Optimization of the Selective Monohydrolysis of Diethyl 4-Aryl-4H-pyran-3,5-dicarboxylates
Abstract
:1. Introduction
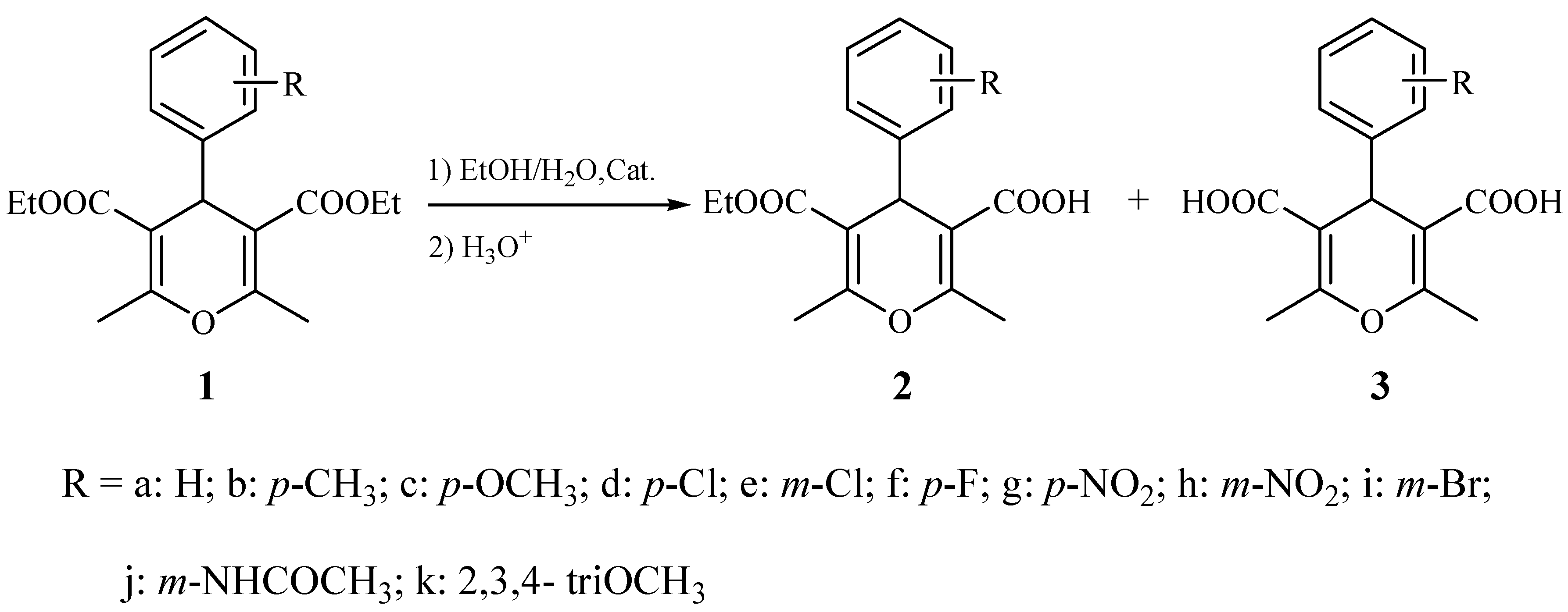
2. Results and Discussion
| Entry | Solvents | Temperature (°C) | Time (h) | Ratio a 2a:3a | Yield b (%) 2a |
|---|---|---|---|---|---|
| 1 | Tetrahydrofuran | 25 | 4.5 | 61:39 | 46 |
| 2 | Tetrahydrofuran | 40 | 5.0 | 70:30 | 50 |
| 3 | Tetrahydrofuran | 55 | 5.0 | 63:37 | 47 |
| 4 | Ethanol | 25 | 4.0 | 78:22 | 62 |
| 5 | Ethanol | 40 | 4.0 | 81:19 | 80 |
| 6 | Ethanol | 55 | 4.0 | 75:25 | 63 |
| 7 | Acetone | 25 | 5.0 | 42:58 | 35 |
| 8 | Acetone | 40 | 5.0 | 50:50 | 40 |
| 9 | Acetone | 55 | 5.0 | 45:55 | 38 |
| 10 | Acetonitrile | 25 | 6.0 | 36:64 | 25 |
| 11 | Acetonitrile | 40 | 5.5 | 41:59 | 32 |
| 12 | Acetonitrile | 55 | 6.0 | 34:66 | 23 |
| Entry | % Water (w/w%) | Time (h) | Ratio a 2a:3a | Yield b (%) 2a |
|---|---|---|---|---|
| 1 | 0 | 12.0 | 46:54 | 35 |
| 2 | 1 | 9.0 | 48:52 | 38 |
| 3 | 5 | 5.5 | 53:47 | 48 |
| 4 | 8 | 5.5 | 74:26 | 65 |
| 5 | 10 | 4.0 | 81:19 | 80 |
| 6 | 15 | 3.5 | 53:47 | 50 |
| 7 | 20 | 3.5 | 36:64 | 28 |
| Entry | NaOH equiv. | Time (h) | Ratio a 2a:3a | Yield b (%) 2a |
|---|---|---|---|---|
| 1 | 0.1 | 10.0 | 51:49 | 34 |
| 2 | 0.3 | 9.5 | 52:48 | 35 |
| 3 | 0.5 | 8.0 | 60:40 | 43 |
| 4 | 1.0 | 6.0 | 73:27 | 70 |
| 5 | 1.2 | 4.0 | 81:19 | 80 |
| 6 | 1.5 | 4.5 | 43:57 | 41 |
| 7 | 2.0 | 3.5 | 25:75 | 23 |
| Entry | Catalyst | Time (h) | Ratio a 2a:3a | Yield b (%) 2a |
|---|---|---|---|---|
| 1 | None | 12.0 | 40:60 | 34 |
| 2 | PEG-400 | 8.0 | 46:54 | 45 |
| 3 | β-CD | 8.5 | 37:63 | 34 |
| 4 | TEAB | 4.0 | 81:19 | 80 |
| 5 | TBAB | 4.0 | 75:25 | 65 |
| Entry | TEAB Equiv. | Time (h) | Ratio a 2a:3a | Yield b(%) 2a |
|---|---|---|---|---|
| 1 | 0.5 | 5.5 | 60:40 | 43 |
| 2 | 1.0 | 4.0 | 81:19 | 80 |
| 3 | 1.5 | 3.5 | 72:28 | 67 |
| 4 | 2.0 | 3.5 | 26:74 | 25 |
| 5 | 2.5 | 3.5 | 10:90 | 9 |
| Substrate | R | Ratio a 2:3 | Yield b(%) | Melting point (°C) |
|---|---|---|---|---|
| 1b | p-CH3 | 72:28 | 69 | 123.6–124.7 |
| 1c | p-OCH3 | 52:48 | 51 | 110.3–112.0 |
| 1d | p-Cl | 69:31 | 66 | 141.8–143.1 |
| 1e | m-Cl | 58:42 | 54 | 132.1–133.4 |
| 1f | p-F | 75:25 | 69 | 137.4–139.1 |
| 1g | p-NO2 | 50:50 | 49 | 110.1–111.7 |
