Investigating Spectrum of Biological Activity of 4- and 5-Chloro-2-hydroxy-N-[2-(arylamino)-1-alkyl-2-oxoethyl]benzamides
Abstract
:1. Introduction
2. Results and Discussion
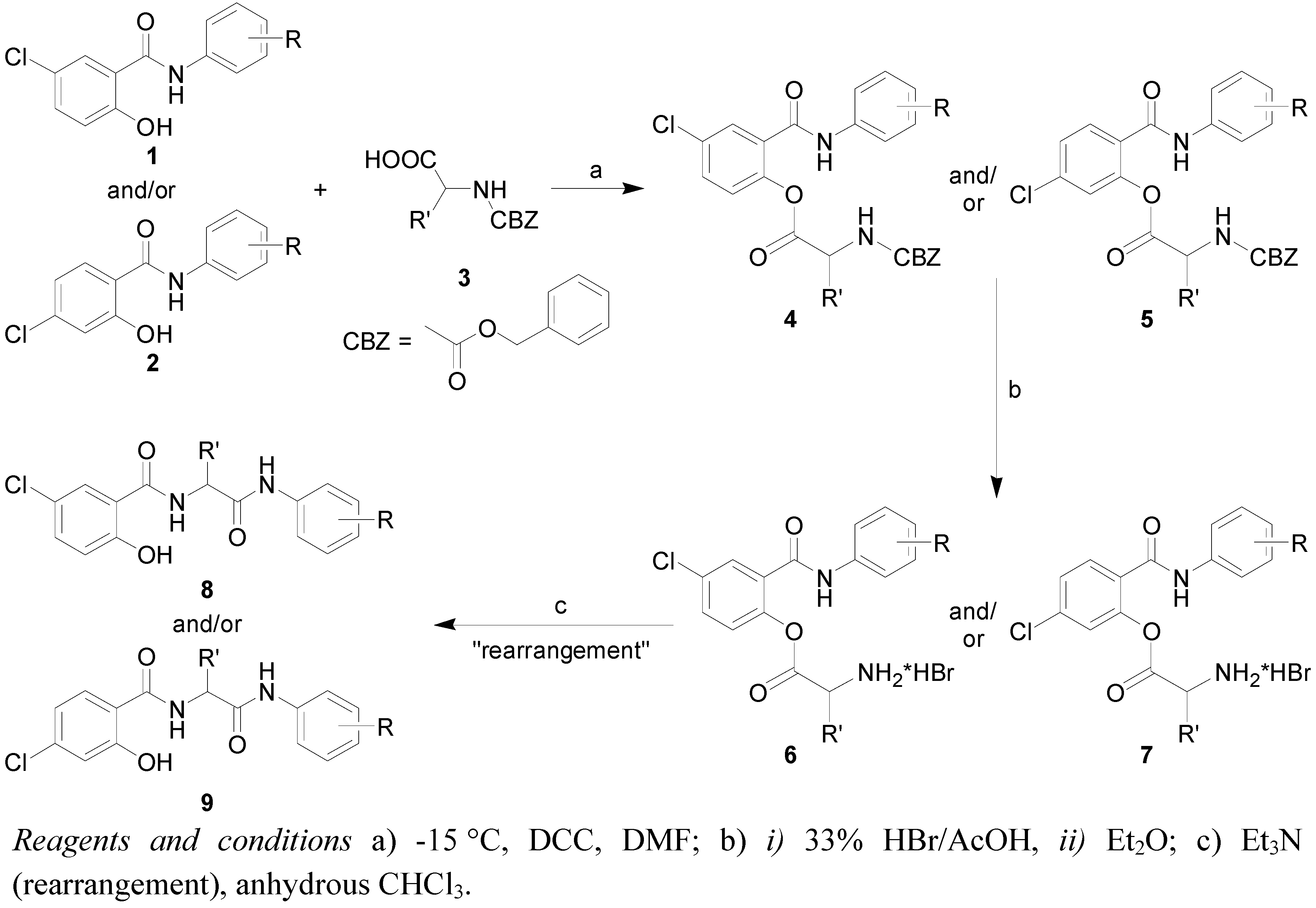
2.1. Chemistry
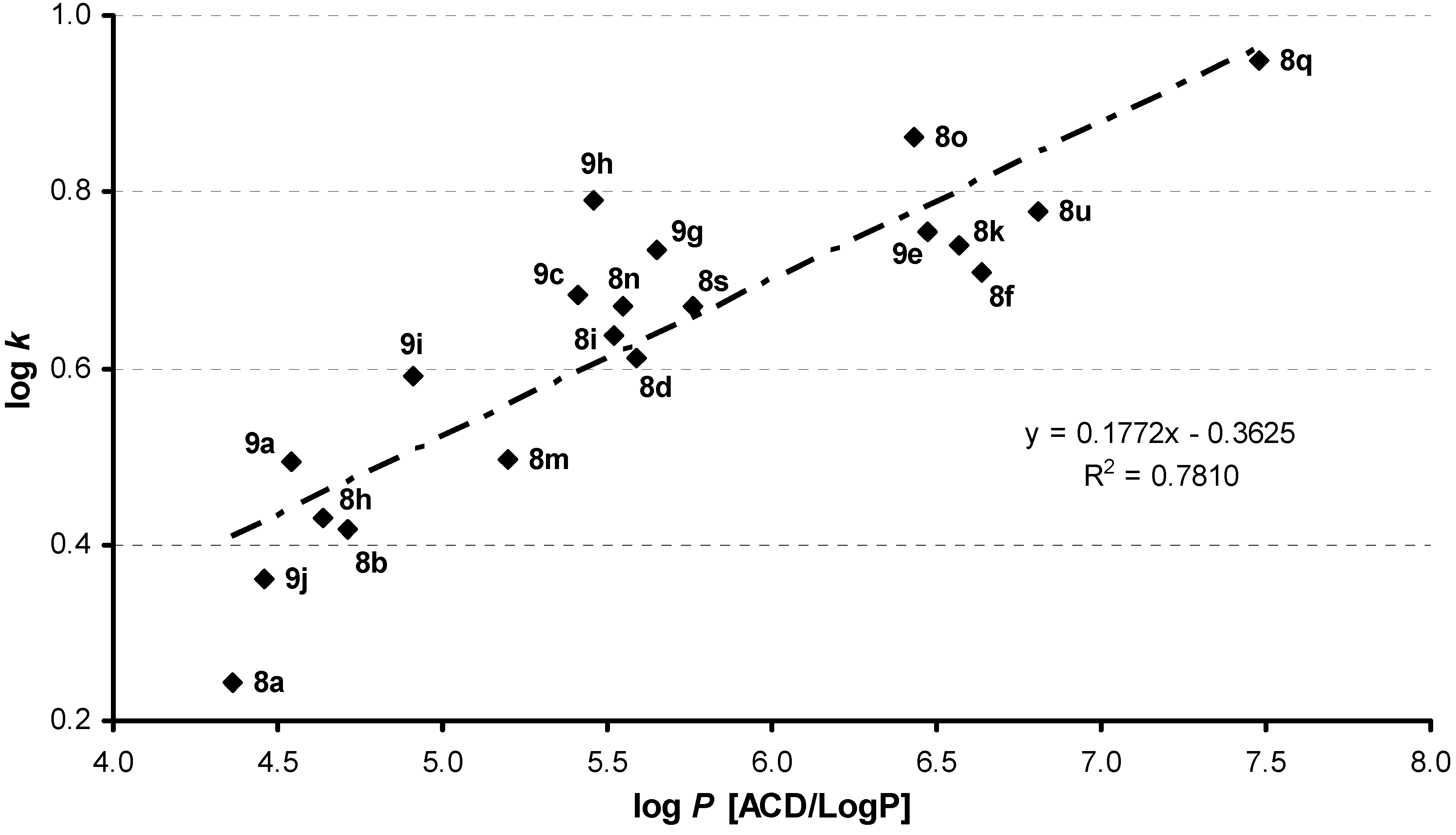
2.2. Lipophilicity
 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Comp. | R1 | R2 | R3 | log k | log P/Clog P ChemOffice | log P ACD/LogP | σR2[33] | MRR3[33] | |||
| GROUP 1 | 8a | 5-Cl | 3-Cl | H | 0.2439 | 2.42 / 4.33156 | 4.36 ± 0.47 | 0.373 | 0 | ||
| 8b | 5-Cl | 3-Cl | (S)-CH3 | 0.4166 | 2.91 / 4.64056 | 4.71 ± 0.48 | 0.373 | 4.7 | |||
| 8c | 5-Cl | 3-Cl | (R)-CH3 | 0.4153 | 2.91 / 4.64056 | 4.71 ± 0.48 | 0.373 | 4.7 | |||
| 8d | 5-Cl | 3-Cl | (S)-CH(CH3)2 | 0.6124 | 3.80 / 5.56856 | 5.59 ± 0.48 | 0.373 | 14.0 | |||
| 8e | 5-Cl | 3-Cl | (R)-CH(CH3)2 | 0.6114 | 3.80 / 5.56856 | 5.59 ± 0.48 | 0.373 | 14.0 | |||
| 8f | 5-Cl | 3-Cl | (S)-CH2C6H5 | 0.7098 | 4.58 / 6.05856 | 6.64 ± 0.49 | 0.373 | 29.0 | |||
| 8g | 5-Cl | 3-Cl | (R)-CH2C6H5 | 0.7066 | 4.58 / 6.05856 | 6.64 ± 0.49 | 0.373 | 29.0 | |||
| GROUP 2 | 8h | 5-Cl | 4-Cl | (R)-CH3 | 0.4308 | 2.91 / 4.64056 | 4.64 ± 0.47 | 0.227 | 4.7 | ||
| 8i | 5-Cl | 4-Cl | (S)-CH(CH3)2 | 0.6371 | 3.80 / 5.56856 | 5.52 ± 0.48 | 0.227 | 14.0 | |||
| 8j | 5-Cl | 4-Cl | (R)-CH(CH3)2 | 0.6125 | 3.80 / 5.56856 | 5.52 ± 0.48 | 0.227 | 14.0 | |||
| 8k | 5-Cl | 4-Cl | (S)-CH2C6H5 | 0.7394 | 4.58 / 6.05856 | 6.57 ± 0.49 | 0.227 | 29.0 | |||
| 8l | 5-Cl | 4-Cl | (R)-CH2C6H5 | 0.7329 | 4.58 / 6.05856 | 6.57 ± 0.49 | 0.227 | 29.0 | |||
| GROUP 3 | 8m | 5-Cl | 3,4-Cl | H | 0.4971 | 2.98 / 5.01472 | 5.20 ± 0.49 | 0.600 | 0 | ||
| 8n | 5-Cl | 3,4-Cl | (S)-CH3 | 0.6698 | 3.47 / 5.32372 | 5.55 ± 0.50 | 0.600 | 4.7 | |||
| 8o | 5-Cl | 3,4-Cl | (S)-CH(CH3)2 | 0.8623 | 4.35 / 6.25172 | 6.43 ± 0.50 | 0.600 | 14.0 | |||
| 8p | 5-Cl | 3,4-Cl | (R)-CH(CH3)2 | 0.8614 | 4.35 / 6.25172 | 6.43 ± 0.50 | 0.600 | 14.0 | |||
| 8q | 5-Cl | 3,4-Cl | (S)-CH2C6H5 | 0.9485 | 5.14 / 6.74172 | 7.48 ± 0.51 | 0.600 | 29.0 | |||
| 8r | 5-Cl | 3,4-Cl | (R)-CH2C6H5 | 0.9408 | 5.14 / 6.74172 | 7.48 ± 0.51 | 0.600 | 29.0 | |||
| GROUP 4 | 8s | 5-Cl | 4-Br | (S)-CH(CH3)2 | 0.6709 | 4.07 / 5.71856 | 5.76 ± 0.54 | 0.232 | 14.0 | ||
| 8t | 5-Cl | 4-Br | (R)-CH(CH3)2 | 0.6646 | 4.07 / 5.71856 | 5.76 ± 0.54 | 0.232 | 14.0 | |||
| 8u | 5-Cl | 4-Br | (S)-CH2C6H5 | 0.7779 | 4.86 / 6.20856 | 6.81 ± 0.55 | 0.232 | 29.0 | |||
