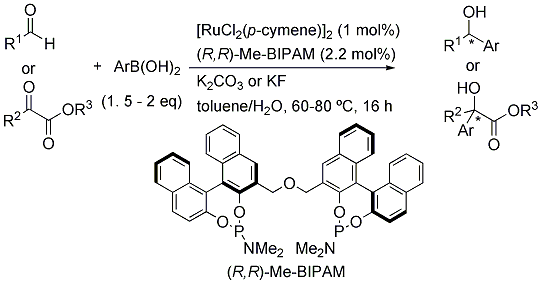
Ru/Me-BIPAM-Catalyzed Asymmetric Addition of Arylboronic Acids to Aliphatic Aldehydes and α-Ketoesters
Abstract
:1. Introduction
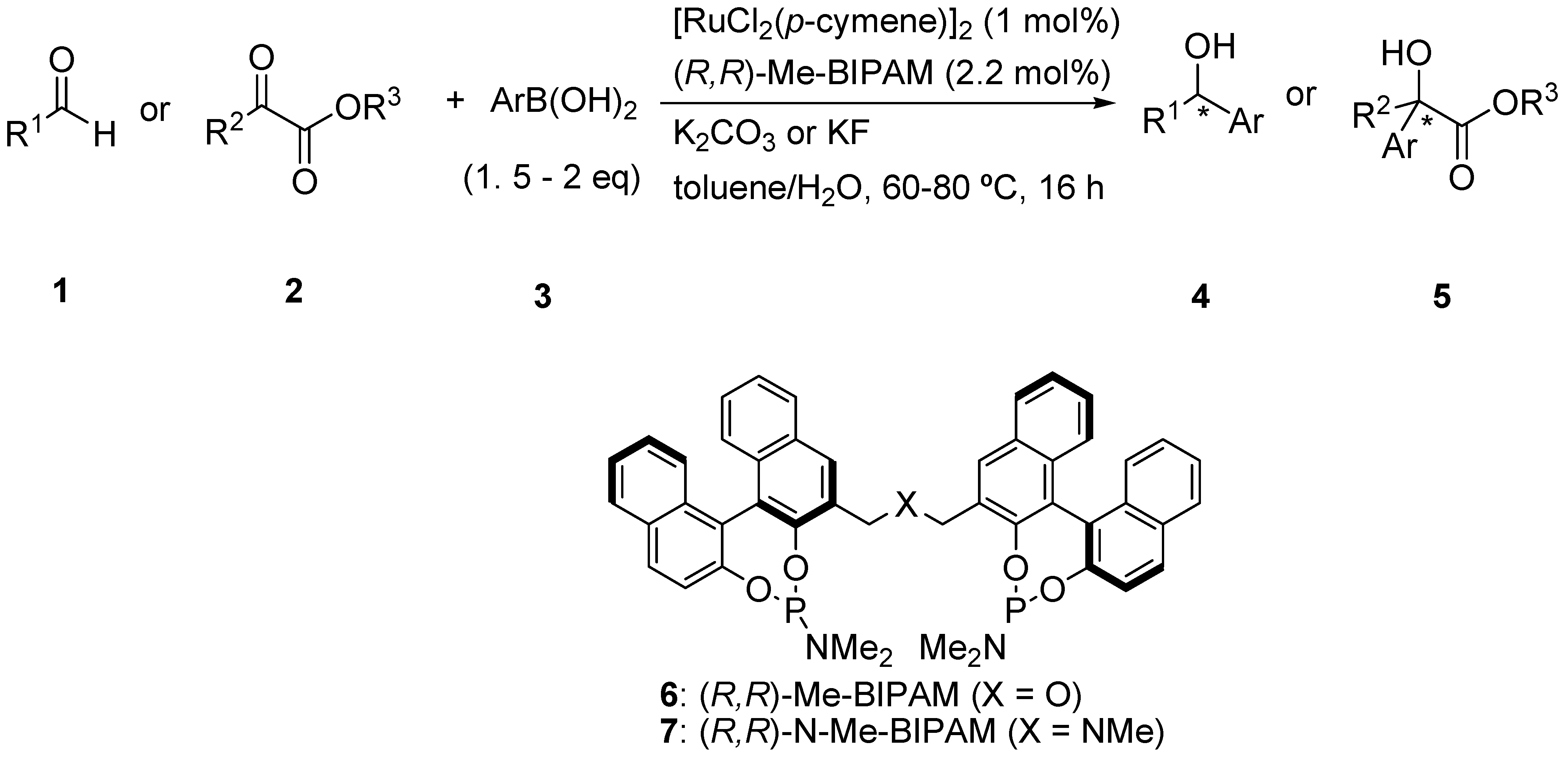
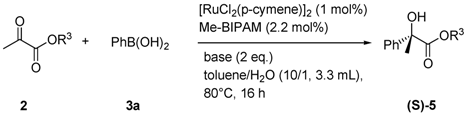
2. Results and Discussion
| Entry | R1 = | Ar = | Yield (%) | ee (%) (abs) |
|---|---|---|---|---|
| 1 b | n-C2H5 (1a) | Ph (3a) | 63 (4aa) | 91 (R) |
| 2 | n-C4H9 (1b) | Ph (3a) | 93 (4ba) | 94 (R) |
| 3 | n-C4H9 (1b) | 2-naphthyl (3b) | 98 (4bb) | 93 (R) |
| 4 | n-C4H9 (1b) | 4-MeC6H4 (3c) | 85 (4bc) | 92 (R) |
| 5 | n-C4H9 (1b) | 4-MeOC6H4 (3d) | 93 (4bd) | 92 (R) |
| 6 b,c | n-C4H9 (1b) | 4-ClC6H4 (3e) | 90 (4be) | 87 (R) |
| 7 | n-C4H9 (1b) | 4-FC6H4 (3f) | 69 (4bf) | 91 (R) |
| 8 | n-C4H9 (1b) | 3-MeOC6H4 (3h) | 62 (4bh) | 90 (R) |
| 9 | n-C4H9 (1b) | 3-ClC6H4 (3i) | 63 (4bi) | 90 (+) |
| 10 b,d | n-C4H9 (1b) | 3-F-4-MeOC6H3 (3k) | 65 (4bk) | 87 (+) |
| 11 b,c | n-C4H9 (1b) | 3,4-(CH2O2)C6H3 (3l) | 58 (4bl) | 99 (R) |
| 12 | n-C5H11 (1c) | Ph (3a) | 91 (4ca) | 94 (R) |
| 13 | n-C6H13 (1d) | Ph (3a) | 93 (4da) | 93 (R) |
| 14 c | n-C8H17 (1e) | Ph (3a) | 87 (4ea) | 92 (R) |
| 15 | PhCH2CH2 (1f) | Ph (3a) | 99 (4fa) | 92 (R) |
| 16 | cyclo-C6H11 (1g) | Ph (3a) | 78 (4ga) | 94 (R) |
| 17 b | i-Pr (1h) | Ph (3a) | 67 (4ha) | 96 (R) |
| 18 b,c | (C2H5)2CH (1j) | Ph (3a) | 54 (4ja) | 91 (R) |
| 19 b,c,e | t-Bu (1k) | Ph (3a) | 40 (4ka) | 99 (R) |
| Entry | Base | R3 = | Yield (%) | ee (%) (abs) |
|---|---|---|---|---|
| 1 b | K2CO3 | Et | 40 | 93 |
| 2 b | K3PO4 | Et | trace | ND |
| 3 b | CsF | Et | 40 | 93 |
| 4 b | KF | Et | 71 | 95 |
| 5 | KF | Et | 78 | 94 |
| 6 | KF | i-Pr (2a) | 85 | 93 (S) |
| 7 | KF | t-Bu | 87 | 90 |
| 8 c | KF | t-Bu | 72 | 70 |
| Entry | R2 = | Ar = | Yield (%) | ee (%) (abs) |
|---|---|---|---|---|
| 1 | Me (2a) | Ph (3a) | 85 (5aa) | 93 (S) |
| 2 | Me (2a) | 4-MeC6H4 (3c) | 84 (5ac) | 89 |
| 3 | Me (2a) | 4-MeOC6H4 (3d) | 84 (5ad) | 91 |
| 4 | Me (2a) | 4-FC6H4 (3f) | 85 (5af) | 93 |
| 5 | Me (2a) | 4-CF3C6H4 (3g) | 64 (5ag) | 92 |
| 6 | Me (2a) | 3-MeOC6H4 (3h) | 73 (5ah) | 92 |
| 7 | Me (2a) | 3-FC6H4 (3j) | 71 (5aj) | 90 |
| 8 | Me (2a) | 3-F-4-MeOC6H3 (3k) | 81 (5ak) | 87 |
| 9 | Et (2b) | Ph (3a) | 88 (5ba) | 95 |
| 10 | Et (2b) | 4-MeC6H4 (3c) | 90 (5bc) | 91 |
| 11 | Et (2b) | 4-FC6H4 (3f) | 90 (5bf) | 93 |
| 12 | Et (2b) | 3-MeOC6H4 (3h) | 88 (5bh) | 91 |
| 13 | i-Pr (2c) | Ph (3a) | 41 (5ca) | 94 |