| 1h | m-NO2 | 46:54 | 40 | 121.4–121.9 |
| 1i | m-Br | 65:35 | 62 | 161.4–162.7 |
| 1j | m-NHCOCH3 | 20:80 | 20 | 181.2–183.2 |
| 1k | 2,3,4-triOCH3 | 45:55 | 42 | 149.1–150.4 |
3. Experimental
3.1. General
3.2. General Procedure for the Synthesis of Compounds 2
4. Conclusions
Acknowledgements
References
- Zamocka, J.; Misikova, E.; Durinda, J. Preparation, structure elucidation and activity of some [(5-hydroxy- or 5-methoxy-4-oxo-4H-pyran-2-yl) methyl]-2-alkoxycarbanilates. Pharmazie 1991, 46, 610. [Google Scholar]
- Wang, J.L.; Liu, D.; Zhang, Z.J.; Shan, S.; Han, X.; Srinivasula, S.M.; Croce, C.M.; Alnemri, E.S.; Huang, Z. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc. Natl. Acad. Sci. USA 2000, 97, 7124–7129. [Google Scholar]
- Hatakeyama, S.; Ochi, N.; Numata, H.; Takano, S. A new route to substituted 3-methoxycarbonyldihydropyrans; enatioselective synthesis of (−)-methyl elenolate. J. Chem. Soc. Chem. Commun. 1988, 17, 1202–1204. [Google Scholar]
- González, R.; Martín, N.; Seoane, C.; Marco, J.L.; Albert, A.; Cano, F.H. The first asymmetric synthesis of polyfunctionalized 4H-pyrans via Michael addition of malononitrile to 2-acyl acrylates. Tetrahedron Lett. 1992, 33, 3809–3812. [Google Scholar]
- Li, A.H.; Ji, X.D.; Kim, H.S.; Melman, N.; Jacobson, K.A. Pyran template approach to the design of novel A3 adenosine receptor antagonists. Drug Dev. Res. 1999, 48, 171–177. [Google Scholar] [CrossRef]
- Urbahns, K.; Horvath, E.; Stasch, J.P.; Mauler, F. 4-Phenyl-4H-pyrans as IKCa channel blockers. Bioorg. Med. Chem. Lett. 2003, 13, 2637–2639. [Google Scholar] [CrossRef]
- Witte, E.C.; Neubert, P.; Roesch, A. 7-(Piperazinylpropoxy)-2H-1-benzopyran-2-ones. DE patent 3427985 1986. [Google Scholar]
- Yan, H.; Ni, C.L.; Wang, H.Q. Preparation of 3,9-dioxapentacyclo[6.4.0.02,7.04,11.05,10] dodecane derivatives as anti-AIDS agents. CN patent 101041662, 2007. [Google Scholar]
- Ni, C.L.; Song, X.H.; Yan, H.; Song, X.Q.; Zhong, R.G. Improved synthesis of diethyl 2,6-dimethyl-4-aryl-4H-pyran-3,5-dicarboxylate under ultrasound irradiation. Ultrason. Sonochem. 2010, 17, 367–369. [Google Scholar] [CrossRef]