| 8v | 5-Cl | 4-Br | (R)-CH2C6H5 | 0.7673 | 4.86 / 6.20856 | 6.81 ± 0.55 | 0.232 | 29.0 | |||
| GROUP 5 | 9a | 4-Cl | 4-Cl | (S)-CH3 | 0.4934 | 2.91 / 4.64056 | 4.54 ± 0.47 | 0.227 | 4.7 | ||
| 9b | 4-Cl | 4-Cl | (R)-CH3 | 0.4921 | 2.91 / 4.64056 | 4.54 ± 0.47 | 0.227 | 4.7 | |||
| 9c | 4-Cl | 4-Cl | (S)-CH(CH3)2 | 0.6839 | 3.80 / 5.56856 | 5.41 ± 0.48 | 0.227 | 14.0 | |||
| 9d | 4-Cl | 4-Cl | (R)-CH(CH3)2 | 0.6832 | 3.80 / 5.56856 | 5.41 ± 0.48 | 0.227 | 14.0 | |||
| 9e | 4-Cl | 4-Cl | (S)-CH2C6H5 | 0.7540 | 4.58 / 6.05856 | 6.47 ± 0.49 | 0.227 | 29.0 | |||
| 9f | 4-Cl | 4-Cl | (R)-CH2C6H5 | 0.7479 | 4.58 / 6.05856 | 6.47 ± 0.49 | 0.227 | 29.0 | |||
| GROUP 6 | 9g | 4-Cl | 4-Br | (R)-CH(CH3)2 | 0.7353 | 4.07 / 5.71856 | 5.65 ± 0.54 | 0.232 | 14.0 | ||
| 9h | 4-Cl | 4-CF3 | (S)-CH(CH3)2 | 0.7892 | 4.16 / 5.93176 | 5.46 ± 0.51 | 0.740 | 14.0 | |||
| 9i | 4-Cl | 4-CH3 | (S)-CH(CH3)2 | 0.5902 | 3.72 / 5.09696 | 4.91 ± 0.46 | -0.170 | 14.0 | |||
| 9j | 4-Cl | 4-OCH3 | (S)-CH(CH3)2 | 0.3623 | 3.11 / 4.67336 | 4.46 ± 0.48 | -0.270 | 14.0 | |||
2.3. Biological activities
2.3.1. Antimycobacterial screening
| Comp. | MIC [µmol/L] | PET IC50 [μmol/L] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MTB a | MA a | MK a | CT b | CK b | TM c | SA d | MRSA d | SE d | EF d | |||
| 14h | 14h | 14h | 24h | 24h | 72h | 24h | 24h | 24h | 24h | |||
| 21h | 21h | 21h | 48h | 48h | 120h | 48h | 48h | 48h | 48h | |||
| GROUP 1 | 8a | 125 | 62.5 | 250 | 125 | 125 | 125 | >250 | >250 | >250 | >250 | 378.6 |
| 125 | 62.5 | 250 | 125 | 125 | 125 | >250 | >250 | >250 | >250 | |||
| 8be | 250 | 500 | 500 | >125 | >125 | 125 | >250 | >250 | >250 | >250 | 41.7 | |
| >500 | >500 | >500 | >125 | >125 | 125 | >250 | >250 | >250 | >250 | |||
| 8c | 62.5 | 125 | 62.5 | 31.25 | 31.25 | 0.49 | >500 | >500 | >500 | >500 | ND | |
| 125 | 250 | 62.5 | 125 | 62.5 | 0.49 | >500 | >500 | >500 | >500 | |||
| 8d | 32 | 125 | 62.5 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | 13.8 | |
| 62.5 | 125 | 125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | |||
| 8e | 32 | 62.5 | 62.5 | 15.62 | 3.9 | 125 | 15.62 | 31.25 | 31.25 | 62.5 | 20.4 | |
| 62.5 | 125 | 125 | 62.5 | 7.81 | 125 | 15.62 | 31.25 | 31.25 | 250 | |||
| 8f | 62.5 | 125 | 62.5 | 31.25 | 1.95 | 125 | >500 | >500 | >500 | >500 | 12.3 | |
| 125 | 250 | 125 | 125 | 3.9 | 125 | >500 | >500 | >500 | >500 | |||
| 8g | 62.5 | 32 | 62.5 | 31.25 | 1.95 | 125 | >500 | >500 | >500 | >500 | ND | |
