Potent Anti-Platelet Constituents from Centaurea iberica
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Acid Hydrolysis of Compound 3
3.5. Preparation of Human Platelets
3.6. Measurement of Platelet Aggregation
3.7. Data Analysis
4. Conclusions
Acknowledgements
References
- Mabberley, D. The Plant-Book, 2nd ed.; Cambridge University Press: Cambridge, UK, 1997; p. 138. [Google Scholar]
- Grieve, M. A Modern Herbal; Dover Publications, Inc.: New York, NY, USA, 1971. [Google Scholar]
- Kumarasamy, Y.; Nahar, L.; Cox, P.J.; Dinan, L.N.; Ferguson, F.R.; Finnie, D.A.; Jaspars, M.; Sarker, S.D. Biological activities of lignans from Centaurea scabiosa. Pharm. Biol. 2003, 41, 203–206. [Google Scholar] [CrossRef]
- Panagouleas, C.; Skaltsa, H.; Lazari, D.; Skaltsounis, A.L.; Sokovie, M. Antifungal activity of secondary metabolites of Centaurea raphanina ssp. Mixta, growing wild in Greece. Pharm. Biol. 2003, 41, 266–270. [Google Scholar] [CrossRef]
- Bitar, I.-E. Mofradat Al-Adwiah Wa Al-Agzia; Al-Zharia Press: Cairo, Egypt, 1980; p. 148. [Google Scholar]
- Wat, J.M.; Breyer-Brandwijik, M.G. The Medicinal and Poisonous Plants of Southern and Eastern Africa, 2nd ed.; E. & S. Livingstone Ltd.: Edinburgh, London, UK, 1962; p. 210. [Google Scholar]
- Monya, M.; Racz, G. Content of flavonosides in some species of the genus Centaurea L.I. Chromatographic studies. Plant Med. Phytother. 1974, 8, 126. [Google Scholar]
- Masso, J.L.; Bertran, M.N.; Adzet, T. Contribution to the chemical and pharmacologic study of some species of Centaurea (Compositae). Plant Med. Phytother. 1979, 13, 41–45. [Google Scholar]
- Kaij-A-Kamb, M.; Amoros, M.; Girrel, L. Chemistry and biological activity of the genus Centaurea. Pharma. Acta Helv. 1992, 67, 178–188. [Google Scholar]
- Gonzalez, A.G.; Bermejo, J.; Caberar, I.; Galido, A.; Masenet, G.M. Sesquiterpene lactones from Centaurea alba and C. conifera. Ann. Quim. 1977, 73, 86. [Google Scholar]