- Iding, H.; Wirz, B.; Rosa-Maria, R.S. Chemoenzymatic preparation of non-racemic N-Boc-piperidine-3,5-dicarboxylic acid 3-methyl esters and their 5-hydroxymethyl derivatives. Tetrahedron: Asymmetry 2003, 14, 1541–1545. [Google Scholar] [CrossRef]
- Guanti, G.; Narisano, E.; Podgorski, T.; Thea, S.; Williams, A. Enzyme catalyzed monohydrolysis of 2-aryl-1,3-propanediol diacetates. A study of structural effects of the aryl moiety on the enantioselectivity. Tetrahedron 1990, 46, 7081–7092. [Google Scholar] [CrossRef]
- Guanti, G.; Banfi, L.; Narisano, E. Enzymes in organic synthesis: Remarkable influence of a π system on the enantioselectivity in PPL catalyzed monohydrolysis of 2-substituted 1,3-diacetoxypropanes. Tetrahedron: Asymmetry 1990, 1, 721–724. [Google Scholar] [CrossRef]
- Rodrigues, D.S.; Mendes, A.A.; Filice, M.; Roberto, F.L.; Guisan, J.M.; Palomo, J.M. Different derivatives of a lipase display different regioselectivity in the monohydrolysis of per-O-acetylated 1-O-substituted-β-galactopyranosides. J. Mol. Catal. B: Enzym. 2009, 58, 36–40. [Google Scholar] [CrossRef]
- Niwayama, S.; Wang, H.Z.; Hiraga, Y.; Clayton, J.C. Influence of co-solvents in the highly efficient selective monohydrolysis of a symmetric diester. Tetrahedron Lett. 2007, 48, 8508–8510. [Google Scholar] [CrossRef]
- Satomi, N.; Hanjoung, C. Practical large scale synthesis of half-esters of malonic acid. Chem. Pharm. Bull. 2009, 57, 508–510. [Google Scholar] [CrossRef]
- Satomi, N.; Hanjoung, C.; Masoud, Z.M.; Bruce, R.M. Remote exo/endo selectivity in selective monohydrolysis of dialkyl bicyclo[2.2.1]heptane-2,3-dicarboxylate derivatives. J. Org. Chem. 2010, 75, 3775–3780. [Google Scholar] [CrossRef]
- Niwayama, S. Highly efficient selective monohydrolysis of symmetric diesters. J. Org. Chem. 2000, 65, 5834–5836. [Google Scholar] [CrossRef]
- Chary, M.V.; Keerthysri, N.C.; Vupallapati, S.V.N.; Lingaiah, N.; Kantevari, S. Tetrabutylammonium bromide (TBAB) in isopropanol: An efficient, novel, neutral and recyclable catalytic system for the synthesis of 2,4,5-trisubstituted imidazoles. Catal.Commun. 2008, 9, 2013–2017. [Google Scholar] [CrossRef]
- Bhalerao, D.S.; Mahajan, U.S.; Chaudhari, K.H.; Akamanchi, K.G. O-Iodoxybenzoic acid- and tetraethylammonium bromide-mediated oxidative transformation of primary carboxamides to one-carbon dehomologated nitriles. J. Org. Chem. 2007, 72, 662–665. [Google Scholar] [CrossRef]
