Lancifoliaine, a New Bisbenzylisoquinoline from the Bark of Litsea lancifolia
Abstract
:1. Introduction
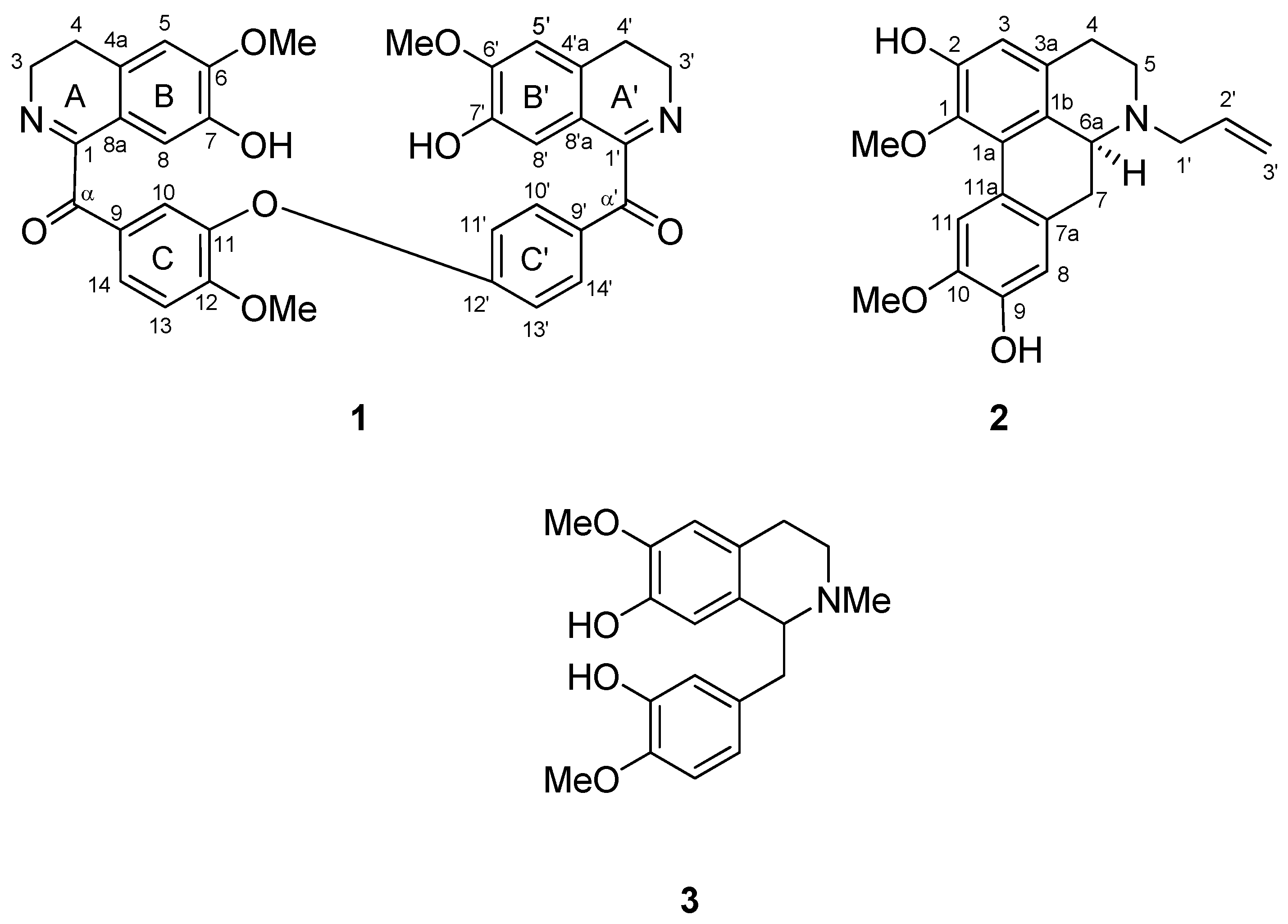
2. Results and Discussion
| Position | 1H (δH, J, Hz) | 13C (δC) | Position | 1H (δH, J, Hz) | 13C (δC) |
| 1 | - | 165.1 | 1' | - | 164.9 |
| 3 | 3.88, m | 47.3 | 3' | 3.88, m | 47.3 |
| 4 | 2.76, m | 25.5 | 4' | 2.76, m | 25.5 |
| 4a | - | 130.2 | 4'a | - | 130.2 |
| 5 | 6.69, s | 110.1 | 5' | 6.71, s | 110.1 |
| 6 | - | 149.5 | 6' | - | 149.4 |
| 6-OMe | 3.92, s | 56.3 | 6'-OMe | 3.91, s | 56.1 |
| 7 | - | 144.4 | 7' | - | 144.4 |
| 8 | 6.89, s | 113.1 | 8' | 6.88, s | 112.2 |
| 8a | - | 119.9 | 8'a | - | 119.9 |
| α | - | 191.9 | α' | - | 192.7 |
| 9 | - | 129.8 | 9' | - | 129.8 |
| 10 | 7.71, d, J = 2.0 Hz | 124.4 | 10' | 7.95, dd, J = 8.8, 2.0 Hz | 132.7 |
| 11 | - | 143.0 | 11' | 6.86, d, J = 8.8 Hz | 116.1 |