| 14 | i-Pr (2c) | 4-MeOC6H4 (3d) | 42 (5cd) | 90 |
| 15 | Ph (2d) | 4-MeC6H4 (3c) | 82 (5dc) | 92 |
| 16 | Ph (2d) | 4-MeOC6H4 (3d) | 95 (5dd) | 86 |
| 17 | Ph (2d) | 4-ClC6H4 (3e) | 90 (5de) | 91 |
| 18 | Ph (2d) | 4-FC6H4 (3f) | 90 (5df) | 94 |
| 19 | Ph (2d) | 3-MeOC6H4 (3h) | 79 (5dh) | 92 |
| 20 | 4-FC6H4 (2e) | 3-ClC6H4 (3i) | 67 (5ei) | 90 |
3. Experimental Section
3.1. General
3.2. General Procedure for Arylation of Aliphatic Aldehydes (Table 1)
3.3. General Procedure for Arylation of α-Ketoesters (Table 3)
4. Conclusions
Acknowledgments
References and Notes
- Miyaura, N.; Yamamoto, Y. Boron in Comprehensive Organometallic Chemistry III; Crabtree, R.H., Mingos, M.P., Knochel, P., Eds.; Elsevier: Oxford, UK, 2007; Volume 9, pp. 146–244. [Google Scholar]
- Miyaura, N. Metal-catalyzed reactions of organoboronic acids and esters. Bull. Chem. Soc. Jpn. 2008, 81, 1535–1553. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Nishikata, T.; Miyaura, N. Rhodium (I)- or palladium (II)-catalyzed 1,4-additions of organoboron, -silicon and -bismuth compounds to electron-deficient alkenes. J. Synth. Org. Chem. Jpn. 2006, 64, 1112–1121. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Nishikata, T.; Miyaura, N. 1,4-Addition of arylboron, -silicon, and -bismuth compounds to α,β-unsaturated carbonyl compounds catalyzed by dicationic palladium(II) complexes. Pure Appl. Chem. 2008, 80, 807–817. [Google Scholar] [CrossRef]
- Miyaura, N. On palladium (II)-catalyzed additions of arylboronic acids to electron-deficient alkenes, aldehydes, imines and nitriles. Synlett 2009, 2039–2050. [Google Scholar]
- Yamamoto, Y.; Kurihara, K.; Sugishita, N.; Oshita, K.; Piao, D.-G.; Miyaura, N. Chiral bis-phosphoramidities based on linked-BINOL for rhodium-catalyzed 1,4-addition of arylboronic acids to α,β-unsaturated carbonyl compounds. Chem. Lett. 2005, 34, 1224–1225. [Google Scholar] [CrossRef]
- Kurihara, K.; Sugishita, N.; Oshita, K.; Piao, D.-G.; Yamamoto, Y.; Miyaura, N. Enantioselective 1,4-addition of arylboronic acids to α,β-unsaturated carbonyl compounds catalyzed by rhodium(I)-chiral phosphoramidite complexes. J. Organomet. Chem. 2007, 692, 428–435. [Google Scholar]
- Kurihara, K.; Yamamoto, Y.; Miyaura, N. N-linked bidentate phosphoramidite ligand (N-Me-BIPAM) for rhodium-catalyzed asymmetric addition of arylboronic acids to N-sulfonylarylaldimines. Adv. Synth. Catal. 2009, 351, 260–270. [Google Scholar] [CrossRef]