| 62.5 | 32 | 62.5 | 125 | 3.9 | 125 | >500 | >500 | >500 | >500 | |||
| GROUP 2 | 8h | 8 | 125 | 125 | >125 | 125 | >125 | >500 | >500 | >500 | >500 | ND |
| 16 | 125 | 250 | >125 | 125 | >125 | >500 | >500 | >500 | >500 | |||
| 8i | 32 | 62.5 | 62.5 | >125 | >125 | >125 | 7.81 | 15.62 | 31.25 | 31.25 | ND | |
| 32 | 62.5 | 62.5 | >125 | >125 | >125 | 15.62 | 15.62 | 250 | 150 | |||
| 8j | 32 | 62.5 | 62.5 | >125 | 62.5 | 62.5 | 7.81 | 7.81 | 31.25 | 250 | ND | |
| 32 | 62.5 | 62.5 | >125 | 125 | 62.5 | 7.81 | 7.81 | 125 | 500 | |||
| 8k | 16 | 32 | 32 | 125 | 125 | 125 | >500 | >500 | >500 | >500 | 29.8 | |
| 16 | 32 | 62.5 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | |||
| 8l | 16 | 32 | 32 | 125 | 125 | 125 | >125 | >125 | >125 | >125 | 33.7 | |
| 16 | 32 | 32 | >125 | >125 | 125 | >125 | >125 | >125 | >125 | |||
| GROUP 3 | 8m | 125 | 62.5 | 125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | ND |
| 500 | 500 | 500 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | |||
| 8n | 32 | 32 | 62.5 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | ND | |
| 32 | 62.5 | 500 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | |||
| 8o | 32 | 62.5 | 32 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | ND | |
| 32 | 125 | 62.5 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | |||
| 8p | 16 | 62.5 | 32 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | ND | |
| 32 | 125 | 62.5 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | |||
| 8qe | 125 | 125 | 250 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | 74.2 | |
| >1000 | >1000 | >1000 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | |||
| 8re | 125 | 125 | 250 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | ND | |
| >1000 | >1000 | >1000 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | |||
| GROUP 4 | 8s | 62.5 | 62.5 | 62.5 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | 26.2 |
| 62.5 | 62.5 | 125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | |||
| 8t | 62.5 | 62.5 | 62.5 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | ND | |
| 62.5 | 62.5 | 125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | |||
| 8u | 32 | 250 | 62.5 | 500 | 500 | 500 | >125 | >125 | >125 | >125 | 31.2 | |
| 32 | 250 | 125 | >500 | >500 | >500 | >125 | >125 | >125 | >125 | |||
| 8v | 32 | 250 | 32 | 500 | 500 | 500 | 3.9 | 7.81 | 62.5 | 62.5 | ND | |
| 32 | 250 | 32 | >500 | >500 | >500 | 15.62 | 15.62 | 125 | 62.5 | |||
