Screening for Compounds with Aromatase Inhibiting Activities from Atractylodes macrocephala Koidz
Abstract
:1. Introduction
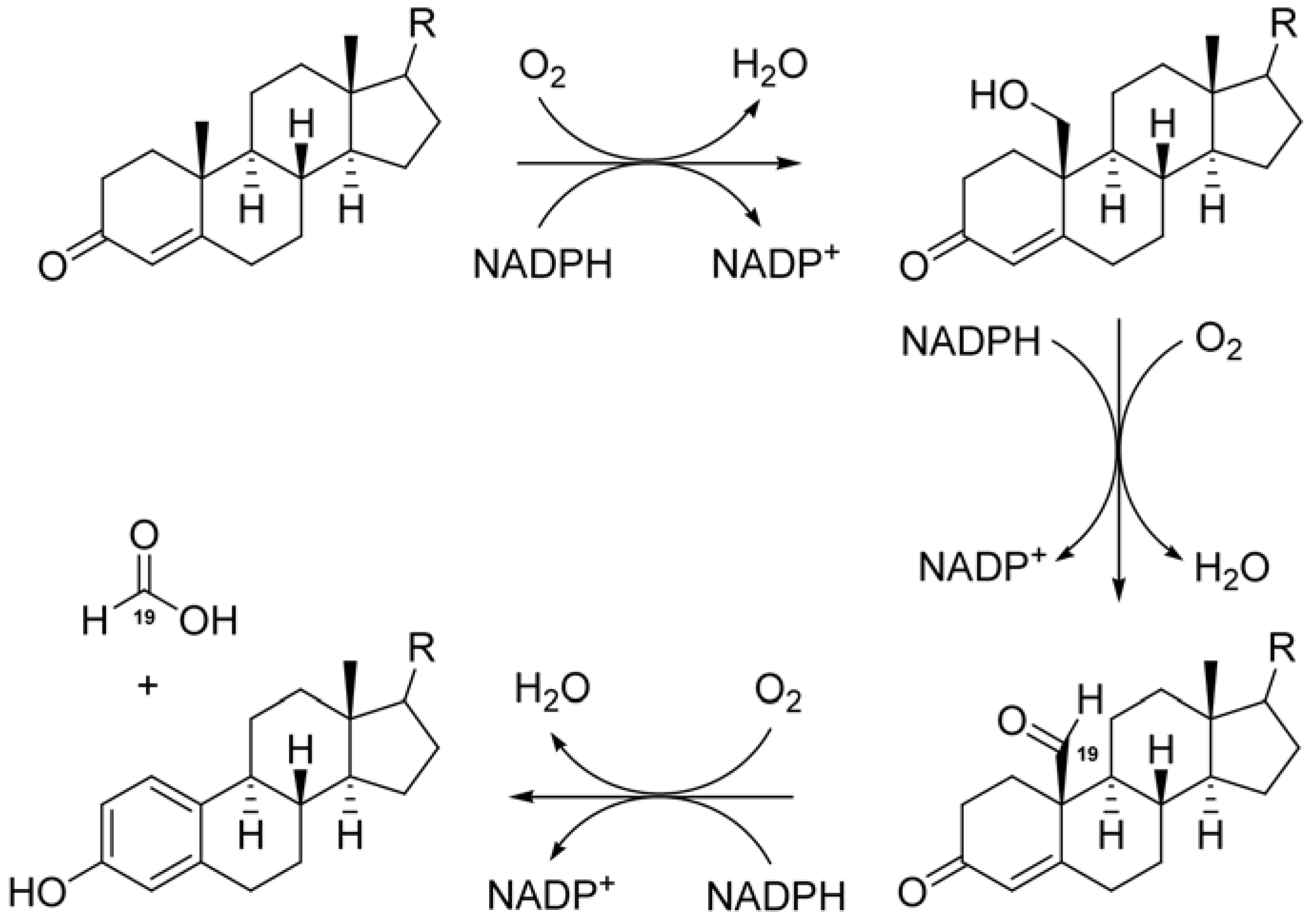
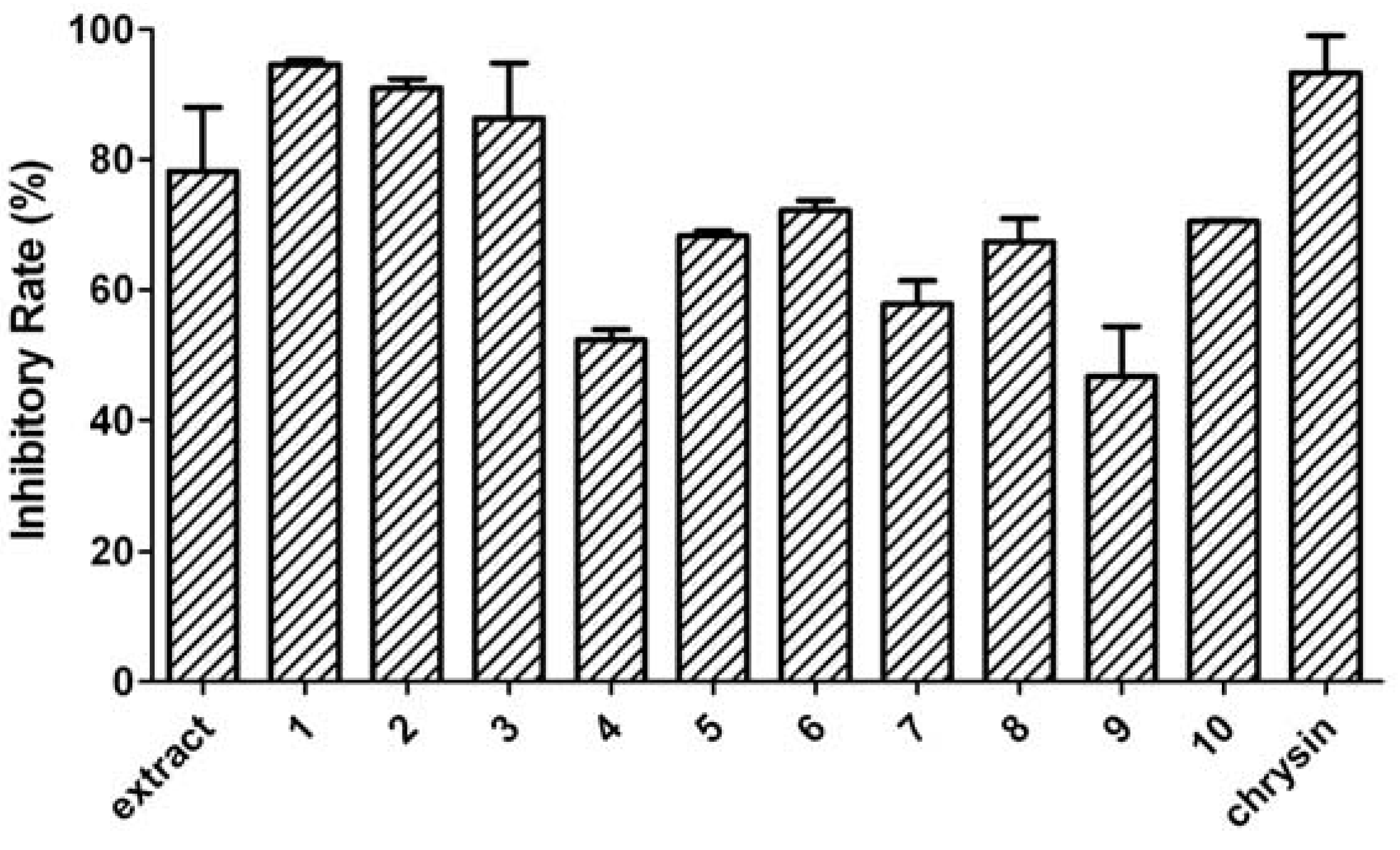
2. Results and Discussion
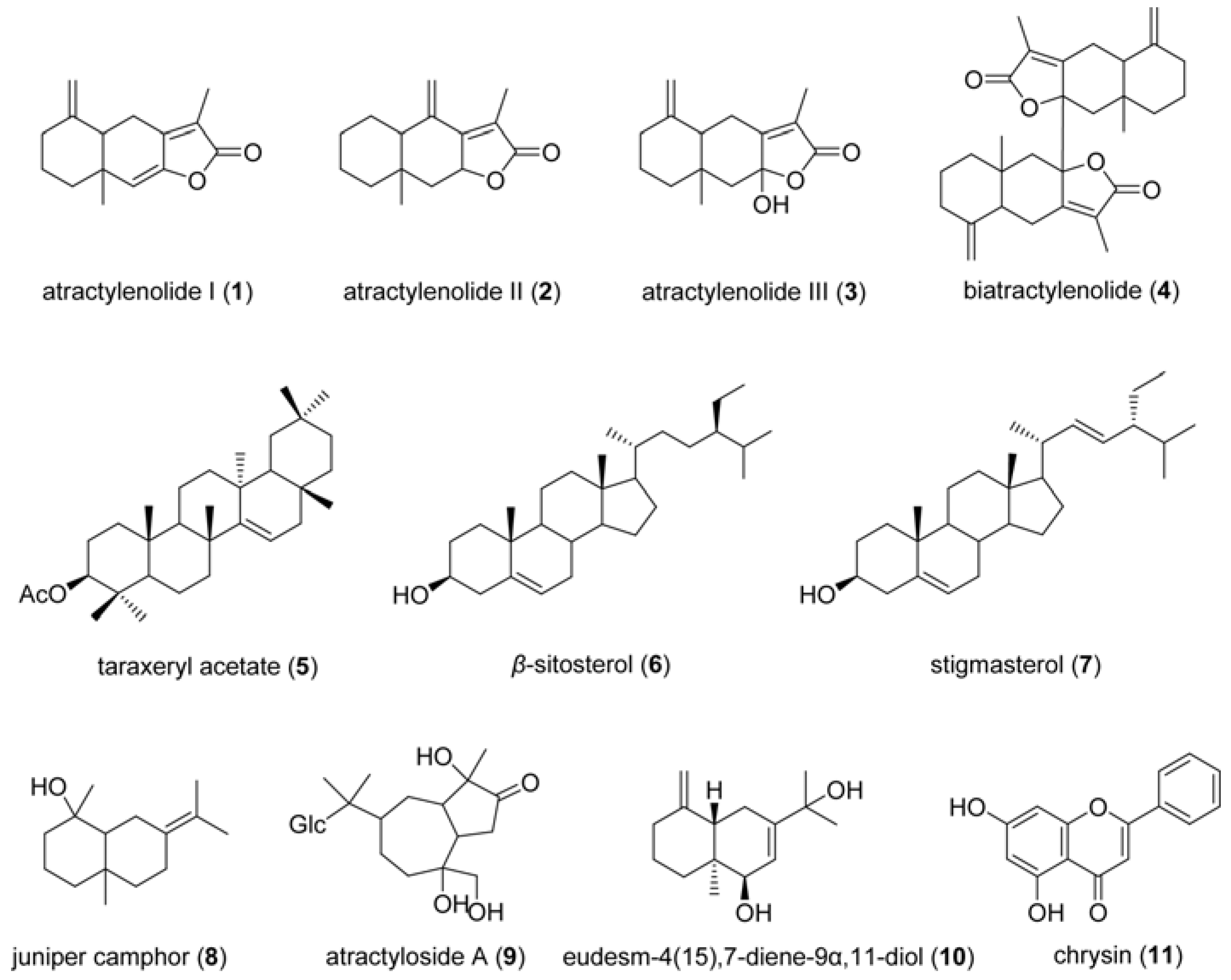
3. Experimental
3.1. General
3.2. Plant materials
3.3. Extraction and isolation
3.4. Aromatase inhibition assay
4. Conclusions
Acknowledgements
References
- Brueggemeier, R.W.; Richards, J.A.; Joomprabutra, S.; Bhat, A.S.; Whetstone, J.L.J. Molecular pharmacology of aromatase and its regulation by endogenous and exogenous agents. J. Steroid Biochem. Mol. Biol. 2001, 79, 75–84. [Google Scholar] [CrossRef]
- Leonetti, F.; Favia, A.; Rao, A.; Aliano, R.; Paluszcak, A.; Hartmann, R.W.; Carotti, A. Design, synthesis, and 3D QSAR of novel potent and selective aromatase inhibitors. J. Med. Chem. 2004, 47, 6792–6803. [Google Scholar] [CrossRef]
- Lonard, D.M.; Smith, C.L. Molecular perspectives on selective estrogen receptor modulators (SERMs): Progress in understanding their tissue-specific agonist and antagonist actions. Steroids 2002, 67, 15–24. [Google Scholar] [CrossRef]
