Synthesis of the Key Intermediate of Coenzyme Q10
Abstract
:1. Introduction
2. Results and Discussion
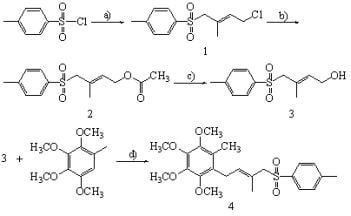
2.1. Synthesis of Compound 1
2.2. Synthesis of Compound 2
2.3. Synthesis of Compound 3
2.4. Synthesis of Compound 4
3. Experimental
3.1. General
3.2. (2E)-1-p-Toluenesulfonyl-2-methyl-4-chloride-2-butene (1)
3.3. (2E)-1-p-Toluenesulfonyl-2-methyl-4-acetoxy-2-butene (2)
3.4. (2E)-1-p-Toluenesulfonyl-2-methyl-4-hydroxy-2-butene (3)
3.5. (2’E)-1-(3-Methyl-4-p-toluenesulfonyl-2-butene)-6-methyl-2,3,4,5-tetramethoxymethylbenzene (4)
4. Conclusions
Acknowledgments
References and Notes
- Anderson, S.S.; Lyle, I.G.; Paterson, R. Electron transfer across membranes using vitamin K1 and coenzyme Q10 as carrier molecules. Nature 1976, 5539, 147–148. [Google Scholar] [CrossRef]
- Littarru, G.P.; Tiano, L. Clinical aspects of coenzyme Q10: An update. Nutrition 2010, 3, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Rüegg, R.; Gloor, U.; Goel, R.N.; Ryser, G.; Wiss, O.; Isler, O. Synthesis of bichinon(45) and ubichinon(50). Helv. Chim. Acta 1959, 42, 2616–2621. [Google Scholar] [CrossRef]
- Yoshizawa, T.; Toyofuka, H.; Tachibana, K.; Kuroda, T. Regioselective polyprenyl rearrangement of polyprenyl 2,3,4,5-tetrasubstituted phenyl ethers promoted by boron trifluoride. Chem. Lett. 1982, 8, 1131–1134. [Google Scholar] [CrossRef]
- Van Liemt, W.B.S.; Steggerda, W.F.; Esmeijer, R.; Lugtenburg, J. Synthesis and spectroscopic characterization of 13C-labelled ubiquinone-0 and ubiquinone-10. Recl. Trav. Chim. Pays-Bas. 1994, 3, 153–161. [Google Scholar]
- Lipshutz, B.H.; Mollard, P.; Pfeiffer, S.S.; Chrisman, W.A. Short, highly efficient synthesis of coenzyme Q10. J. Am. Chem. Soc. 2002, 124, 14282–14283. [Google Scholar] [CrossRef] [PubMed]
- Negishi, E.; Liou, S.Y.; Xu, C.; Huo, S. A novel, highly selective, and general methodology for the synthesis of 1,5-diene-containing oligoisoprenoids of all possible geometrical combinations exemplified by an iterative and convergent synthesis of coenzyme Q10. Org. Lett. 2002, 2, 261–264. [Google Scholar] [CrossRef]
- Terao, S.; Kato, K.; Shiraishi, M.; Morimoto, H. Synthesis of ubiquinones. 2. An efficient preparation of ubiquinone-10. J. Org. Chem. 1979, 44, 868–869. [Google Scholar] [CrossRef]
- Fujita, Y.; Ishiguro, M.; Onishi, T.; Nishida, T. A new efficient and stereoselective synthesis of ubiquinone-10. Bull. Chem. Soc. Jpn. 1982, 55, 1325–1326. [Google Scholar] [CrossRef]
- Min, J.H.; Lee, J.S.; Yang, J.D.; Koo, S. The Friedel-Crafts alkylation of a prenyl group stabilized by a sulfone moiety: Expeditious synthesis of ubiquinones and menaquinones. J. Org. Chem. 2003, 68, 7925–7927. [Google Scholar] [CrossRef] [PubMed]
- Lipshutz, B.H.; Lower, A.; Berl, V.; Schein, K.; Wetterich, F. An improved synthesis of the “Miracle Nutrient”, coenzyme Q10. Org. Lett. 2005, 7, 4095–4097. [Google Scholar] [CrossRef] [PubMed]
- Ravada, S.R.; Emani, L.R.; Garaga, M.R.; Meka, B.; Golakoti, T. Synthesis of coenzyme Q10. Am. J. Infect. Dis. 2009, 2, 83–89. [Google Scholar] [CrossRef]
- Chen, Y.; Zu, Y.G.; Fu, Y.J.; Zhang, X.; Yu, P.; Sun, G.Y.; Efferth, T. Efficient lewis acid ionic liquid-catalyzed synthesis of the key intermediate of coenzyme Q10 under microwave irradiation. Molecules 2010, 12, 9486–9495. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |

| Number | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| CuCl / g | 0.052 | 0.105 | 0.157 | 0.209 | 0.313 | 0.522 |
| Yield / % | - | 58.6 | 75.9 | 75.0 | 70.4 | 68.8 |
| Number | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Temperature / °C | 110 | 115 | 120 | 125 | 130 |
| Yield / % | 53.3 | 72.0 | 90.7 | 80.4 | 62.6 |
| Number | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Na2CO3 adding time / h | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 |
| Yield / % | 20.1 | 43.4 | 52.8 | 68.8 | 68.7 | 68.7 |
| Number | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| BF3·OEt2 / mL | 0.06 | 0.12 | 0.18 | 0.24 | 0.3 |
| Yield / % | - | 32.2 | 58.8 | 44.8 | 29.4 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mu, F.-S.; Luo, M.; Fu, Y.-J.; Zhang, X.; Yu, P.; Zu, Y.-G. Synthesis of the Key Intermediate of Coenzyme Q10. Molecules 2011, 16, 4097-4103. https://doi.org/10.3390/molecules16054097
Mu F-S, Luo M, Fu Y-J, Zhang X, Yu P, Zu Y-G. Synthesis of the Key Intermediate of Coenzyme Q10. Molecules. 2011; 16(5):4097-4103. https://doi.org/10.3390/molecules16054097
Chicago/Turabian StyleMu, Fan-Song, Meng Luo, Yu-Jie Fu, Xuan Zhang, Ping Yu, and Yuan-Gang Zu. 2011. "Synthesis of the Key Intermediate of Coenzyme Q10" Molecules 16, no. 5: 4097-4103. https://doi.org/10.3390/molecules16054097
APA StyleMu, F.-S., Luo, M., Fu, Y.-J., Zhang, X., Yu, P., & Zu, Y.-G. (2011). Synthesis of the Key Intermediate of Coenzyme Q10. Molecules, 16(5), 4097-4103. https://doi.org/10.3390/molecules16054097