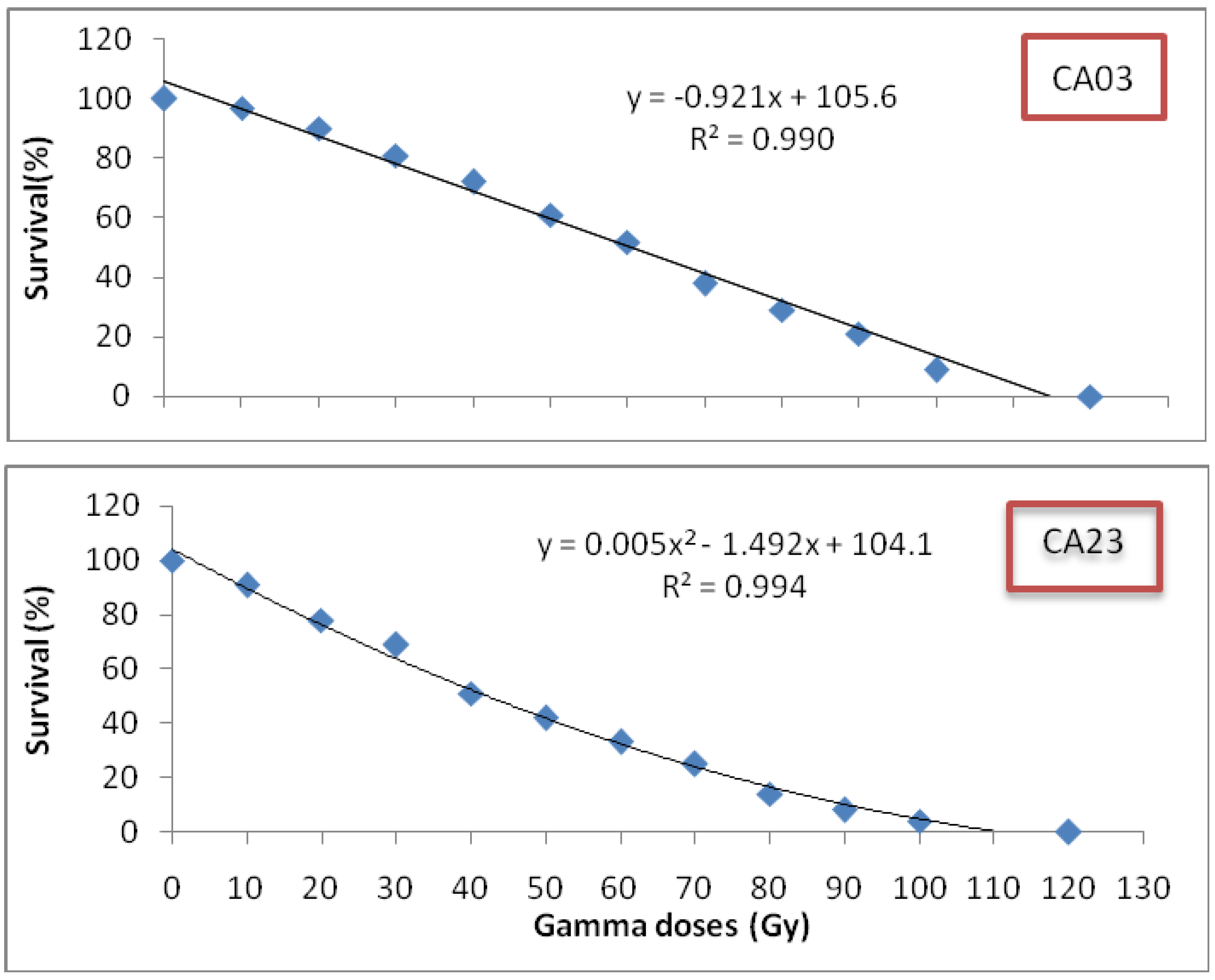
2.2. Determination of Plant Biomass and Total Flavonoid Content
Statistical tests of flavonoid contents of
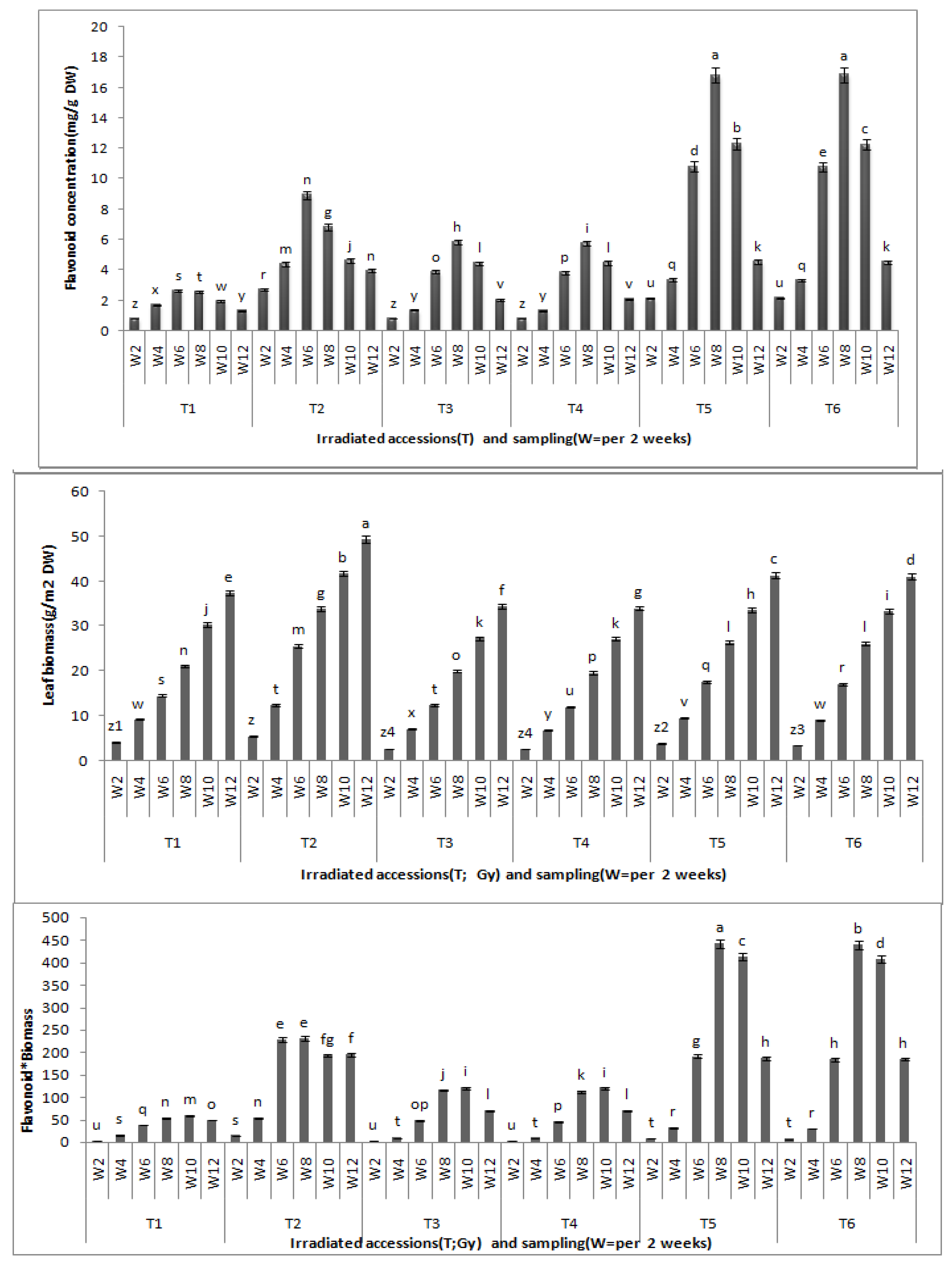
Centella asiatica leaves between the growth stages and between the gamma radiation doses revealed significant differences at (P < 0.05). The total flavonoid content was found to be highest after eight weeks of growth, and this, accordingly, stands as the best time for leaf harvest. Biochemical differentiation based on total flavonoid content revealed that irradiated CA23 plantlets at 20 and 30 Gy after eight weeks contained the highest total flavonoid concentrations (16.827 ± 0.02; 16.837 ± 0.008 mg/g DW, respectively) whereas CA03 exposed to 30 and 40 Gy was found to have the lowest total flavonid content (5.83 ± 0.11; 5.75 ± 0.03 mg/g DW) (
Figure 2).
