Effective Ostrich Oil Bleaching Technique Using Peroxide Value as an Indicator
Abstract
:1. Introduction
2. Results and Discussion
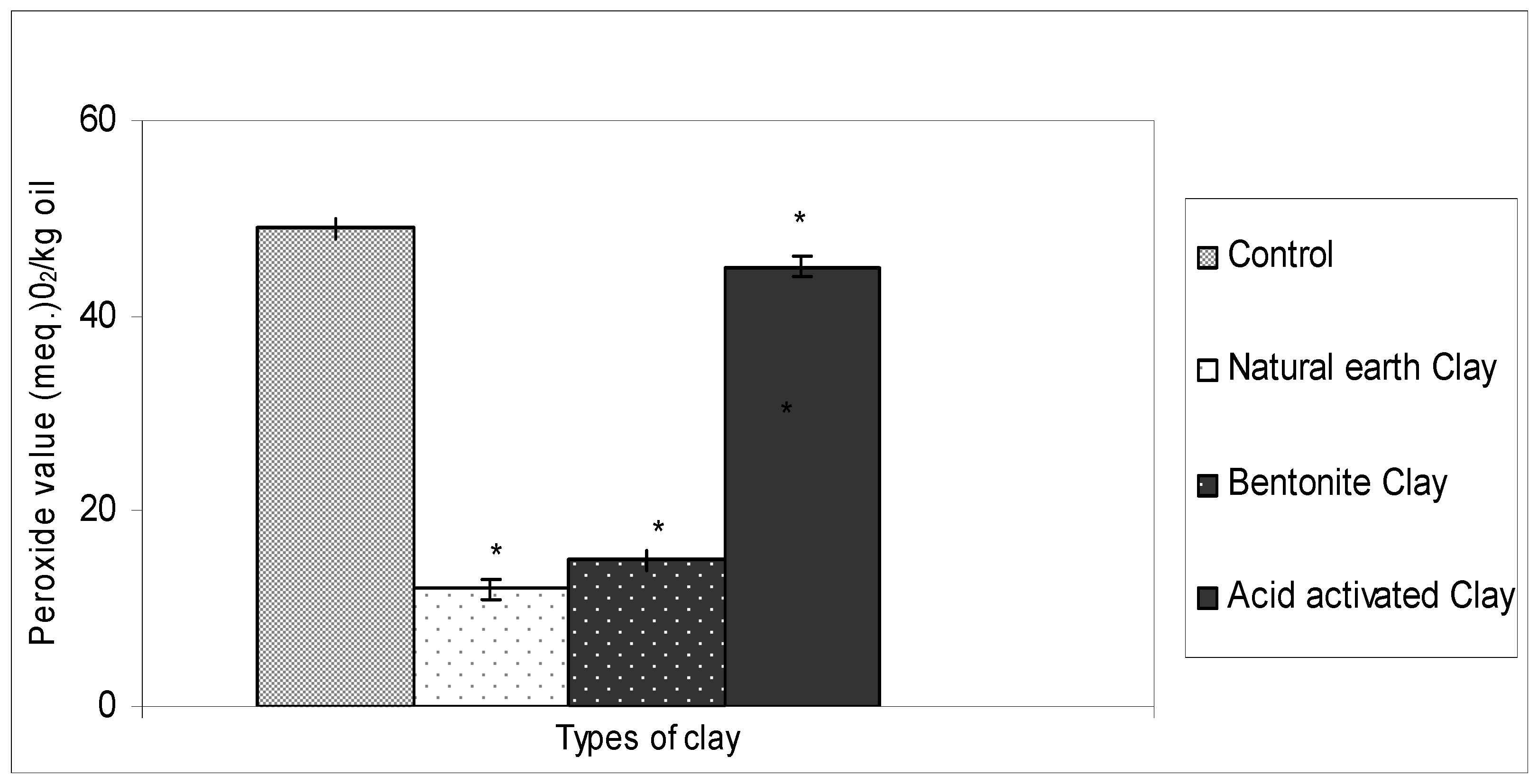
2.1. Ability of Different Types of Clay in Bleaching Crude Ostrich Oil
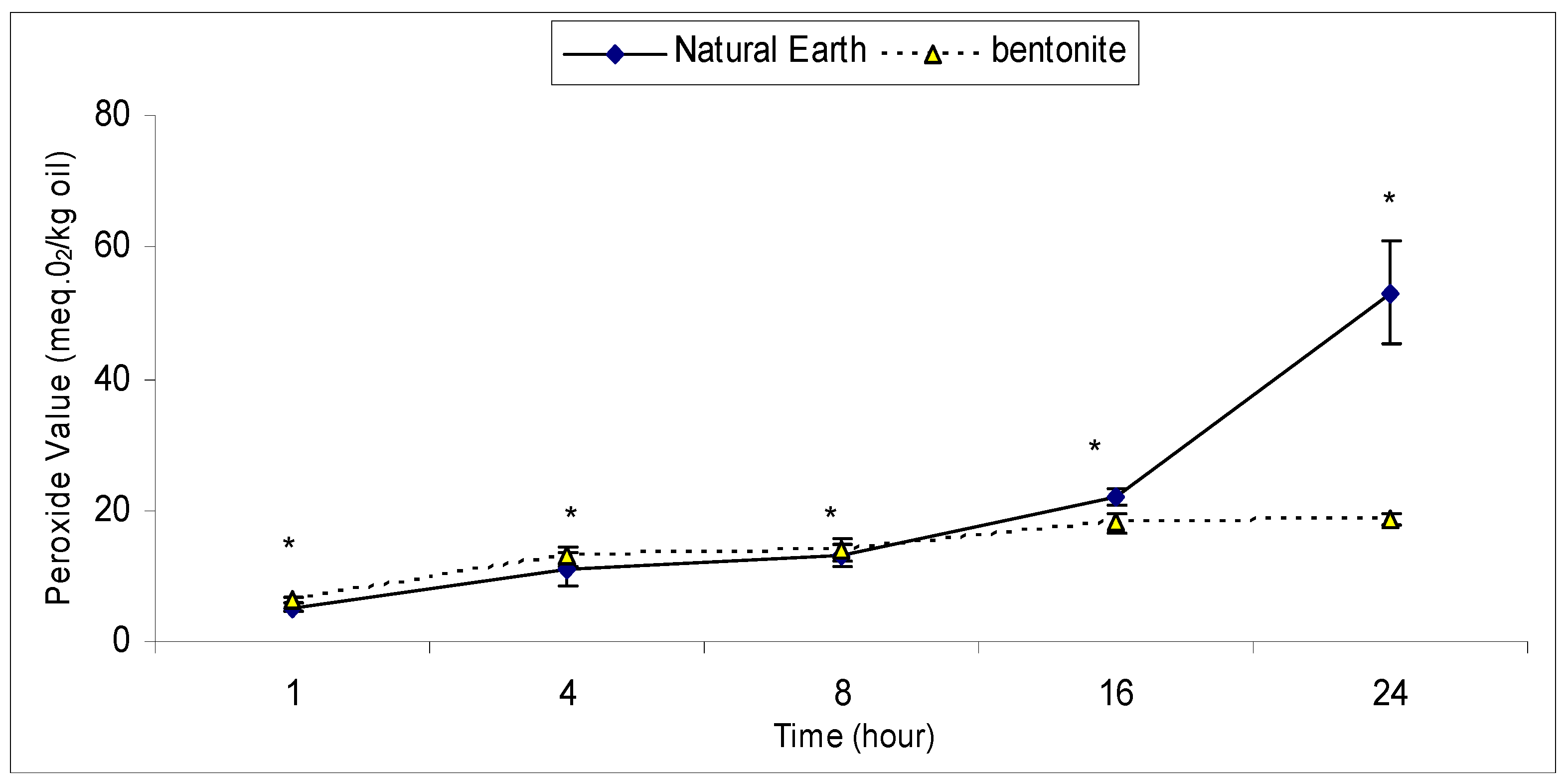
2.2. An Effective Bleaching Time with Bentonite and Natural Earth Clay
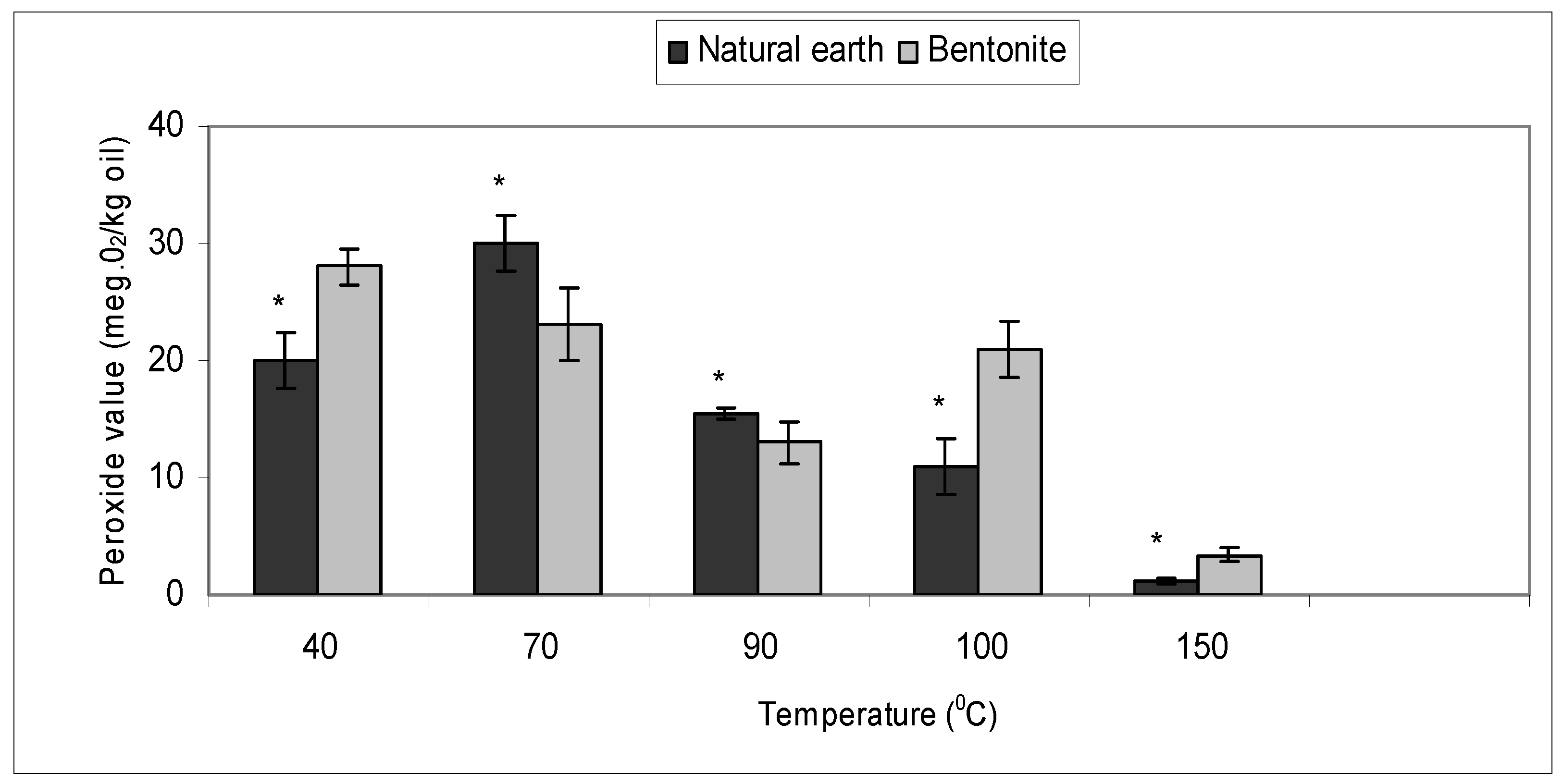
2.3. Effect of Bleaching Temperature with Bentonite and Natural Earth Clay
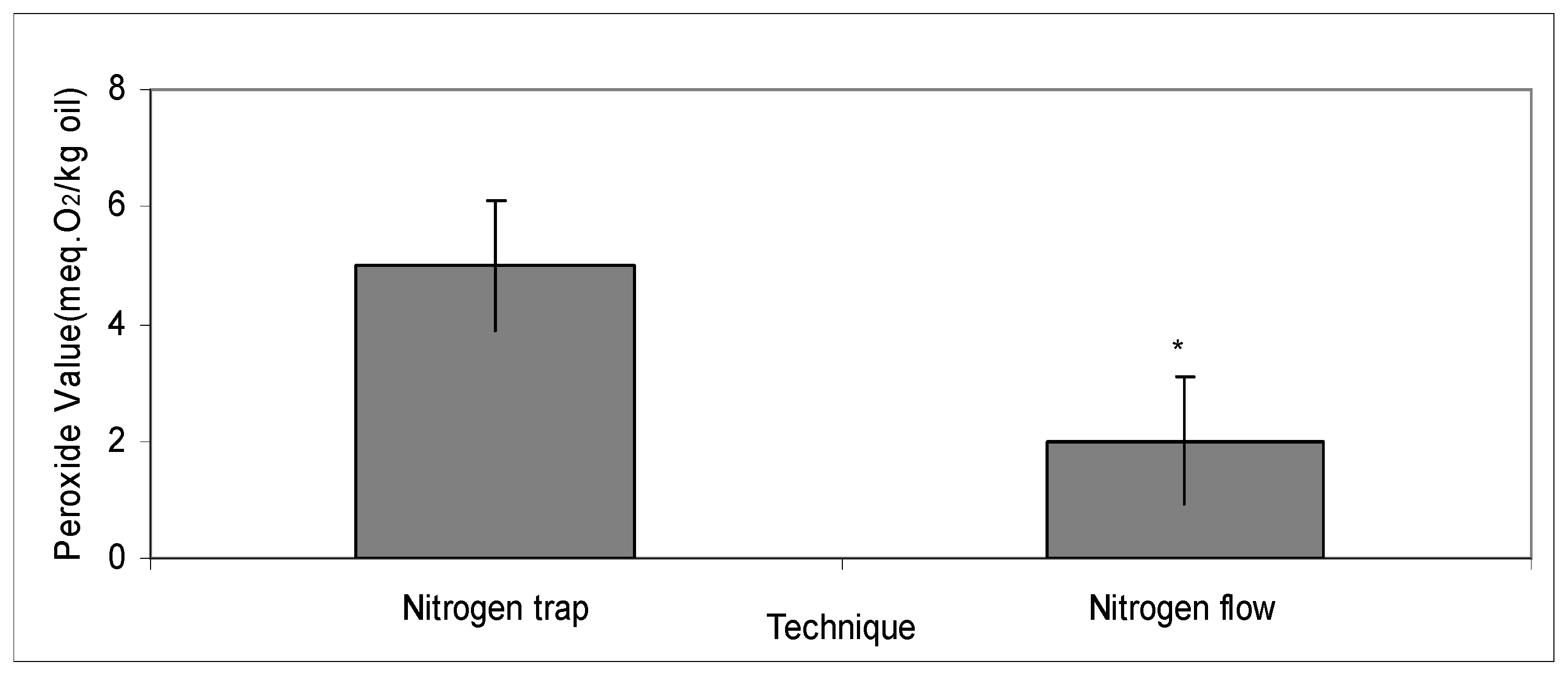
2.4. Comparing the Vacuum Techniques of Continuous Flow and Nitrogen Trap
3. Experimental
4. Conclusions
Acknowledgements
References
- Whitehouse, M.W.; Turner, A.G.; Davis, C.K.; Roberts, M.S. Emu oil(s): A source of non-toxic transdermal anti-inflammatory agents in aboriginal medicine. Inflammopharmacology 1998, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.P. Stability and Quality of Fish Oil during Typical Domestic Application; Wonsan University of Fisheries: Kangwoon Province, D.P.R. of Korea, 2005. [Google Scholar]