| 12 | - | 156.6 | 12' | - | 162.6 |
| 12-OMe | 3.84, s | 56.3 | |||
| 13 | 7.03, d, J = 8.8 Hz | 112.2 | 13' | 6.89, d, J = 8.8 Hz | 116.1 |
| 14 | 7.95, dd, J = 8.8, 2.0 Hz | 132.7 | 14' | 7.95, dd, J = 8.8, 2.0 Hz | 132.7 |
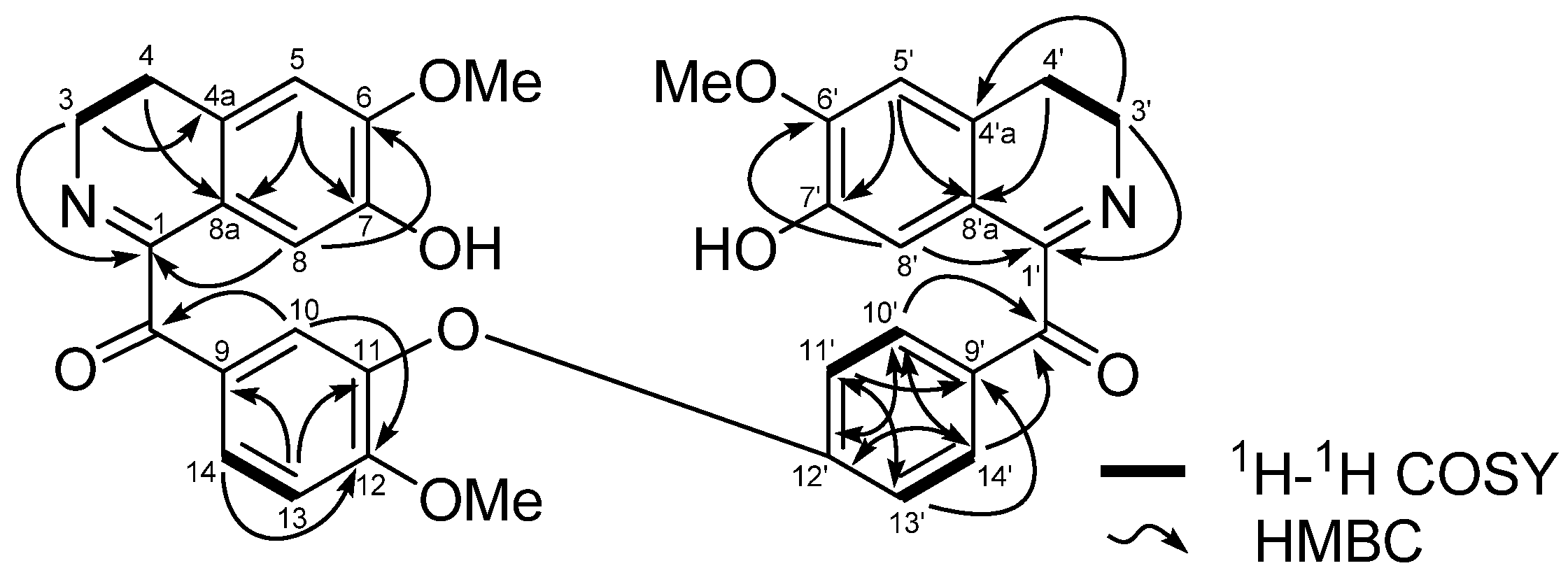
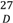 = +33.9º (c 1.0, MeOH) was isolated as a brownish amorphous solid. The LCMS-IT-TOF spectrum of 2 showed [M+H]+ peak at m/z 354.1823, corresponding to the molecular formula of C21H24NO4. 1H-NMR data of a synthetic compound of 2 were reported previously and we report herein the complete 13C-NMR assignments of 2, which were established by thorough analysis of DEPT, HSQC and HMBC spectra [6]. This is the first communication on N-allyllaurolitsine as a natural compound and the 13C-NMR data is listed in Table 2.