Figure 2.
Effects of gamma irradiation on leaf total flavonoid, leaf biomass and flavonoid * leaf biomass in C.asiatica. n = 4 (T1 = CA03 (Control); T2 = CA23 (Control); T3 = CA03 irradiated to 30 Gy; T4 = CA03 irradiated to 40 Gy; T5 = CA23 irradiated to 20 Gy; T6 = CA23 irradiated to 30 Gy; W2 = 2 weeks after planting; W4 = 4 weeks after planting; W6 = 6 weeks after planting; W8 = 8 weeks after planting; W10 = 10 weeks after planting; W12 = 12 weeks after planting).
Figure 2.
Effects of gamma irradiation on leaf total flavonoid, leaf biomass and flavonoid * leaf biomass in C.asiatica. n = 4 (T1 = CA03 (Control); T2 = CA23 (Control); T3 = CA03 irradiated to 30 Gy; T4 = CA03 irradiated to 40 Gy; T5 = CA23 irradiated to 20 Gy; T6 = CA23 irradiated to 30 Gy; W2 = 2 weeks after planting; W4 = 4 weeks after planting; W6 = 6 weeks after planting; W8 = 8 weeks after planting; W10 = 10 weeks after planting; W12 = 12 weeks after planting).
Comparing total flavonoid contents of control with the irradiated plantlets, results demonstrate that the irradiated plantlets exhibited significantly greater total flavonoid contents (in eight weeks) than the control where the control also exhibited the highest total flavonoid contents in the sixth week of growth: 2.64 ± 0.02 mg/g DW in CA03 and 8.94 ± 0.04 mg/g DW in CA23, respectively. These contents were 54.7% and 46.8% lower, respectively, than the total flavonoids contents of the irradiated plants in their eighth week of growth. However, there were no significant differences in total flavonoid contents between the irradiated plants with 30 and 40 Gy in CA03 and between those irradiated with 20 and 30 Gy in CA23. From the results obtained in the present study, it can be concluded that plants irradiated with gamma radiation showed the higher total flavonoid content as compared to non-irradiated plants. Moreover, at the eighth week of growth, the irradiated plants were found to have the highest flavonoid contents.
Flavonoid biosynthesis is stimulated by enhancing phenylalanine content and phenylalanine ammonia-lyase activity (PAL). Flavonoids are secondary metabolites broadly distributed in plants. They result from the addition of malonyl CoA to the phenylpropanoid molecule coumaroyl CoA [
12]. These polyphenolic compounds are categorized by two aromatic rings (A and B rings) linked via a heterocycle (C ring). They are grouped based on the degree of oxidation of the C ring and comprise flavonols, anthocyanins, and flavan-3-ols. The carbon atoms on the B and C rings are from phenylalanine that is from the shikimic acid way. As primary factors in flavonoid synthetic pathway, the phenylalanine content influences flavonoid content directly.