- Hoffman, C.; Joubert, M.; Brand, T.S.; Manley, M. The effect of dietary fish oil rich in n-3 fatty acids on the organoleptic, fatty acid and physicochemical characteristics of ostrich meat. Meat Sci. 2005, 70, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Gunstone, F.D.; Russell, W.C. Animal fats. 3. The component acids of ostrich fat. Biochem. J. 1954, 57, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Pechtold, L.A.R.M.; Potts, R.O.; Guy, R.H. Mechanisme of oleic acid-induced skin penetration enhancement in vivo in humans. J. Control Release 1995, 37, 299–306. [Google Scholar] [CrossRef]
- Alfred, T. Fats and Fatty Oils. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Baden-Württemberg, Germany, 2005. [Google Scholar]
- Calder, P.C. n-3 fatty acids, inflammation, and immunity – relevance to postsurgical and critically ill patients. Lipids 2004, 39, 1147–1161. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-Y.; Kim, S.-R.; Kang, H.-G.; Noh, Y.S.; Park, M.C.; Finkel, D.; An, G. Characterization of two rice MADS box genes homologous to GLOBOSA. Plant Sci. 1995, 109, 45–56. [Google Scholar] [CrossRef]
- David, F.H. Fatty acid metabolism in health and disease: The role of Δ-6-desaturase. Am. J. Clin. Nutr. 1993, 57, 732S–737S. [Google Scholar]
- Laada, M.; Saowanee, S. The use of perlite to remove dark colour of repeatedly used palm oil. Sci. Asia 2010, 36, 33–39. [Google Scholar]
- Pezzuto, J.M.; Park, E.J. Autooxidation and antioxidation. In Encyclopedia of Pharmaceutical Technology, 2nd ed.; Swarbrick, J., Boylan, J.C., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2002; Volume 1, pp. 97–113. [Google Scholar]
- Morris, M.C.; Sacks, F.; Rosner, B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation 1993, 88, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.A.; Bao, D.Q.; Burke, V.; Puddey, I.B.; Beilin, L.J. Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension 1993, 34, 253–260. [Google Scholar] [CrossRef]