| GROUP 5 | 9a | 62.5 | 125 | 125 | >250 | >250 | >250 | 250 | 500 | 250 | 500 | 61.5 |
| 62.5 | 125 | 125 | >250 | >250 | >250 | 500 | 500 | 250 | 500 | |||
| 9b | 32 | 125 | 125 | 250 | 250 | 250 | 7.81 | 7.81 | 1.95 | 500 | ND | |
| 62.5 | 125 | 125 | 250 | 250 | 250 | 7.81 | 7.81 | 1.95 | 500 | |||
| 9c | 62.5 | 62.5 | 62.5 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | 23.8 | |
| 62.5 | 125 | 62.5 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | |||
| 9d | 62.5 | 62.5 | 62.5 | >125 | >125 | 7.81 | >500 | >500 | >500 | >500 | ND | |
| 62.5 | 62.5 | 62.5 | >125 | >125 | 7.81 | >500 | >500 | >500 | >500 | |||
| 9e | 32 | 32 | 32 | 125 | 125 | 125 | >250 | >250 | >250 | >500 | ND | |
| 32 | 62.5 | 62.5 | 125 | 125 | 125 | >250 | >250 | >250 | >500 | |||
| 9f | 32 | 32 | 32 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | 23.5 | |
| 32 | 32 | 32 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | |||
| GROUP 6 | 9g | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 24 |
| 9h | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 14.2 | |
| 9i | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 37.3 | |
| 9j | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 35.7 | |
| INH | 0.5 | >250 | >250 | – | – | – | – | – | – | – | – | |
| 0.5 | >250 | >250 | ||||||||||
| FLU | – | – | – | 0.12 | 3.91 | 1.95 | – | – | – | – | – | |
| >125 | 15.62 | 3.91 | ||||||||||
| PEN | – | – | – | – | – | – | 0.24 | 125 | 31.62 | 7.81 | – | |
| 0.24 | 125 | 125 | 15.62 | |||||||||
| CPF | – | – | – | – | – | – | 0.98 | 500 | 250 | 0.98 | – | |
| 0.98 | 500 | 250 | 0.98 | |||||||||
| DCMU | – | – | – | – | – | – | – | – | 1.9 | |||

2.3.2. In vitro antifungal susceptibility testing
2.3.3. In vitro antibacterial susceptibility testing
2.3.4. Inhibition of photosynthetic electron transport (PET) in spinach chloroplasts
3. Experimental
3.1. Synthesis
3.2. Lipophilicity determination by HPLC (capacity factor k/calculated log k)
3.3. Lipophilicity calculations
3.4. In vitro antimycobacterial evaluation
3.5. In vitro antifungal susceptibility testing
3.6. In vitro antibacterial susceptibility testing
3.7. Study of inhibition photosynthetic electron transport (PET) in spinach chloroplasts
4. Conclusions
Acknowledgements
References
- Vinsova, J.; Imramovsky, A. Salicylanilides: Still a topical potential antibacterially active group. Ces. Slov. Farm. 2004, 53, 294–299. [Google Scholar]