= +33.9º (c 1.0, MeOH) was isolated as a brownish amorphous solid. The LCMS-IT-TOF spectrum of 2 showed [M+H]+ peak at m/z 354.1823, corresponding to the molecular formula of C21H24NO4. 1H-NMR data of a synthetic compound of 2 were reported previously and we report herein the complete 13C-NMR assignments of 2, which were established by thorough analysis of DEPT, HSQC and HMBC spectra [6]. This is the first communication on N-allyllaurolitsine as a natural compound and the 13C-NMR data is listed in Table 2.| Position | *1H (δH) | 13C (δC) |
| 1 | - | 142.2 |
| 1a | - | 126.3 |
| 1b | - | 127.3 |
| 1-OMe | 3.56, s | 60.3 |
| 2 | - | 148.2 |
| 3 | - | 113.3 |
| 3a | - | 130.4 |
| 4 | - | 28.8 |
| 5 | - | 49.1 |
| 6a | - | 59.7 |
| 7 | - | 33.9 |
| 7a | - | 130.4 |
| 8 | 6.81, s | 114.2 |
| 9 | - | 145.2 |
| 10 | - | 145.8 |
| 10-OMe | 3.90, s | 56.2 |
| 11 | 7.86, s | 110.1 |
| 11a | - | 123.8 |
| 1' (N-CH2CH=CH2) | 3.05, br d, J = 6.6 Hz | 57.3 |
| 2' (N-CH2CH=CH2) | 5.96, ddt, J = 17.2, 10.1, 6.6 Hz | 134.3 |
| 3' | ||
| (N-CH2CH=CHE HZ) | 5.26, br d, J = 17.2 Hz | 118.5 |
| (N-CH2CH=CHEHZ) | 5.19, br d, J = 10.1 Hz |
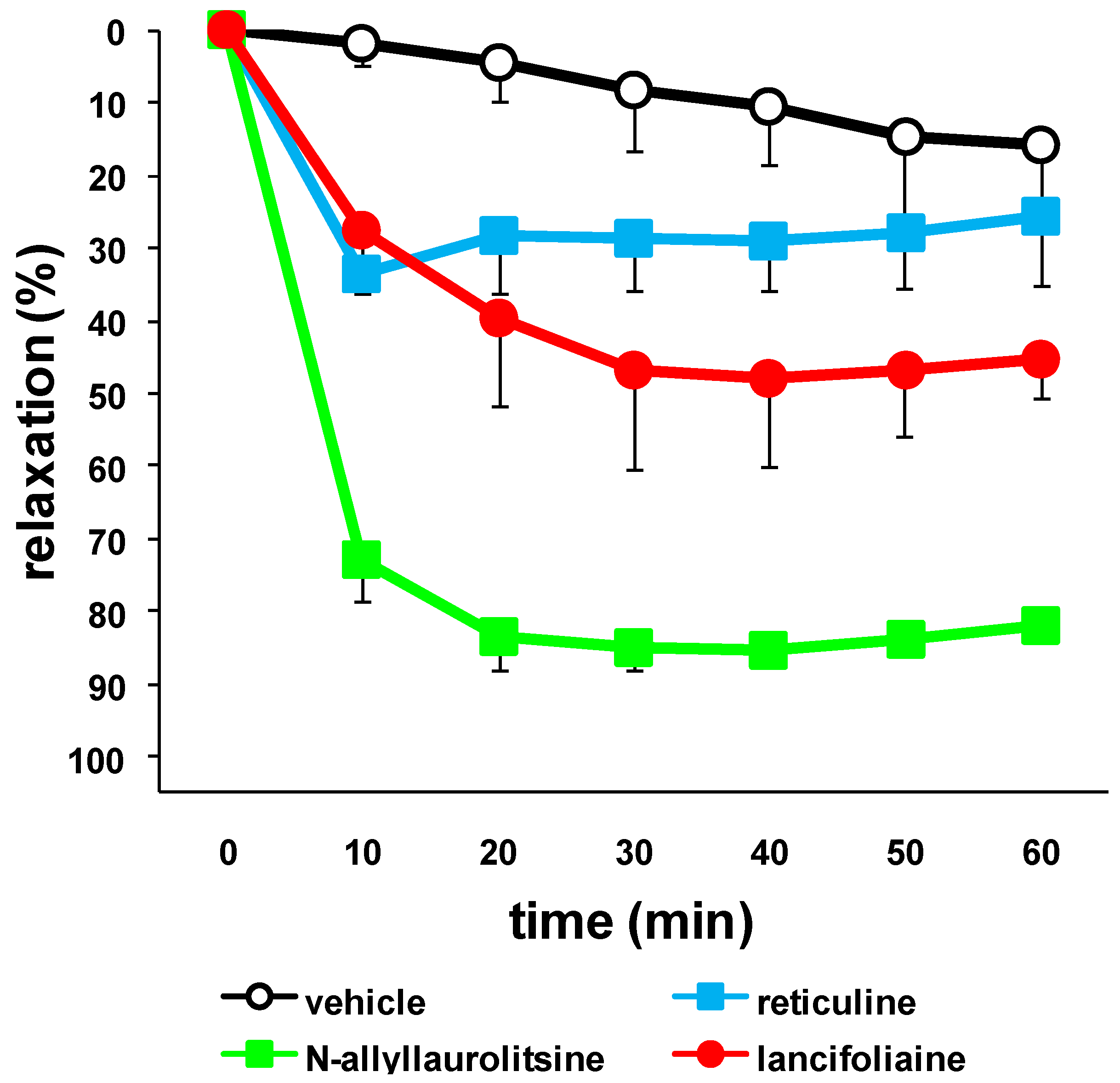
3. Experimental
3.1. General
3.2. Plant material
3.3. Extraction and Isolation
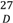 = +33.9º (c=1.0, MeOH), LCMS-IT-TOF at m/z 354.1823 ([M+H]+; calcd. for C21H24NO4, 354.1705); UV (MeOH) 307 nm; IR (CHCl3) λmax: 3584, 3372, 2955, 2352 and 1652 cm−1; 1H and 13C-NMR: see Table 2.
= +33.9º (c=1.0, MeOH), LCMS-IT-TOF at m/z 354.1823 ([M+H]+; calcd. for C21H24NO4, 354.1705); UV (MeOH) 307 nm; IR (CHCl3) λmax: 3584, 3372, 2955, 2352 and 1652 cm−1; 1H and 13C-NMR: see Table 2.3.4. Vasodilation Assay
4. Conclusions
Acknowledgements
References and Notes
- Whitmore, T.C. Tree Flora of Malaya-A Manual for Foresters; Longman: Kuala Lumpur, Malaysia, 1972; Volume 1, p. 98. [Google Scholar]
- Mukhtar, M.R.; Martin, M.T.; Domansky, M.; Pais, M.; Awang, K.; Hadi, A.H.A. Phoebegrandines A and B, proaporphine-tryptamine dimers, from Phoebe grandis. Phytochemistry 1997, 45, 1543–1546. [Google Scholar] [CrossRef]