Flavonoid biosynthesis, which is a significant step in the phenylpropanoid pathway, is the conversion of phenylalanine to cinnamic acid in the presence of PAL as catalyst. The activity of PAL affects the flavonoid synthesis in response to irradiation (gamma and UV-B stress) whereas the flavonoids alleviate the damage induced by the irradiation stress. For instance, the flavonoid content of soybean seedlings increases in response to UV-B radiation. With prolonged stress, the damage induced by UV-B radiation to soybean seedlings cannot be ameliorated completely by increased flavonoid concentrations. As a result, chlorophyll synthesis is inhibited and assimilation in leaves is constantly reduced by the UV-B stress, which ultimately results in a decline in the efficiency of the secondary metabolism biosynthesis system. Hence, biosynthesis and accumulation of flavonoids decrease [
13].
A significant difference was observed between irradiated and non-irradiated plants for total biomass where the former had lower leaf biomass than the control. The data obtained after 12 weeks of growth showed that CA23 achieved the highest leaf dry weight (49.4 ± 0.2 g/m
2 DW) and the CA03 at 40 Gy (CA03G40) had the slowest growth (34.0 ± 0.2 g/m
2 DW). Furthermore, the flavonoid*biomass (flavonoids content multiplied by leaf biomass at the same harvest time) were significantly different between the irradiated accessions and the control. The CA23 had the highest flavonoid*biomass (442.56 ± 8.53) in the eighth week and at 20 Gy (CA23G20). On the other hand, no significant differences were observed in the flavonoid*biomass between the irradiated plants of each accession (
Figure 2).
It can be inferred that the biomass of Centella asiatica leaves was significantly inhibited by γ-radiation as compared with the control (p < 0.01). Nonetheless, there were no significant differences in leaf biomass between the irradiated plants in each of accession at the same harvest time.
Irradiation of Arabidopsis caused increases in the levels flavonoids, which accumulated in the aerial parts of the plants [
14]. Jia and Li [
15] found that plant height, number of first-class branches, and rhizome biomass in buckwheat were restrained drastically by gamma irradiation (p < 0.01). Reduction in growth of red stem buckwheat mutants, in contrast with increases in dry mass of soybean and secondary metabolite of
Lithospermum erythrorhizon, may possibly be the reason for slow cell division; lower hormone synthesis; unusual nutrimental transportation; and metabolic disorders by apical meristem injure under γ-irradiation [
15].
Banerji and Datta [
16] and Shukla and Datta [
17] reported a decline in Chrysanthemum height and numbers of branches and leaves when it was irradiated with low doses of gamma rays (1.5, 2, and 2.5 k-rad). Likewise, Ramachandran and Goud [
18] showed that higher doses of gamma irradiation (G.I). (40-120 k-rad) decreased plant height, number of leaves and the branching capacity of safflower. A diminution in plant height, branches number, leaves number and size was observed when root cuttings of Chrysanthemum were irradiated with 20 or 25 k-rad gamma rays (16).
The growth of Arabidopsis seedlings exposed to low-dose gamma irradiation (1 or 2 Gy) was faintly increased compared with that of the control, while the seedling growth was perceptibly decreased by a high-dose irradiation of 50 Gy. Although no certain explanations for the stimulatory effects of low-dose gamma irradiation are accessible until now, a number of researchers support the assumption that low dose irradiation will stimulate growth by altering the hormonal signalling network in the plant cells or via enhancing the antioxidative capacity of the cells to simply overcome daily stress factors such as variations in light intensity and temperature in the growing medium/environment [
7]. By contrast, the growth inhibition induced through the high-dose irradiation has been attributed to the cell cycle arrest at the G2/M phase during somatic cell division and/or to a variety of damages in the entire genome [
19].
The association between growth of irradiated plants and dose of gamma irradiation has been demonstrated by investigating the morphological alteration and seedling growth of the irradiated plants. No significant morphological abnormalities were observed in the phenotype of the plants irradiated with relatively low doses (1–5 Gy) of gamma rays whereas a high-dose (50 Gy) irradiation restrained seedling growth considerably. As an illustration, Kim
et al. [
9] claimed that the growth of red pepper was raised at 2 and 4 Gy but slowed down at 8 and 16 Gy and that the level of stress resistance of gamma-irradiated plants may depend on the species/cultivar or stress circumstances [
7].