- Dahlgren, M.K.; Kauppi, A.M.; Olsson, I.M.; Linusson, A.; Elofsson, M. Design, synthesis, and multivariate quantitative structure–activity relationship of salicylanilidess–potent inhibitors of type III secretion in Yersinia. J. Med. Chem. 2007, 50, 6177–6188. [Google Scholar] [CrossRef]
- Stephenson, K.; Yamaguchi, Y.; Hoch, J.A. The mechanism of action of inhibitors of bacterial two-component signal transduction systems. J. Biol. Chem. 2000, 275, 38900–38904. [Google Scholar] [CrossRef]
- Vinsova, J.; Imramovsky, A.; Buchta, V.; Ceckova, M.; Dolezal, M.; Staud, F.; Jampilek, J.; Kaustova, J. Salicylanilide acetates: Synthesis and antibacterial evaluation. Molecules 2007, 12, 1–12. [Google Scholar] [CrossRef]
- Imramovsky, A.; Vinsova, J.; Ferriz, J.M.; Dolezal, R.; Jampilek, J.; Kaustova, J.; Kunc, F. New antituberculotics originated from salicylanilides with promising in vitro activity against atypical mycobacterial strains. Bioorg. Med. Chem. 2009, 17, 3572–3579. [Google Scholar] [CrossRef]
- Ferriz, J.M.; Vavrova, K.; Kunc, F.; Imramovsky, A.; Stolarikova, J.; Vavrikova, E.; Vinsova, J. Salicylanilide carbamates: Antitubercular agents active against multidrug-resistant Mycobacterium tuberculosis strains. Bioorg. Med. Chem. 2010, 18, 1054–1061. [Google Scholar] [CrossRef]
- Imramovsky, A.; Vinsova, J.; Ferriz, J.M.; Buchta, V.; Jampilek, J. Salicylanilide esters of N-protected amino acids as novel antimicrobial agents. Bioorg. Med. Chem. Lett. 2009, 19, 348–351. [Google Scholar] [CrossRef]
- Brown, M.E.; Fitzner, J.N.; Stevens, T.; Chin, W.; Wright, C.D.; Boyce, J.P. Salicylanilides: Selective inhibitors of interleukin-12p40 production. Bioorg. Med. Chem. 2008, 16, 8760–8764. [Google Scholar] [CrossRef]
- Liechti, C.; Sequin, U.; Bold, G.; Furet, P.; Meyer, T.; Traxler, P. Salicylanilides as inhibitors of the protein tyrosine kinase epidermal growth factor receptor. Eur. J. Med. Chem. 2004, 39, 11–26. [Google Scholar] [CrossRef]
- Deng, W.; Guo, Z.; Guo, Y.; Feng, Z.; Jiang, Y.; Chu, F. Acryolylamino-salicylanilides as EGFR PTK inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 469–472. [Google Scholar] [CrossRef]
- Kamath, S.; Buolamwini, J.K. Targeting EGFR and HER-2 receptor tyrosine kinases for cancer drug discovery and development. Med. Res. Rev. 2006, 26, 569–594. [Google Scholar] [CrossRef]