- Awang, K.; Mukhtar, M.R.; Mustafa, M.R.; Litaudon, M.; Shaari, K.; Mohamad, K.; Hadi, A.H.A. New Alkaloids from Phoebe scortechinii. Nat. Prod. Res. 2007, 21, 704–709. [Google Scholar] [CrossRef]
- Mukhtar, M.R.; Nafiah, M.A.; Awang, K.; Zaima, K.; Morita, H.; Hadi, A.H.A. α’-Oxoperakensimines A – C, New Bisbenzylisoquinoline from Alseodaphne perakensis (Gamble) Kosterm. Heterocycles 2009, 78, 2085–2092. [Google Scholar] [CrossRef]
- Mukhtar, M.R.; Zahari, A.; Nafiah, M.A.; Hadi, A.H.A.; Thomas, N.F.; Arai, H.; Morita, H.; Litaudon, M.; Awang, K. 3’,4’-Dihydronorstephasubine, A New Bisbenzylisoquinoline from the Bark of Alseodaphne corneri. Heterocycles 2009, 78, 2571–2578. [Google Scholar] [CrossRef]
- Chiou, C.M.; Kang, J.J.; Lee, S.S. Litebamine N-Homologues: Preparation and Anti-Acetylcholinesterase Activity. J. Nat. Prod. 1998, 61, 46–50. [Google Scholar] [CrossRef]
- Cava, M.P.; Rao, K.V.; Douglas, B.; Weisbach, J.A. Alkaloids of Cassytha americana. J. Org. Chem. 1968, 33, 2443–2446. [Google Scholar] [CrossRef]
- Oscar Castro, C.; José López, V.; Armando Vergara, G. Aporphine Alkaloids from Phoebe pittieri. Phytochemistry 1985, 24, 203–204. [Google Scholar]
- Janssen, R.H.A.M.; Wijkens, P.; Kruk, C.; Biessels, H.W.A.; Menichini, F.; Theuns, H.G. Assignments of 1H and 13C NMR Resonances of Some Isoquinoline Alkaloids. Phytochemistry 1990, 29, 3331–3339. [Google Scholar] [CrossRef]
- Guinaudeau, H.; Leboeuf, M.; Cavé, A. Aporphine Alkaloids. II. J. Nat. Prod. 1979, 42, 325–360. [Google Scholar] [CrossRef]
- Tewari, S.; Bhakuni, D.S.; Dhar, M.M. The Aporphine Alkaloids of Litsea glutenosa. Phytochemistry 1972, 11, 1149–1152. [Google Scholar] [CrossRef]
- Kozuka, M.; Miyazawa, S.; Yokoyama, K.; Odani, T.; Kubo, M. Alkaloids from Lindera umbellata, Lindera sericea, and Their Varieties. J. Nat. Prod. 1985, 48, 160–161. [Google Scholar] [CrossRef]
- Ross, S.A.; Minard, R.D.; Shamma, M.; Fagbule, M.O.; Olatunji, G.; Gbile, Z. Thaliporphinemethine: A New Phenanthrene Alkaloid from Illigera pentaphylla. J. Nat. Prod. 1985, 48, 835–836. [Google Scholar] [CrossRef]
- Guinaudeau, H.; Leboeuf, M.; Cavé, A. Aporphinoid Alkaloids, III. J. Nat. Prod. 1983, 46, 761–835. [Google Scholar] [CrossRef]
