Synthesis of Ginkgolic Acid Analogues and Evaluation of Their Molluscicidal Activity
Abstract
:1. Introduction
2. Results and Discussion
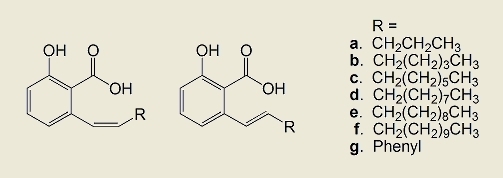
2.1. Synthesis of the compounds
2.2. Assay for molluscicidal activity
3. Experimental
3.1. General
3.2. Synthesis
3.2.1. Ethyl 6-methylsalicylate (1)
3.2.2. Ethyl O-acetyl-6-methylsalicylate (2)
3.2.3. Ethyl O-acetyl-6-bromomethylsalicylate (3)
3.2.4. 3-Acetoxy-2-ethoxycarbonylbenzyltriphenylphosphonium bromide (4)
3.2.5. General procedure for preparation of ethyl O-acetyl-6-(1-alkenyl)salicylates 5a–5g
3.2.6. Separation of Z and E isomers 6a–6g and 7a–7g of ethyl O-acetyl-6-(1-alkenyl)salicylates
3.2.7. General procedure for preparation of 6-(1-alkenyl)salicylic acids
3.2.8. (Z)-6-(1-Pentenyl)salicylic acid (8a)
3.2.9. (E)-6-(1-Pentenyl)salicylic acid (9a)
3.2.10. (Z)-6-(1-Heptenyl)salicylic acid (8b)
3.2.11. (E)-6-(1-Heptenyl)salicylic acid (9b)
3.2.12. (Z)-6-(1-Nonenyl)salicylic acid (8c)
3.2.13. (E)-6-(1-Nonenyl)salicylic acid (9c)
3.2.14. (Z)-6-(1-Undecenyl)salicylic acid (8d)
3.2.15. (E)-6-(1-Undecenyl)salicylic acid (9d)
3.2.16. (Z)-6-(1-Dodecenyl)salicylic acid (8e)
3.2.17. (E)-6-(1-Dodecenyl)salicylic acid (9e)
3.2.18. (Z)-6-(1-Tridecenyl)salicylic acid (8f)
3.2.19. (E)-6-(1-Tridecenyl)salicylic acid (9f)
3.2.20. (Z)-6-Phenylethenyl salicylic acid (8g)
3.2.21. (E)-6-Phenylethenyl salicylic acid (9g)
3.3. Molluscicidal activity tests
4. Conclusions
Conflict of Interest
Acknowledgments
References
- Lardans, V.; Dissous, C. Snail control strategies for reduction of schistosomiasis transmission. Parasitol. Today 1998, 14, 413–417. [Google Scholar] [CrossRef]
- Yi, Y.A.; Xu, X.J.; Dong, H.F.; Jiang, M.S.; Zhu, H.G. Transmission control of schistosomiasis japonica: Implementation and evaluation of different snail control interventions. Acta Trop. 2005, 96, 191–197. [Google Scholar]
- WHO. The Control of Schistosomiasis: Second Report of the WHO Expert Committee; WHO Technical Report Series, No. 830; WHO: Geneva, Swizerland, 1993. [Google Scholar]
- Perrett, S.; Whitfield, P.J. Currently available molluscicides. Parasitol. Today 1996, 12, 156–159. [Google Scholar] [CrossRef]
- Webbe, G.; Lambert, J.D.H. Plants that kill snails and prospects for disease control. Nature 1983, 302, 754–754. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, P.J. Novel anthelmintic compounds and molluscicides from medicinal plants. Trans. Roy. Soc. Trop. Med. Hyg. 1996, 90, 596–600. [Google Scholar] [CrossRef]
- Melendez, P.A.; Capriles, V.A. Molluscicidal activity of plants from Puerto Rico. Ann. Trop. Med. Parasitol. 2002, 96, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.M.S.; Batista, M.M.; Camara, C.A.; Agra, M.F. Molluscicidal activity of some Brazilian Solanum spp. (Solanaceae) against Biomphalaria glabrata. Ann. Trop. Med. Parasitol. 2005, 99, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Barsoum, F.F.; Hosni, H.M.; Girgis, A.S. Novel bis(1-acyl-2-pyrazolines) of potential anti-inflammatory and molluscicidal properties. Bioorg. Med. Chem. 2006, 14, 3929–3937. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, T.P.; Camara, C.A.; Silva, T.M.S.; Martins, R.M.; Pinto, A.C.; Vargas, M.D. New 1,2,3,4-tetrahydro-1-aza-anthraquinones and 2-aminoalkyl compounds from norlapachol with molluscicidal activity. Bioorg. Med. Chem. 2005, 13, 6464–6469. [Google Scholar] [CrossRef] [PubMed]
- Abass, M.; Mostafa, B.B. Synthesis and evaluation of molluscicidal and larvicidal activities of some novel enaminones derived from 4-hydroxyquinolinones: Part IX. Bioorg. Med. Chem. 2005, 13, 6133–6144. [Google Scholar] [CrossRef] [PubMed]