- Negrette, R.E.; Latorre, I.; Backhouse, N.; Delpote, C. Guaianolides from Centaurea scoparia. Plant Med. Phytother. 1988, 53, 503. [Google Scholar]
- Ahmed, N.; Bibi, R. Chemical investigation of Centaurea iberica. Fitoterapia 1979, 50, 199–200. [Google Scholar]
- Tekeli, Y.; Sezgin, M.; Aktumsek, A.; Guler, G.O.; Sanda, M.A. Fatty acid composition of six Centaurea species growing in Konya, Turkey. Nat. Prod. Res. 2010, 24, 1883–1889. [Google Scholar] [CrossRef] [PubMed]
- Senatore, F.; Arnold, N.A.; Bruno, M. Volatile components of Centaurea eryngioides Lam. and Centaurea iberica Trev. Var. hermonis Boiss. Lam., two Asteraceae growing wild in Lebanon. Nat. Prod. Res. 2005, 19, 749–754. [Google Scholar] [PubMed]
- Sham’yanov, D.; Akhmedov, U.A.; Saidkhodzhaev, A.I. Sesquiterpene lactones and other components of Centaurea iberica. Chem. Nat. Compd. 1998, 34, 339–340. [Google Scholar] [CrossRef]
- Dumlu, M.U.; Gürkan, E. A new active compound from Centaurea species. Z. Naturforsch. C. 2006, 61, 44–46. [Google Scholar] [CrossRef]
- Koca, U.; Süntar, I.P.; Keles, H.; Yesilada, E.; Akkol, E.K. In vivo anti-inflammatory and wound healing activities of Centaurea iberica Trev. ex Spreng. J. Ethnopharmacol. 2009, 126, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Waheed, A.; Qureshi, R.A.; Burdi, D.K.; Verspohl, E.J.; Khan, N.; Hasan, M. The effect of medicinal plants of Islamabad and Murree region of Pakistan on insulin secretion from INS-1 cells. Phytother. Res. 2004, 18, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.N.; Ferrigni, N.R.; Putman, J.E.; Jacobson, L.B.; Nichols, D.E.; McLaughlin, J.L. Brine shrimp: a convenient general bioassay for active plants constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Pakrashi, S.C.; Bhattacharyya, J. Studies on indian medicinal plants. Tetrahedron 1968, 24, 1–5. [Google Scholar] [CrossRef]