- Kurihara, K.; Yamamoto, Y.; Miyaura, N. A chiral bidentate phosphoramidite (Me-BIPAM) for Rh-catalyzed asymmetric hydrogenation of α-dehydroamino esters, enamides and dimethyl itaconate. Tetrahedron Lett. 2009, 50, 3158–3160. [Google Scholar]
- Yamamoto, Y.; Kurihara, K.; Miyaura, N. Me-bipam for enantioselective ruthenium (II)-catalyzed arylation of aldehydes with arylboronic acids. Angew. Chem. Int. Ed. 2009, 48, 4414–4416. [Google Scholar] [CrossRef]
- Abenhaïm, D.; Boireau, G.; Deberyl, A. Asymmetric synthesis using chiral lithium alkoxytrialkylaluminates: Obtention of (2S)-2-hydroxy-2-phenyl-4-methylpentanoic acid with 85% optical purity. J. Org. Chem. 1985, 50, 4045–4047. [Google Scholar] [CrossRef]
- Mash, E.A.; Fryling, J.A. Enantioselective pure acetals in organic synthesis. 2. Diastereoselective alkylation of enantiomeric lithio alkyl lactyl tetrahydropyranosides and related enolates. J. Org. Chem. 1991, 56, 1094–1098. [Google Scholar] [CrossRef]
- Rzeszotarski, W.J.; Gibson, R.E.; Eckelman, W.C.; Simms, D.A.; Jagoda, E.M.; Ferreira, N.L.; Reba, R.C. Analogues of 3-quiuclidinyl benzilate. J. Med. Chem. 1982, 25, 1103–1106. [Google Scholar] [CrossRef]
- He, X.-C.; Elitel, E.L. Highly enantioselective synthesis of α-hydroxyacids using N-benzyl-4,4,7α-trimethyl-trans-octahydro-1,3-benzoxazine as a chiral adjuvant. Tetrahedron 1987, 43, 4979–4987. [Google Scholar] [CrossRef]
- Muramatsu, Y.; Harada, T. Catalytic asymmetric alkylation of aldehydes with Grignard reagents. Angew. Chem. Int. Ed. 2008, 47, 1088–1090. [Google Scholar] [CrossRef]
- DiMauro, E.; Kozlowski, M.C. Development of bifunctional salen catalysts: Rapid, chemoselective alkylations of α-ketoesters. J. Am. Chem.Soc. 2002, 124, 12668–12669. [Google Scholar] [CrossRef]
- DiMauro, E.; Kozlowski, M.C. The first catalytic asymmetric addition of dialkylzincs to α-ketoesters. Org. Lett. 2002, 4, 3781–3784. [Google Scholar] [CrossRef]
- Funabashi, K.; Jachmann, M.; Kanai, M.; Shibasaki, M. Multicenter strategy for the development of catalytic enantioselective nucleophilic alkylation of ketones: Me2Zn addition to a ketoesters. Angew. Chem. Int. Ed. 2003, 42, 5489–5492. [Google Scholar] [CrossRef]
- Fennie, M.W.; DiMauro, E.F.; O’Brien, E.M.; Annamalai, V.; Kozlowski, M.C. Mechanism and scope of salen bifunctional catalysts in asymmetric aldehydes and α-ketoester alkylation. Tetrahedron 2005, 61, 6249–6265. [Google Scholar] [CrossRef]