- Iso, H.; Rexrode, K.M.; Stampfer, M.J.; Manson, J.E.; Colditz, G.A.; Speizer, F.E.; Hennekens, C.H.; Willett, W.C. Intake of fish and omega-3 fatty acids and risk of stroke in women. JAMA 2001, 285, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Fortin, P.R.; Lew, R.A.; Liang, M.H.; Wright, E.A.; Beckett, L.A.; Chalmers, T.C.; Sperling, R.I. Validation of a meta-analysis: The effects of fish oil in rheumatoid arthritis. J. Clin. Epidemiol. 1995, 48, 1379–1390. [Google Scholar] [CrossRef]
- Kremer, J.M.; Bigauoette, J.; Michalek, A.V.; Timchalk, M.A.; Lininger, L.; Rynes, R.I.; Huyck, C.; Zieminski, J.; Bartholomew, L.E. Effects of manipulation of dietary fatty acids on clinical manifestations of rheumatoid arthritis. Lancet 1985, 1, 184–187. [Google Scholar] [CrossRef]
- Berquin, I.M.; Min, Y.; Wu, R.; Wu, J.; Perry, D.; Cline, J.M.; Thomas, M.J.; Thornburg, T.; Kulik, G.; Smith, A.; et al. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J. Clin. Invest. 2007, 117, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- David, T.W. Oleic acid – the anti-breast cancer component in olive oil. Assumption Univ. J. Technol. 2005, 9, 75–78. [Google Scholar]
- Hwang, K.T.; Regenstein, J.M. Protection of menhaden mince lipids from rancidity during frozen storage. J. Food Sci. 1989, 54, 1120–1124. [Google Scholar] [CrossRef]
- International Union of Pure and Applied Chemistry (IUPAC). Standard Methods of Analysis of Oils, Fats and Derivatives, 7th revised and enlarged ed.; Paquat, C., Hautfenne, A., Eds.; Blackwell Scientific: London, UK, 1987. [Google Scholar]
- Puah, C.W.; Choo, Y.M.; Ma, A.N.; Chuah, C.H. Degumming and bleaching: Effect on selected constituents of palm oil. J. Oil Palm. Res. 2004, 16, 57–63. [Google Scholar]
- Cardinale, F.; Donna, L. Injection of rhea and ostrich oils in animals. US Patent 5834027, 1998. [Google Scholar]
- Konishi, I. Method for separating, extracting and purifying ostrich oil from Ostrich. Japan Patent 2005146236, 2005. [Google Scholar]
- Siew, W.I.; Cheah, K.Y.; Roddy, R. Evaluation of bleaching clays for refining of oils. Malaysian Palm Oil Board Information Series June 2007, 40ISSN: 1511-7871.