- Imramovsky, A.; Vinsova, J.; Ferriz, J.M.; Kunes, J.; Pour, M.; Dolezal, M. Salicylanilide esterification: Unexpected formation of novel seven-membered rings. Tetrahedron Lett. 2006, 47, 5007–5011. [Google Scholar] [CrossRef]
- Vinsova, J.; Imramovsky, A.; Kratky, M.; Ferriz, J.M.; Palat, K.; Lycka, A.; Ruzicka, A. An unprecedented rearrangement of salicylanilide derivatives: Imidazolinone intermediate formation. Tetrahedron Lett. 2010, 51, 23–26. [Google Scholar]
- Imramovsky, A.; Ferriz, J.M.; Pauk, K.; Kratky, M.; Vinsova, J. Synthetic route for the preparation of 2-hydroxy-N-[1-(2-hydroxyphenylamino)-1-oxoalkan-2-yl]benzamides. J. Comb. Chem. 2010, 12, 414–416. [Google Scholar] [CrossRef]
- Good, N.E. Inhibitors of the Hill reaction. Plant Physiol. 1961, 36, 788–803. [Google Scholar] [CrossRef]
- Kralova, K.; Sersen, F.; Cizmarik, J. Inhibitory effect of piperidinoethylesters of alkoxyphenylcarbamic acids on photosynthesis. Gen. Physiol. Biophys. 1992, 11, 261–267. [Google Scholar]
- Kralova, K.; Sersen, F.; Kubicova, L.; Waisser, K. Inhibitory effects of substituted benzanilides on photosynthetic electron transport in spinach chloroplasts. Chem. Pap. 1999, 53, 328–331. [Google Scholar]
- Kralova, K.; Sersen, F.; Kubicova, L.; Waisser, K. Inhibition of photosynthetic electron transport in spinach chloroplasts by 3- and 4-halogeno substituted benzanilides and thiobenzanilides. J. Trace Microprobe Technol. 2000, 18, 251–256. [Google Scholar]
- Black, C.C. Photosynthetic phosphorylation and associated reactions in the presence of a new group of uncouplers: Salicylanilides. Biochim. Biophys. Acta 1968, 162, 294–296. [Google Scholar] [CrossRef]
- Dolezal, M.; Miletin, M.; Kunes, J.; Kralova, K. Substituted amides of pyrazine-2-carboxylic acids, their synthesis and biological activity. Molecules 2002, 7, 363–373. [Google Scholar] [CrossRef]
- Dolezal, M.; Palek, L.; Vinsova, J.; Buchta, V.; Jampilek, J.; Kralova, K. Substituted pyrazinecarboxamides: Synthesis and biological evaluation. Molecules 2006, 11, 242–256. [Google Scholar] [CrossRef]
- Dolezal, M.; Tumova, L.; Kesetovicova, D.; Tuma, J.; Kralova, K. Substituted N-phenylpyrazine-2-carboxamides, their synthesis and evaluation as herbicides and abiotic elicitors. Molecules 2007, 12, 2589–2598. [Google Scholar] [CrossRef]
- Dolezal, M.; Cmedlova, P.; Palek, L.; Vinsova, J.; Kunes, J.; Buchta, V.; Jampilek, J.; Kralova, K. Synthesis and antimycobacterial evaluation of substituted pyrazinecarboxamides. Eur. J. Med. Chem. 2008, 43, 1105–1113. [Google Scholar] [CrossRef]