2.3. Leaf Gas Exchange
In the eighth week of growth, net photosynthetic rate (PN), stomatal conductance (gs), intercellular CO
2 concentration (C
i), and transpiration rate (E) were determined by a portable infrared photosynthesis system LI-6400 (LI-COR, Lincoln, NE, USA) from 8:30 am to 10:30 am. Photosynthetic photon flux density (PPFD) and leaf temperature were maintained at 1000 μmol m
–2 s
–1 and 30 °C, respectively. Water use efficiency (WUE) was calculated according to Penuelas
et al. [
20]. Based on the results gathered in this study, significant differences were found between irradiated accessions and control ones in relation to the leaf gas. As shown in
Figure 3, the highest PN and gs were detected in CA23 as control (17.45 ± 0.03, 0.923 ± 0.002, respectively), followed by CA23 at 20 Gy (CA23G20) (12.48 ± 0.06, 0.742 ± 0.011) and CA23 at 30 Gy (CA23G30) (12.33 ± 0.06, 0.725 ± 0.009) and the lowest PN and gs were observed in CA03 at 40 Gy (CA03G40) (4.44 ± 0.04, 0.31 ± 0.003; respectively). Moreover, there were no significant differences in terms of PN and gs among the irradiated plants in each accession. The highest C
i (intercellular carbon dioxide) was found in CA03 (285.0 ± 0.8), while the lowest was found in CA23 (274.0 ± 0.8).
There were significant differences between irradiated accessions and control ones in terms of C
i, E and WUE. The WUE of both irradiated accessions of
Centella asiatica were reduced as compared with the control plants (p < 0.01) while Ci and E were enhanced. The transpiration rate increased but C
i and gs decreased in response to γ-radiation (
Figure 3). There were no significant differences in the gas exchange parameters among radiated plants in each accession. However, the CA03G30 was observed to have the highest C
i (295.5 ± 0.6). Still, this concentration was not significantly different from that of CA03G40 (294.0 ± 0.3) while CA23 had the lowest C
i (273.25 ± 1.03). Meanwhile, CA23G30 was found to have the highest E (10.075 ± 0.005) while the lowest E was detected in CA03 (4.375 ± 0.015). As illustrated in
Figure 3, the highest WUE was found in CA23 (1.783 ± 0.006) and the lowest was detected in CA03G40 (0.905 ± 0.006).
In buckwheat mutants’ reduction of Chl contents showed that Chl accumulation was reduced by γ-radiation, which stimulated activities of chlorophyllase, increased degradation of Chl, and ultimately reduced the photosynthetic activity of the plants. Although photosynthetic efficiency and biomass were declined notably, plants could acclimate to the varying growth environmental conditions through a defensive mechanism (secondary metabolites) against over-damage of the PSII reaction centres and excessive reduction of photosynthetic productivity. Jia and Li [
15] showed that the photosynthetic rate of buckwheat fell down while that of
Capsicum annuum accelerated by effect of a low dose of γ-radiation.
Figure 3.
Effects of gamma irradiation on leaf gas exchange and WUE of irradiated and non irradiated accessions n = 4 (CA03G30 = CA03 irradiated to 30 Gy; CA03G40 = CA03 irradiated to 40 Gy; CA23G20 = CA23 irradiated to 20 Gy; CA23G30 = CA23 irradiated to 30 Gy).
Figure 3.
Effects of gamma irradiation on leaf gas exchange and WUE of irradiated and non irradiated accessions n = 4 (CA03G30 = CA03 irradiated to 30 Gy; CA03G40 = CA03 irradiated to 40 Gy; CA23G20 = CA23 irradiated to 20 Gy; CA23G30 = CA23 irradiated to 30 Gy).