- Park, W.C.; Jordan, V.C. Selective estrogen receptor modulators (SERMS) and their roles in breast cancer prevention. Trends Mol. Med. 2002, 8, 82–88. [Google Scholar] [CrossRef]
- Ghosh, D.; Griswold, J.; Erman, M.; Pangborn, W. Structural basis for androgen specificity and oestrogen synthesis in human aromatase. Nature 2009, 457, 219–223. [Google Scholar]
- Santen, R.J.; Samojlik, E.; Lipton, A.; Harvey, H.; Ruby, E.B.; Wells, S.A.; Kendall, J. Kinetic, hormonal and clinical studies with aminoglutethimide in breast-cancer. Cancer 1977, 39, 2948–2958. [Google Scholar] [CrossRef]
- O’Reilly, J.M.; Brueggemeier, R.W. 7α-Arylaliphatic androsta-1,4-diene-3,17-diones as enzyme-activated irreversible inhibitors of aromatase. J. Steroid Biochem. Mol. Biol. 1996, l59, 93–102. [Google Scholar]
- Chen, Z.L. The acetylenes from Atractylodes macrocephala. Planta Med. 1987, 53, 493–494. [Google Scholar] [CrossRef]
- Endo, K.; Taguchi, T.; Taguchi, F.; Hikino, H.; Yamahara, J.; Fujimura, H. Anti-inflammatory principles of Atractylodes rhizomes. Chem. Pharm. Bull. 1979, 27, 2954–2958. [Google Scholar] [CrossRef]