- Blay, G.; Fernández, I.; Marco-Aleixandre, A.; Pedro, J.R. Enantioselective addition of dimethylzinc to α-keto esters. Synthesis 2007, 3754–3757. [Google Scholar]
- Wieland, L.C.; Deng, H.; Snapper, M.L.; Hoveyda, A.H. Al-catalyzed enantioselective alkylation of α-ketoesters by dialkylzinc reagents. Enhancement of enantioselectivity and reactivity by an achiral Lewis base additive. J. Am. Chem. Soc. 2005, 127, 15453–15456. [Google Scholar]
- Oi, S.; Moro, M.; Fukuhara, H.; Kawanishi, T.; Inoue, Y. Rhodium-catalyzed addition of arylstannanes to carbon-heteroatom double bond. Tetrahedron 2003, 59, 4351–4361. [Google Scholar] [CrossRef]
- Sakai, M.; Ueda, M.; Miyaura, N. Rhodium-catalyzed addition of organoboronic acids to aldehydes. Angew. Chem. Int. Ed. 1998, 37, 3279–3281. [Google Scholar] [CrossRef]
- Ganci, G.R.; Chisholm, J.D. Rhodium-catalyzed addition of aryl boronic acids to 1,2-diketonesters and 1,2-ketoesters. Tetrahedron Lett. 2007, 48, 8266–8269. [Google Scholar] [CrossRef]
- Miyamura, S.; Satoh, T.; Miura, M. Rhodium-catalyzed diarylation of oxalates using arylboron compounds. J. Org. Chem. 2007, 72, 2255–2257. [Google Scholar] [CrossRef]
- He, P.; Lu, Y.; Hu, Q.-S. Phosphinite- and phosphite-based type I palladacycles as highly active catalysts for addition reactions of arylboronic acids with aldehydes, α,β-unsaturated ketones, α-ketoesters, and aldimines. Tetrahedron Lett. 2007, 48, 5283–5288. [Google Scholar] [CrossRef]
- He, P.; Lu, Y.; Dong, C.-G.; Hu, Q.-S. Anionic four-electron donor-based palladacycles as catalysts for addition reactions of arylboronic acids with α,β-unsaturated ketones, aldehydes and α-ketoesters. Org. Lett. 2007, 9, 343–346. [Google Scholar] [CrossRef]
- Bennett, M.A.; Huang, T.-N.; Matheson, T.W.; Smith, A.K. (η6-Hexamethylbenzene)ruthenium complexes. Inorg. Synth. 1982, 21, 74–78. [Google Scholar] [CrossRef]
- Yong, K.H; Taylor, N.J.; Chong, J.M. Enantioselective alkylation of aldehydes with chiral organomagnesium amides (COMAs). Org. Lett. 2002, 4, 3553–3556. [Google Scholar] [CrossRef]
- Li, Y.; Ding, K.; Sandoval, C.A. Hybrid NH2-benzimidazole ligands for efficient Ru-catalyzed asymmetric hydrogenation of aryl ketones. Org. Lett. 2009, 11, 907–910. [Google Scholar] [CrossRef]
- Jones, G.; Heaton, S.R. Catalytic asymmetric induction part 2. Chiral tricarbonyl (η6 arene) chromium (0) complexes as enantioselective catalysts. Tetrahedron Asymmetry 1993, 4, 261–272. [Google Scholar] [CrossRef]