Whether stomatal or non-stomatal restriction is the significant factor in reducing PN could be judged upon by the altering patterns of both C
i and gs [
21]. If both C
i and PN reduced through a lowering of gs, the decline of PN would be caused mostly via stomatal limitation. And it would be caused by non-stomatal limitation when C
i increased or remained almost unchanged with low PN and gs. In view of the above theory, the photoinhibition of buckwheat leaves was influenced by stomatal and non-stomatal limitation. On the one hand, stomata closure and gs decline due to rapid transpiration and excessive loss of water restricted the quantity of CO
2 entering the plant leaves through lower C
i which consequently reduced photosynthesis rate due to a lack of photosynthetic substrate. On the other hand, the PN still declined through higher C
i values owing to lower gs as a consequence of limited carboxylation efficiency and CO
2 assimilation because of the excessive accumulation of free radicals in mesophyll cells by irradiation [
22]. Ursino
et al. [
23] revealed that the diminished rates of CO
2 uptake in soybean plants were the result of a radiation impact on the photosynthetic apparatus rather than to increased stomatal resistance or to accelerated CO
2 evolution from dark respiration. Furthermore, decreases in the rate of shoot growth and leaf expansion were apparent even at a dose of 750 rads.
2.4. Determination of Lipid Oxidation (Malondialdehyde (MDA))
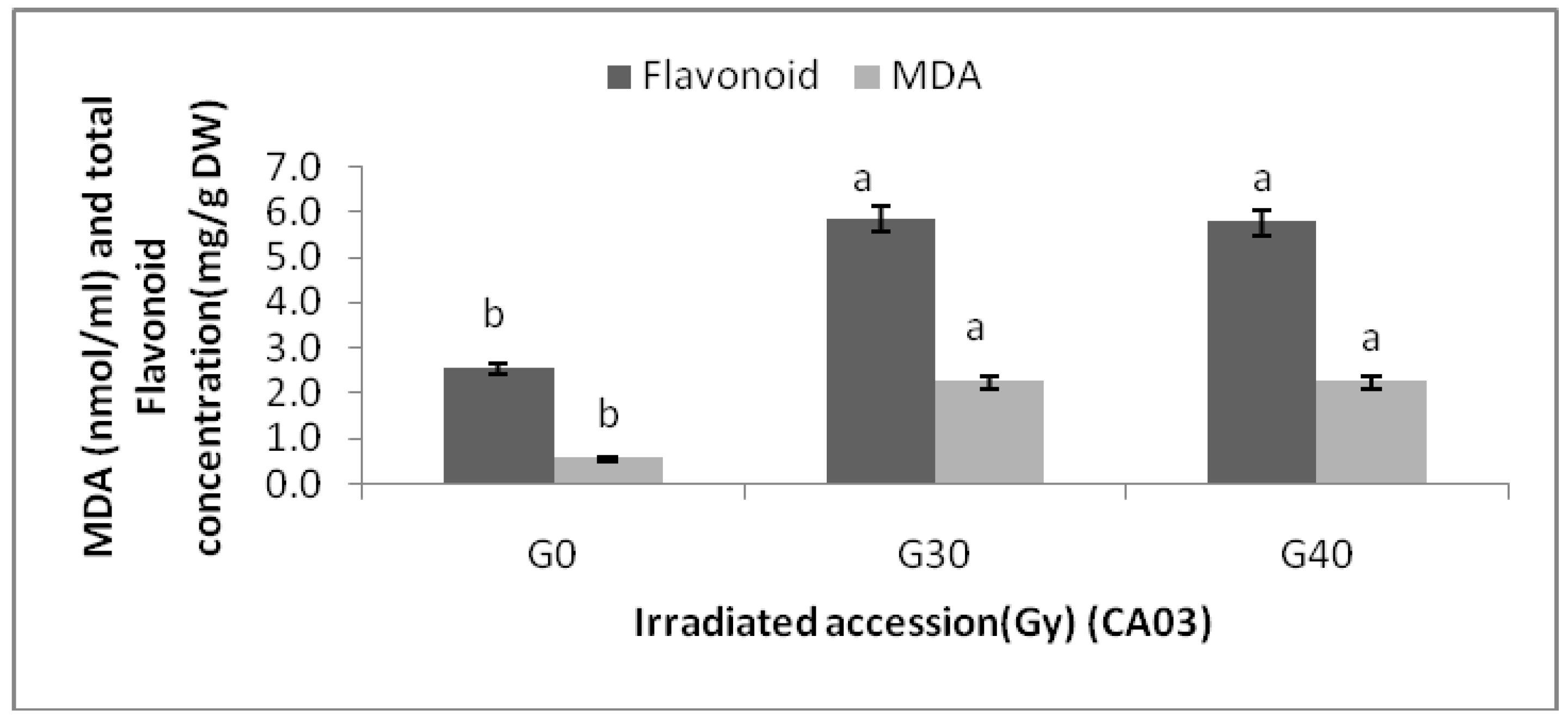
Changes in MDA content of the two
Centella asiatica accessions under different gamma treatments were determined. The MDA contents of the
Centella asiatica accessions after gamma treatments were significantly higher than the control. However, no significant differences in MDA contents among the irradiated plants in both accessions were detected. The highest MDA (3.20 ± 0.00 nmol/mL) was observed in CA23G30, while the lowest were detected in CA03 (0.543 ± 0.000 nmol/mL) and CA23 (0.540 ± 0.000 nmol/mL). For additional information about the relationships between MDA and flavonoids, graphs (
Figure 4 and
Figure 5) are provided, where it can be seen that a higher levels of MDA, flavonoid content was higher.