- Otevrel, J.; Mandelova, Z.; Pesko, M.; Guo, J.; Kralova, K.; Sersen, F.; Vejsova, M.; Kalinowski, D.; Kovacevic, Z.; Coffey, A.; Csollei, J.; Richardson, D.R.; Jampilek, J. Investigating the spectrum of biological activity of ring-substituted salicylanilides and carbamoylphenylcarbamates. Molecules 2010, 15, 8122–8142. [Google Scholar] [CrossRef]
- Dolezal, M.; Zitko, J.; Osicka, Z.; Kunes, J.; Vejsova, M.; Buchta, V.; Dohnal, J.; Jampilek, J.; Kralova, K. Synthesis, antimycobacterial, antifungal and photosynthesis-inhibiting activity of chlorinated N-phenylpyrazine-2-carboxamides. Molecules 2010, 15, 8567–8581. [Google Scholar] [CrossRef]
- Kralova, K.; Sersen, F.; Miletin, M.; Dolezal, M. Inhibition of photosynthetic electron transport in spinach chloroplasts by 2,6-disubstituted pyridine-4-thiocarboxamides. Chem. Pap. 2002, 56, 214–217. [Google Scholar]
- Draber, W.; Tietjen, K.; Kluth, J.F.; Trebst, A. Herbicides in photosynthesis research. Angew. Chem. 1991, 3, 1621–1633. [Google Scholar]
- Tischer, W.; Strotmann, H. Relationship between inhibitor binding by chloroplasts and inhibition of photosynthetic electron-transport. Biochim. Biophys. Acta 1977, 460, 113–125. [Google Scholar] [CrossRef]
- Trebst, A.; Draber, W. Structure activity correlations of recent herbicides in photosynthetic reactions. In Advances in Pesticide Science; Greissbuehler, H., Ed.; Pergamon Press: Oxford, UK, 1979; pp. 223–234. [Google Scholar]
- Bowyer, J.R.; Camilleri, P.; Vermaas, W.F.J. Herbicides, Topics in Photosynthesis; Baker, N.R., Percival, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 10, pp. 27–85. [Google Scholar]
- Govindjee, S. Sixty-three years since Kautsky: Chlorophyll a fluorescence. Aust. J. Plant Physiol. 1995, 22, 131–160. [Google Scholar] [CrossRef]
- Kerns, E.H.; Li, D. Drug-like Properties: Concept, Structure Design and Methods; Elsevier: San Diego, CA, USA, 2008. [Google Scholar]
- Norrington, F.E.; Hyde, R.M.; Williams, S.G.; Wotton, R. Physicochemical-activity relations in practice. 1. Rational and self-consistent data bank. J. Med. Chem. 1975, 18, 604–607. [Google Scholar] [CrossRef]
- Kaustova, J. Quantitative micromethod for drug susceptibility testing of mycobacteria in Sula’s medium. Klin. Mikrobiol. Infeck. Lek. 1997, 3, 115–117. [Google Scholar]