- Hatano, M.; Miyamto, T.; Ishihara, K. 3,3’-Diphosphoryl-1,1’-bi-2-naphthol-Zn(II) complexes as conjugate acid-base catalysts for enantioselective dialkylzinc addition to aldehydes. J. Org. Chem. 2006, 71, 6474–6484. [Google Scholar] [CrossRef]
- Glynn, D.; Shannon, J.; Woodward, S. On the scope of trimethylaluminium-promoted 1,2-additions of ArZnX reagents to aldehydes. Chem. Eur. J. 2010, 16, 1053–1060. [Google Scholar]
- Coldham, I.; Patel, J.J.; Raimbault, S.; Whittaker, D.T.E.; Adams, H.; Fang, G.Y.; Aggarwal, V.K. Asymmetric lithiation-substitution of amines involving rearrangement of borates. Org. Lett. 2008, 10, 141–143. [Google Scholar] [CrossRef]
- DeBerardinis, A.M.; Turlington, M.; Pu, L. Activation of functional arylzincs prepared from aryl iodides and highly enantioselective addition to aldehydes. Org. Lett. 2008, 10, 2709–2712. [Google Scholar] [CrossRef]
- Koul, S.; Koul, J.L.; Singh, B.; Kapoor, M.; Parshad, R.; Manhas, K.S.; Taneja, S.C.; Qazi, G.N. Trichosporon beigelli esterase (TBE): A versatile esterase for the resolution of economically important racemates. Tetrahedron Asymmetry 2005, 16, 2575–2591. [Google Scholar] [CrossRef]
- Inagaki, T.; Phong, L.T.; Furuta, A.; Ito, J.-I.; Nishiyama, H. Iron- and cobalt-catalyzed asymmetric hydrosilylation of ketones and enones with bis(oxazolinylphenyl)amine ligands. Chem. Eur. J. 2010, 16, 3090–3096. [Google Scholar] [CrossRef]
- Dahmen, S.; Lormann, M. Triarylborane ammonia complexes as ideal precursors for arylzinc reagents in asymmetric catalysis. Org. Lett. 2005, 7, 4597–4600. [Google Scholar] [CrossRef]
- Onodera, G.; NIshibayashi, Y.; Uemura, S. Ir- and Ru-catalyzed sequential reactions: Asymmetric α-alkylative reduction of ketones with alcohols. Angew. Chem. Int. Ed. 2006, 45, 3819–3822. [Google Scholar] [CrossRef]
- Vettel, S.; Vaupel, A.; Knochel, P. Nickel-catalyzed preparations of functionalized organozincs. J. Org. Chem. 1996, 61, 7473–7481. [Google Scholar] [CrossRef]
- Huang, H.; Okuno, T.; Tsuda, K.; Yoshimura, M.; Kitamura, M. Enantioselective hydrogenation of aromatic ketones catalyzed by Ru complexes of Goodwin-Lions-type sp2N/sp3N hybrid ligands R-BINAN-R’-Py. J. Am. Chem. Soc. 2006, 128, 8716–8717. [Google Scholar]