- Shamma, M.; Chinnasamy, P.; Hussain, S.F.; Khan, F. Norpallidine, A New Morphinandienone Alkaloid from Fumaria vaillantii. Phytochemistry 1976, 15, 1802–1803. [Google Scholar] [CrossRef]
- Tojo, E.; Dominguez, D.; Castedo, L. O-Methylpallidine N-oxide, the First Morphinandienone N-oxide Alkaloid. J. Nat. Prod. 1989, 52, 415–416. [Google Scholar] [CrossRef]
- Gözler, B.; Öziç, P.; Freyer, A.J.; Shamma, M. Morphinandienone Alkaloids from Roemeria refracta. J. Nat. Prod. 1990, 53, 986–988. [Google Scholar]
- Schiff, P.L. Bisbenzylisoquinoline Alkaloids. J. Nat. Prod. 1991, 54, 645–749. [Google Scholar]
- Uprety, H.; Bhakuni, D.S.; Dhar, M.M. Aporphine Alkaloids of Litsea sebifera, L. wightiana and Actinodaphne obovata. Phytochemistry 1972, 11, 3057–3059. [Google Scholar] [CrossRef]
- Leboeuf, M.; Caré, A.; Tohami, M.E.; Pusset, J.; Forgacs, P.; Provost, J. Alkaloids of Annonaceae. XXXV. Alkaloids of Desmos tiebaghiensis. J. Nat. Prod. 1982, 45, 617–662. [Google Scholar] [CrossRef]
- Chowdhury, B.K.; Sethi, M.L.; Lloyd, H.A.; Kapadia, G.J. Aporphine and Tetrahydrobenzylisoquinoline Alkaloids in Sassafras albidum. Phytochemistry 1976, 15, 1803–1804. [Google Scholar]
- Patra, A.; Freyer, A.J.; Guinaudeau, H.; Shamma, M.; Tantisewie, B.; Pharadai, K. The Bisbenzylisoquinoline Alkaloids of Stephania suberosa. J. Nat. Prod. 1986, 49, 424–427. [Google Scholar]
- Diego, C.; Henry, D.; Rose, L.R.P.; Alaide, B.O. Nouveaux Alcaloides Bis-benzylisoquinoleiques Isoles des Feuilles de Aristolochia gigantean. J. Nat. Prod. 1987, 50, 910–914. [Google Scholar] [CrossRef]
- Pudjiastuti, P.; Mukhtar, M.R.; Hadi, A.H.A.; Saidi, N.; Morita, H.; Litaudon, M.; Awang, K. (6,7-Dimethoxy-4-methylisoquinolinyl)-(4’-methoxyphenyl)-methanone, A New Benzylisoquinoline Alkaloid from Beilschmiedia brevipes. Molecules 2010, 15, 2339–2346. [Google Scholar] [CrossRef]
- Morita, H.; Iizuka, T.; Choo, C.Y.; Chan, K.L.; Takeya, K.; Kobayashi, J. Vasorelaxant Activity of Cyclic Peptide, Cyclosquamosin B, from Annona squamosa. Bioorg. Med. Chem. Lett. 2006, 16, 4609–4611. [Google Scholar] [CrossRef]