- Huang, B.S.; Sun, J.S.; Chen, Z.L. Isolation and identification of atractylenolide VI from Atractylodes macrocephala Koidz. Acta Bot. Sin. 1992, 34, 614–617. [Google Scholar]
- Wang, H.X.; Liu, C.M.; Liu, Q.; Gao, K. Three types of sesquiterpenes from rhizomes of Atractylodes lancea. Phytochemistry 2008, 69, 2088–2094. [Google Scholar] [CrossRef]
- Li, C.Q.; He, L.C.; Dong, H.Y.; Jin, J.Q. Screening for the anti-inflammatory activity of fractions and compounds from Atractylodes macrocephala koidz. J. Ethnopharmacol. 2007, 114, 212–217. [Google Scholar] [CrossRef]
- Dong, H.Y.; He, L.C.; Huang, M.; Dong, Y.L. Anti-inflammatory components isolated from Atractylodes macrocephala Koidz. Nat. Prod. Res. 2008, 22, 1418–1427. [Google Scholar] [CrossRef]
- Peng, W.; Han, T.; Wang, Y.; Xin, W.B.; Zheng, C.J.; Qin, L.P. Chemical constituents of the aerial part of Atractylodes macrocephala. Chem. Nat. Compd. 2011, 46, 959–960. [Google Scholar] [CrossRef]