- Stymiest, J.L.; Dutheuil, G.; Mahmood, A.; Aggarwal, V.K. Lithiated carbamates: Chiral carbenoids for iterative homologation of boranes and boronic esters. Angew. Chem. Int. Ed. 2007, 46, 7491–7494. [Google Scholar] [CrossRef]
- Fontes, M.; Verdaguer, X.; Solà, L.; Pericàs, M.A.; Riera, A. 2-Piperidino-1,1,2-triphenylethanol: A highly effective catalyst for the enantioselective arylation of aldehydes. J. Org. Chem. 2004, 69, 2532–2543. [Google Scholar] [CrossRef]
- Blay, G.; Fernández, I.; Marco-Aleixandre, A.; Pedro, J.R. Catalytic asymmetric addition of dimethylzinc to α-ketoesters, using mandelamides as ligands. Org. Lett. 2006, 8, 1287–1290. [Google Scholar] [CrossRef]
- Wu, H.-L.; Wu, P.-Y.; Shen, Y.-Y.; Uang, B.-J. Asymmetric addition of dimethylzinc to α-ketoesters catalyzed by (−)-MITH. J. Org. Chem. 2008, 73, 6445–6447. [Google Scholar] [CrossRef]
- Zheng, B.; Hou, S.; Li, Z.; Guo, H.; Zhong, J.; Wang, M. Enantioselective synthesis of quaternary stereogenic centers though catalytic asymmetric addition of dimethylzinc to α-ketoesters with chiral cis-cyclopropane-based amide alcohol as ligand. Tetrahedron Asymmetry 2009, 20, 2125–2129. [Google Scholar] [CrossRef]
- Duan, H.-F.; Xie, J.-H.; Qiao, X.-C.; Wang, L.-X.; Zhou, Q.-L. Enantioselective rhodium-catalyzed addition of arylboronic acids to α-ketoesters. Angew. Chem. Int. Ed. 2008, 47, 4351–4353. [Google Scholar]
- Sample Availability: Me-BIPAM and N-Me-BIPAM are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yamamoto, Y.; Shirai, T.; Watanabe, M.; Kurihara, K.; Miyaura, N. Ru/Me-BIPAM-Catalyzed Asymmetric Addition of Arylboronic Acids to Aliphatic Aldehydes and α-Ketoesters. Molecules 2011, 16, 5020-5034. https://doi.org/10.3390/molecules16065020
Yamamoto Y, Shirai T, Watanabe M, Kurihara K, Miyaura N. Ru/Me-BIPAM-Catalyzed Asymmetric Addition of Arylboronic Acids to Aliphatic Aldehydes and α-Ketoesters. Molecules. 2011; 16(6):5020-5034. https://doi.org/10.3390/molecules16065020
Chicago/Turabian StyleYamamoto, Yasunori, Tomohiko Shirai, Momoko Watanabe, Kazunori Kurihara, and Norio Miyaura. 2011. "Ru/Me-BIPAM-Catalyzed Asymmetric Addition of Arylboronic Acids to Aliphatic Aldehydes and α-Ketoesters" Molecules 16, no. 6: 5020-5034. https://doi.org/10.3390/molecules16065020
APA StyleYamamoto, Y., Shirai, T., Watanabe, M., Kurihara, K., & Miyaura, N. (2011). Ru/Me-BIPAM-Catalyzed Asymmetric Addition of Arylboronic Acids to Aliphatic Aldehydes and α-Ketoesters. Molecules, 16(6), 5020-5034. https://doi.org/10.3390/molecules16065020