Peng and Zhou [
24] found that flavonoid content of soybean seedlings exposed to UV-B treatment during the stress period was enhanced in the beginning and then decreased in comparison with that of the control. Membrane permeability and MDA contents increased at first (first to fifth day) and then decreased (6th–11th day). The main causal factor of the stress damage is free radicals. Flavonoids proved to have the ability to scavenge free radicals.
Figure 4.
Flavonoid content and MDA in irradiated and non-irradiated accessions of CA03 n = 3 (CA03G30 = CA03 irradiated to 30 Gy; CA03G40 = CA03 irradiated to 40 Gy). Notice: Each observation compared with its counterparts.
Figure 4.
Flavonoid content and MDA in irradiated and non-irradiated accessions of CA03 n = 3 (CA03G30 = CA03 irradiated to 30 Gy; CA03G40 = CA03 irradiated to 40 Gy). Notice: Each observation compared with its counterparts.
Figure 5.
Flavonoid content and MDA in irradiated and non-irradiated accessions of CA23 n = 3 (CA23G20 = CA23 irradiated to 20 Gy; CA23G30 = CA23 irradiated to 30 Gy). Notice: Each observation compared with its counterparts (e.g, MDA compared with MDA in different accessions).
Figure 5.
Flavonoid content and MDA in irradiated and non-irradiated accessions of CA23 n = 3 (CA23G20 = CA23 irradiated to 20 Gy; CA23G30 = CA23 irradiated to 30 Gy). Notice: Each observation compared with its counterparts (e.g, MDA compared with MDA in different accessions).
The excess ROS can react with nearly all cell constituents. Such interaction triggers free radical chain reactions, eventually causing membrane lipid peroxidation. The product of lipid peroxidation, MDA, can react with the amino acid residues of membrane protein and nucleic acid, decline membrane stability, and boost membrane permeability. As a result, cell structure and normal physiological functioning are damaged and accordingly, cell aging and death of organism as a consequence of pathological changes occur. Flavonoids can scavenge surplus free radicals. Under irradiation stress (e.g., gamma, UV), flavonoids improve the plant’s ability of self-protection by inhibiting formation of cellular membrane lipid peroxidation product-MDA and by retaining membrane permeability [
24].