- Endringer, D.C.; Guimaraes, K.G.; Kondratyuk, T.P.; Pezzuto, J.M.; Braga, F.C. Selective inhibition of aromatase by a dihydroisocoumarin from Xyris pterygoblephara. J. Nat. Prod. 2008, 71, 1082–1084. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1-10 are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jiang, H.; Shi, J.; Li, Y. Screening for Compounds with Aromatase Inhibiting Activities from Atractylodes macrocephala Koidz. Molecules 2011, 16, 3146-3151. https://doi.org/10.3390/molecules16043146
Jiang H, Shi J, Li Y. Screening for Compounds with Aromatase Inhibiting Activities from Atractylodes macrocephala Koidz. Molecules. 2011; 16(4):3146-3151. https://doi.org/10.3390/molecules16043146
Chicago/Turabian StyleJiang, Hai, Jing Shi, and Yuanyuan Li. 2011. "Screening for Compounds with Aromatase Inhibiting Activities from Atractylodes macrocephala Koidz" Molecules 16, no. 4: 3146-3151. https://doi.org/10.3390/molecules16043146
APA StyleJiang, H., Shi, J., & Li, Y. (2011). Screening for Compounds with Aromatase Inhibiting Activities from Atractylodes macrocephala Koidz. Molecules, 16(4), 3146-3151. https://doi.org/10.3390/molecules16043146