- de Souza, L.C.; dos Santos, A.F.; Sant’Ana, A.E.G.; Imbroisi, D.D. Synthesis and evaluation of the molluscicidal activity of the 5,6-dimethyl-dihydro-pyran-2,4-dione and 6-substituted analogous. Bioorg. Med. Chem. 2004, 12, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Mao, Z.; Yu, P.; Xie, D.; Gao, Q.; Pan, X.; Sun, K. Use of ginkgolic acids in preparing biotic pesticide for killing snails and preventing schistosomiasis. WO 2007095842, 30 August 2007. [Google Scholar]
- Mao, Z.; Yu, P.; Sun, K.; Pan, X.; Jiang, Q.; Pan, J. Preparation of five ginkgolic acid monomers and their molluscicidal effects against Oncomelania hupensis. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2007, 25, 274–278. [Google Scholar] [PubMed]
- Kubo, I.; Komatsu, S.; Ochi, M. Molluscicides from the cashew anacardiumoccidentale and their large-scale isolation. J. Agric. Food Chem. 1986, 34, 970–973. [Google Scholar] [CrossRef]
- Hamada, Y.; Hara, O.; Kawai, A.; Kohno, Y.; Shioiri, T. Efficient total synthesis of AI-77-B, a gastroprotective substance from bacillus pumilus AI-77. Tetrahedron 1991, 47, 8635–8652. [Google Scholar] [CrossRef]
- de Villiers, J.P.; Rossouw, M.M. Strucutre and activity in activity in molluscicides. Nature 1967, 213, 1208–1209. [Google Scholar] [CrossRef]
- Hauser, F.M.; Pogany, S.A. 2-Hydroxy-6-methylbenzoic acid derivatives. Synthesis 1980, 814–815. [Google Scholar] [CrossRef]
- Lazar, C.; Kluczyk, A.; Kiyota, T.; Konishi, Y. Drug evolution concept in drug design: 1. Hybridization method. J. Med. Chem. 2004, 47, 6973–6982. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for the evaluation of plant molluscicides. In Phytolacca dodecandra (endod); Lemma, A., Heyneman, D., Silangwa, S.M., Eds.; Tycooly International: Dublin, Ireland, 1984; pp. 121–124. [Google Scholar]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
Sample Availability: Contact the authors. |

| Compound | LD10 (C.L.)a | LD50 (C.L.)a | LD90 (C.L.)a | |||
| 8 (Z) | 9 (E) | 8 (Z) | 9 (E) | 8 (Z) | 9 (E) | |
| a | 27.2 (9.3–44.1) | 14.6 (7.6–57.8) | 107.2 (32.4–133.8) | 56.1 (16.7–122.6) | 786.6 (322.6–1213.8) | 115.9 (35.1–643.1) |
| b | 33.6 (7.5–75.6) | 22 (6.9–35.3) | 73.1 (48.4–130.4) | 54.2 (21.4–132.2) | 243.6 (134.9–1215) | 214.5 (78.3–462.8) |
| c | 12.5 (4.8–19.6) | 6.5 (0.8–9.1) | 50.9 (6.5–68.7) | 27.5 (14.8–87.1) | 98.2 (25.5–150.6) | 74.3 (34.2–95.0) |
| d | 18.4 (1.7–27.7) | 10.9 (1.6–17.3) | 43.7 (22.9–89.2) | 23.6 (10.5–26.9) | 135.7 (14.2–199.3) | 68.3 (63.4–801.6) |
| e | 11.1 (3.0–17.0) | 7.7 (0.3–15.0) | 56.9 (24.9–63.6) | 31.4 (21.1–80.3) | 362.9 (83.4–595.5) | 88.7 (46.7–121.1) |
| f | 13.3 (0.3–26.6) | 5.5 (1.5–6.2) | 47.2 (31.4–57.7) | 22.4 (4.3–25.3) | 338.7 (82.2–364.6) | 50.9 (23.3–62.8) |
| g | 14.6 (3.3–25.9) | 11.4 (3.6–18.8) | 64.4 (40.1–127.7) | 37.2 (23.9–57.0) | 283.9 (138.6–2001) | 122.0 (74.6–366.6) |
| GA C13:0 * | 1.6 (0.8–8.6) | 29.2 (4.2–60.8) | 64.6 (37.7–180.2) | |||
| Niclosamide | 0.7 (0.3–0.9) | 1.5 (1.1–2.1) | 3.4 (2.3–7.9) | |||
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, P.; Pan, J.; Duan, W.; Li, X.; Zhang, Y.; Zhou, Y.; Jiang, Q.; Mao, Z.; Yu, P. Synthesis of Ginkgolic Acid Analogues and Evaluation of Their Molluscicidal Activity. Molecules 2011, 16, 4059-4069. https://doi.org/10.3390/molecules16054059
Zhang P, Pan J, Duan W, Li X, Zhang Y, Zhou Y, Jiang Q, Mao Z, Yu P. Synthesis of Ginkgolic Acid Analogues and Evaluation of Their Molluscicidal Activity. Molecules. 2011; 16(5):4059-4069. https://doi.org/10.3390/molecules16054059
Chicago/Turabian StyleZhang, Peng, Jiahu Pan, Wanxing Duan, Xuedong Li, Yao Zhang, Yibiao Zhou, Qingwu Jiang, Zuohua Mao, and Peizhong Yu. 2011. "Synthesis of Ginkgolic Acid Analogues and Evaluation of Their Molluscicidal Activity" Molecules 16, no. 5: 4059-4069. https://doi.org/10.3390/molecules16054059
APA StyleZhang, P., Pan, J., Duan, W., Li, X., Zhang, Y., Zhou, Y., Jiang, Q., Mao, Z., & Yu, P. (2011). Synthesis of Ginkgolic Acid Analogues and Evaluation of Their Molluscicidal Activity. Molecules, 16(5), 4059-4069. https://doi.org/10.3390/molecules16054059