- Sheehan, D.J.; Espinel-Ingroff, A.; Steele, M.; Webb, C.D. Antifungal susceptibility testing of yeasts: A brief overview. Clin. Infect. Dis. 1993, 17, 494–500. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards. Reference Method for Broth Dilution Testing of Yeasts: Approved Standard M27-A2, 2nd edNational Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2002.
- National Committee for Clinical Laboratory Standards. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts: Approved Guideline M44-A; National Committee for Clinical Laboratory Standards, Wayne, PA, USA, 2004.
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically Approved Standard: Approved Standard M7-A4, 4th edNational Committee for Clinical Laboratory Standards: Villanova, PA, USA, 1997.
- Masarovicova, E.; Kralova, K. Approaches to measuring plant photosynthesis activity. In Handbook of Photosynthesis, 2nd; Pessarakli, M., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2005; pp. 617–656. [Google Scholar]
- Kralova, K.; Sersen, F.; Sidoova, E. Photosynthesis inhibition produced by 2-alkylthio-6-R-benzothiazoles. Chem. Pap. 1992, 46, 348–350. [Google Scholar]
- Fedke, C. Biochemistry and Physiology of Herbicide Action; Springer Verlag: New York, NY, USA, 1982. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Imramovsky, A.; Pesko, M.; Kralova, K.; Vejsova, M.; Stolarikova, J.; Vinsova, J.; Jampilek, J. Investigating Spectrum of Biological Activity of 4- and 5-Chloro-2-hydroxy-N-[2-(arylamino)-1-alkyl-2-oxoethyl]benzamides. Molecules 2011, 16, 2414-2430. https://doi.org/10.3390/molecules16032414
Imramovsky A, Pesko M, Kralova K, Vejsova M, Stolarikova J, Vinsova J, Jampilek J. Investigating Spectrum of Biological Activity of 4- and 5-Chloro-2-hydroxy-N-[2-(arylamino)-1-alkyl-2-oxoethyl]benzamides. Molecules. 2011; 16(3):2414-2430. https://doi.org/10.3390/molecules16032414
Chicago/Turabian StyleImramovsky, Ales, Matus Pesko, Katarina Kralova, Marcela Vejsova, Jirina Stolarikova, Jarmila Vinsova, and Josef Jampilek. 2011. "Investigating Spectrum of Biological Activity of 4- and 5-Chloro-2-hydroxy-N-[2-(arylamino)-1-alkyl-2-oxoethyl]benzamides" Molecules 16, no. 3: 2414-2430. https://doi.org/10.3390/molecules16032414
APA StyleImramovsky, A., Pesko, M., Kralova, K., Vejsova, M., Stolarikova, J., Vinsova, J., & Jampilek, J. (2011). Investigating Spectrum of Biological Activity of 4- and 5-Chloro-2-hydroxy-N-[2-(arylamino)-1-alkyl-2-oxoethyl]benzamides. Molecules, 16(3), 2414-2430. https://doi.org/10.3390/molecules16032414