- Uchiyama, Y.; Fujinami, M.; Sawada, T.; Lsao, T. Observation of dynamic molecular behavior in a phase transfer catalytic reaction at a liquid/liquid interface by using the time-resolved quasi-elastic laser scattering method. J. Phys. Chem. B 2000, 104, 4699–4702. [Google Scholar]
- Wu, W.J.; Ding, H.Y.; Li, Q.R. Synthesis of 2,6-dimethyl-4-(3-nitrophenyl)-5-methoxycarbonyl-1,4-dihydropyridine-3-carboxylic acid. J. Guangdong Pharm. 2008, 24, 493–495. [Google Scholar]
- Hasegawa, T.; Yamamoto, H. A selective partial hydrolysis of dimenthyl ester using dry tetrabutylammonium hydroxide. Synlett 1999, 1, 84–86. [Google Scholar] [CrossRef]
- Sirovski, F.; Gorokhova, M.; Ruban, S. Phase-transfer catalysis: kinetics and mechanism of dichlorocyclopropane formation in liquid/liquid and solid/liquid systems. J. Mol. Catal. A-Chem. 2003, 197, 213–222. [Google Scholar] [CrossRef]
- Lygo, B.; Crosby, J.; Lowdon, T.R.; Peterson, J.A.; Wainwright, P.G. Studies on the enantioselective synthesis of α-amino acids via asymmetric phase-transfer catalysis. Tetrahedron 2001, 57, 2403–2409. [Google Scholar] [CrossRef]
- Samples Availability: Sample of compounds 2a–2k are available from authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Duan, J.; Song, X.; Yan, H.; Song, X. Optimization of the Selective Monohydrolysis of Diethyl 4-Aryl-4H-pyran-3,5-dicarboxylates. Molecules 2011, 16, 3845-3854. https://doi.org/10.3390/molecules16053845
Duan J, Song X, Yan H, Song X. Optimization of the Selective Monohydrolysis of Diethyl 4-Aryl-4H-pyran-3,5-dicarboxylates. Molecules. 2011; 16(5):3845-3854. https://doi.org/10.3390/molecules16053845
Chicago/Turabian StyleDuan, Jiaojiao, Xiaohui Song, Hong Yan, and Xiuqing Song. 2011. "Optimization of the Selective Monohydrolysis of Diethyl 4-Aryl-4H-pyran-3,5-dicarboxylates" Molecules 16, no. 5: 3845-3854. https://doi.org/10.3390/molecules16053845
APA StyleDuan, J., Song, X., Yan, H., & Song, X. (2011). Optimization of the Selective Monohydrolysis of Diethyl 4-Aryl-4H-pyran-3,5-dicarboxylates. Molecules, 16(5), 3845-3854. https://doi.org/10.3390/molecules16053845