- Kheok, S.C.; Lim, E.E. Mechanisms of palm oil bleaching by Montmorillonite clay activated at various acid concentrations. J. Am. Oil Chem. Soc. 1982, 59, 129–131. [Google Scholar] [CrossRef]
- Sarier, N.; Guler, C. B-Carotene adsorption on acid-activated Montmorillonite. J. Am. Oil Chem. Soc. 1988, 65, 776–779. [Google Scholar] [CrossRef]
- Ying, H.T.; Chun, I.L. Variation of peroxide value in water-degummed and alkali-refined soy oil during bleaching under vacuum. Sep. Purif. Technol. 2007, 56, 257–264. [Google Scholar]
- Yun, K.W.; Chun, I.L. Variation of peroxide value in water-degummed and alkali-refined soy oil during bleaching under nitrogen stream. Sep. Purif. Technol. 2006, 51, 64–71. [Google Scholar]
- Hraš, A.R.; Hadolin, M.; Knez, Ž.; Bauman, D. Comparison of antioxidative and synergistic effects of rosemary extract with α-tocopherol, ascorbyl palmitate and citric acid in sunflower oil. Food Chem. 2000, 71, 229–233. [Google Scholar] [CrossRef]
- Pearson, D. The Chemical Analysis of Food, 6th ed.; J. and A. Churchill: London, UK, 1970; p. 508. [Google Scholar]
- Duh, P.D.; Yen, Y.C. Antioxidant efficacy of methanolic extracts of peanut hulls in soybean and peanut oils. J. Am. Oil Chem. Soc. 1997, 74, 745–748. [Google Scholar] [CrossRef]
- Mariod, A.A.; Ibrahim, R.M.; Norsharina, I. Antioxidant activities of phenolics rich fractions (PRFs) obtained from black mahlab (Monechma ciliatum) and white mahlab (Prunus mahaleb) seedcakes. Food Chem. 2010, 118, 120–127. [Google Scholar] [CrossRef]
- AOCS. Sampling and Analysis of Commercial Fats and Oils. AOCS Official Method Cd 8-53 Surplus Peroxide Value Acetic Acid–Chloroform Method Definition; AOCS Cold Spring Harbour: New York, NY, USA, 2003. [Google Scholar]
- Siew, W.L.; Cheah, K.Y. Optimization of degumming with attapulgite nad acid activated clay in refining palm oil. J. Oil Palm Res. 2007, 19, 373–380. [Google Scholar]
- Kirali, E.G.; Lacin, O. Statistical modelling of acid activation on cotton oil bleaching by Turkish bentonite. J. Food Eng. 2006, 75, 137–141. [Google Scholar] [CrossRef]
Sample Availability: Samples are available from the authors. |

© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Palanisamy, U.D.; Sivanathan, M.; Radhakrishnan, A.K.; Haleagrahara, N.; Subramaniam, T.; Chiew, G.S. An Effective Ostrich Oil Bleaching Technique Using Peroxide Value as an Indicator. Molecules 2011, 16, 5709-5719. https://doi.org/10.3390/molecules16075709
Palanisamy UD, Sivanathan M, Radhakrishnan AK, Haleagrahara N, Subramaniam T, Chiew GS. An Effective Ostrich Oil Bleaching Technique Using Peroxide Value as an Indicator. Molecules. 2011; 16(7):5709-5719. https://doi.org/10.3390/molecules16075709
Chicago/Turabian StylePalanisamy, Uma Devi, Muniswaran Sivanathan, Ammu Kutty Radhakrishnan, Nagaraja Haleagrahara, Thavamanithevi Subramaniam, and Gan Seng Chiew. 2011. "An Effective Ostrich Oil Bleaching Technique Using Peroxide Value as an Indicator" Molecules 16, no. 7: 5709-5719. https://doi.org/10.3390/molecules16075709
APA StylePalanisamy, U. D., Sivanathan, M., Radhakrishnan, A. K., Haleagrahara, N., Subramaniam, T., & Chiew, G. S. (2011). An Effective Ostrich Oil Bleaching Technique Using Peroxide Value as an Indicator. Molecules, 16(7), 5709-5719. https://doi.org/10.3390/molecules16075709