- Likhitwitayawaid, K.; Angerhofer, C.K.; Cordell, G.A.; Pezzuto, J.M.; Ruangrungsi, N. Cytotoxic and Antimalarial Bisbenzylisoquinoline Alkaloids from Stephania erecta. J. Nat. Prod. 1993, 56, 30. [Google Scholar] [CrossRef]
- Ke, J.; Weng, S.A.; Zhang, G.Q. Effects of Tetrandrine on Experimental Arrhythmias. Acta Pharmacol. Sin. 1981, 2, 235–237. [Google Scholar]
- Li, N.; Li, Y. Effects of Berbamine on Isolated Myocardium in Guinea Pigs and Humans. Acta Pharmacol. Sin. 1986, 7, 222–226. [Google Scholar]
- Seow, W.K.; Ferrante, A.; Goh, D.B.H.; Chalmers, A.H.; Li, S.; Thong, Y.H. In vitro Immunosuppressive Properties of the Plant Alkaloid Tetrandrine. Int. Arch Allergy Apopl Immunol. 1988, 85, 410–415. [Google Scholar] [CrossRef]
- Marini-Bettolo, G.B.; Chiavarelli, S. Synthesis of bisquinolines derivatives on the model of natural alkaloids. I. Isoquinoline derivatives of diphenyl ether. Ann. Ist. Super. Sanita 1952, 15, 1041–1053. [Google Scholar]
- Sample Availability: Samples of compound 3 are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sulaiman, S.N.; Mukhtar, M.R.; Hadi, A.H.A.; Awang, K.; Hazni, H.; Zahari, A.; Litaudon, M.; Zaima, K.; Morita, H. Lancifoliaine, a New Bisbenzylisoquinoline from the Bark of Litsea lancifolia. Molecules 2011, 16, 3119-3127. https://doi.org/10.3390/molecules16043119
Sulaiman SN, Mukhtar MR, Hadi AHA, Awang K, Hazni H, Zahari A, Litaudon M, Zaima K, Morita H. Lancifoliaine, a New Bisbenzylisoquinoline from the Bark of Litsea lancifolia. Molecules. 2011; 16(4):3119-3127. https://doi.org/10.3390/molecules16043119
Chicago/Turabian StyleSulaiman, Syazreen Nadia, Mat Ropi Mukhtar, A Hamid A Hadi, Khalijah Awang, Hazrina Hazni, Azeana Zahari, Marc Litaudon, Kazumasa Zaima, and Hiroshi Morita. 2011. "Lancifoliaine, a New Bisbenzylisoquinoline from the Bark of Litsea lancifolia" Molecules 16, no. 4: 3119-3127. https://doi.org/10.3390/molecules16043119
APA StyleSulaiman, S. N., Mukhtar, M. R., Hadi, A. H. A., Awang, K., Hazni, H., Zahari, A., Litaudon, M., Zaima, K., & Morita, H. (2011). Lancifoliaine, a New Bisbenzylisoquinoline from the Bark of Litsea lancifolia. Molecules, 16(4), 3119-3127. https://doi.org/10.3390/molecules16043119