- Laumer, J.Y.S.; Frerot, E.; Herrmann, A. Controlled release of perfumery alcohols by neighboring-group participation. Comparison of the rate constants for the alkaline hydrolysis of 2-Acyl-,2-(hydroxymethyl)-, and 2-carbamoylbenzoates. Helv. Chim. Acta 2003, 86, 2871–2899. [Google Scholar] [CrossRef]
- Juraj, H.; Milos, B.; Antonin, T. Plant substances XLV. The structure of yatein. Determination of the positions and configurations of benzyl groups in lignans of the 2,3-dibenzylbutyrolactone type. Collect. Czech Chem. Commun. 1982, 47, 644–663. [Google Scholar]
- Maiada, M.A.R.; Paul, M.D.; David, E.J. Lignan of Forsythia intermedia. Phytochemistry 1990, 29, 1971–1980. [Google Scholar]
- Ricci, A.; Fiorentino, A.; Piccolella, S.; Golino, A.; Pepi, F.; D’Abrosca, B.; Letizia, M.; Monaco, P. Furofuranic glycosylated lignans: A gas-phase ion chemistry investigation by tandem mass spectrometry. Rapid Commun. Mass. Spectrom. 2008, 22, 3382–3392. [Google Scholar] [CrossRef] [PubMed]
- Farndale, R.W.; Hargreaves, P.G.; Dietrich, J.L.; Keogh, R.J. Measurement of platelet arachidonic acid metabolism. Methods Mol. Biol. 2004, 272, 121–133. [Google Scholar] [PubMed]
- Rasheed, H.; Saeed, S.A. Involvement of thromboxane A2 and tyrosine kinase in the synergistic interaction of platelet activating factor and calcium ionophore A23187 in human platelet aggregation. Exp. Mol. Med. 2004, 114, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Mehmood, S.; Fatima, I.; Malik, A.; Ifzal, R.; Afza, N.; Iqbal, L.; Latif, M.; Nizami, T.A. Structural determination of salsolins A and B, new antioxidant polyoxygenated triterpenes from Salsola baryosma, by 1D and 2 D NMR spectroscopy. Magn. Reson. Chem. 2008, 46, 94–98. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of compounds 1–3 are available from the authors. |
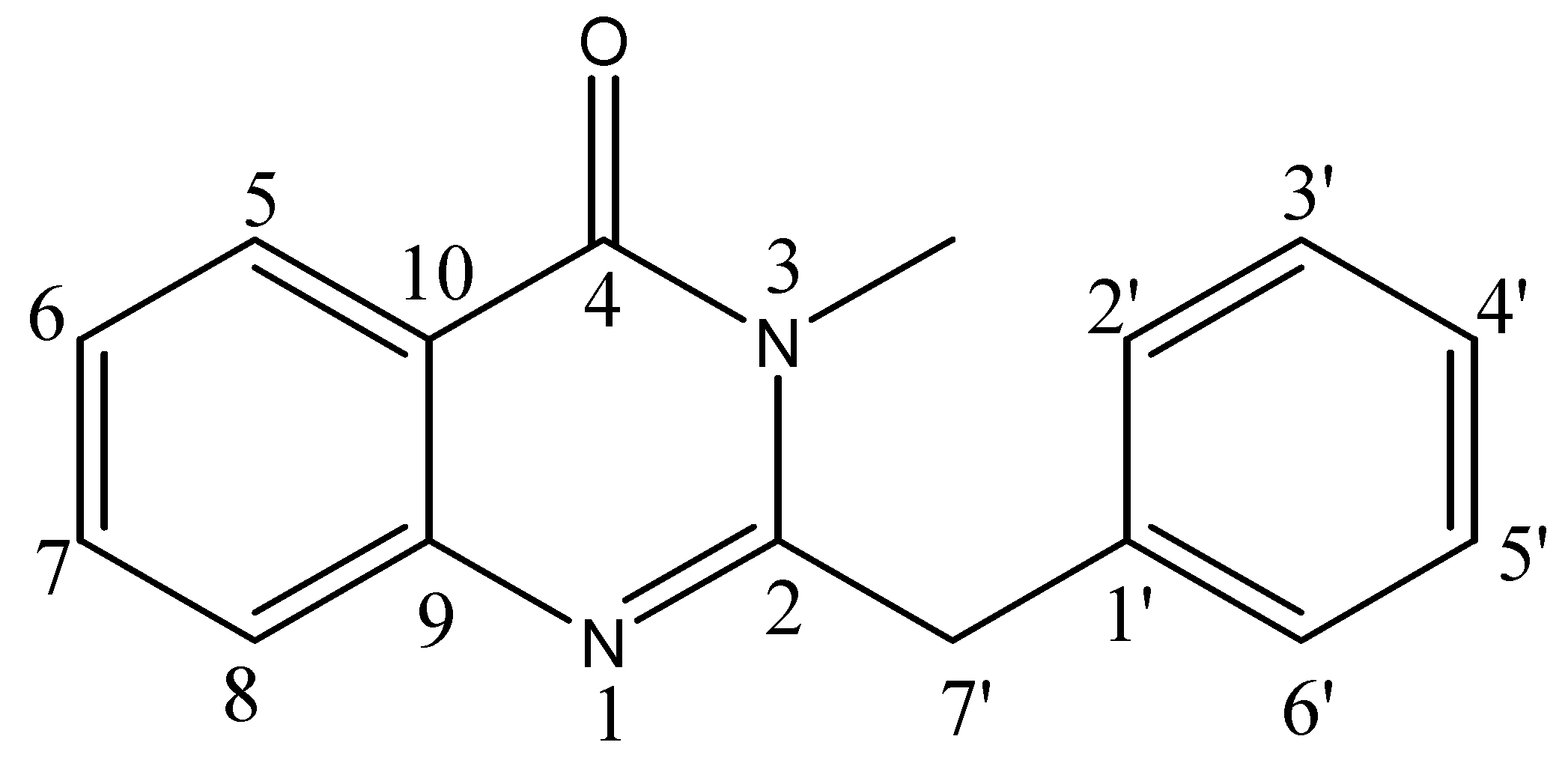

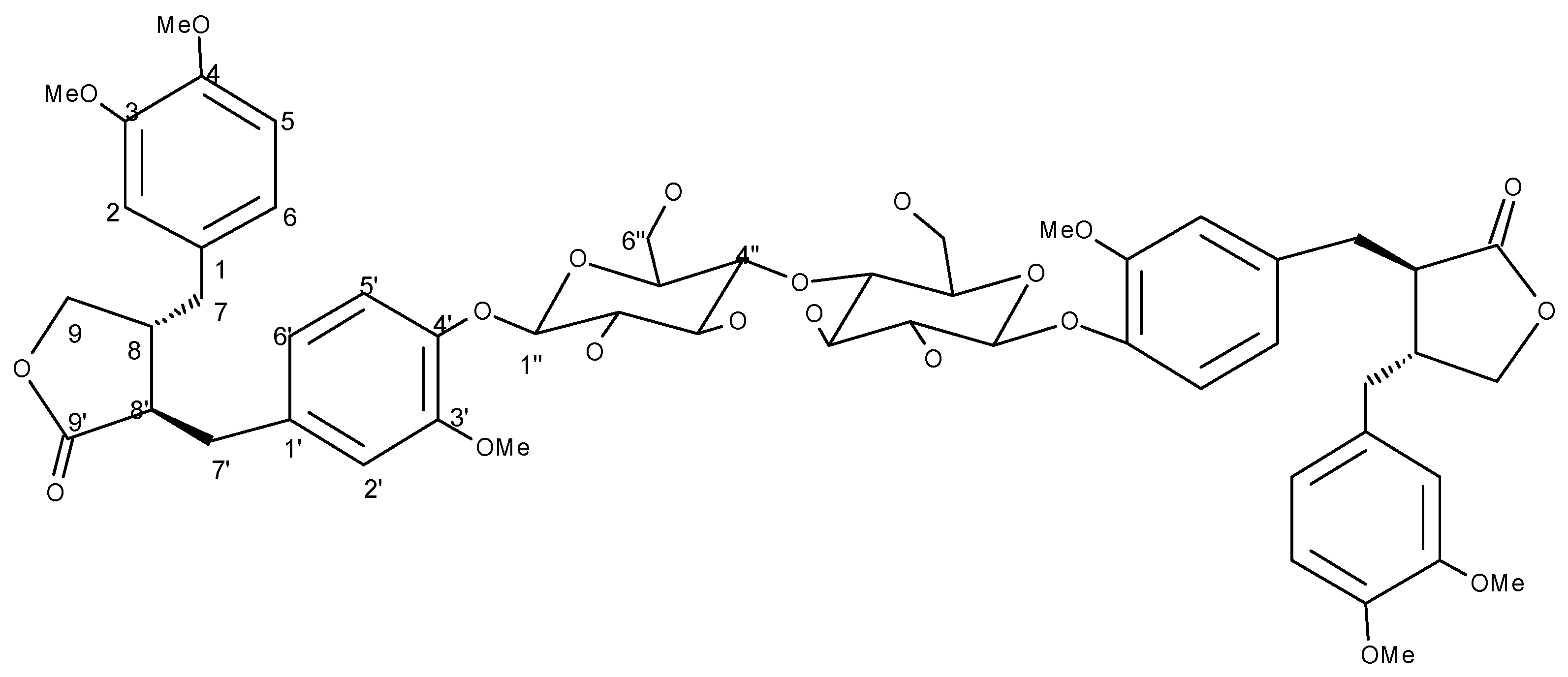
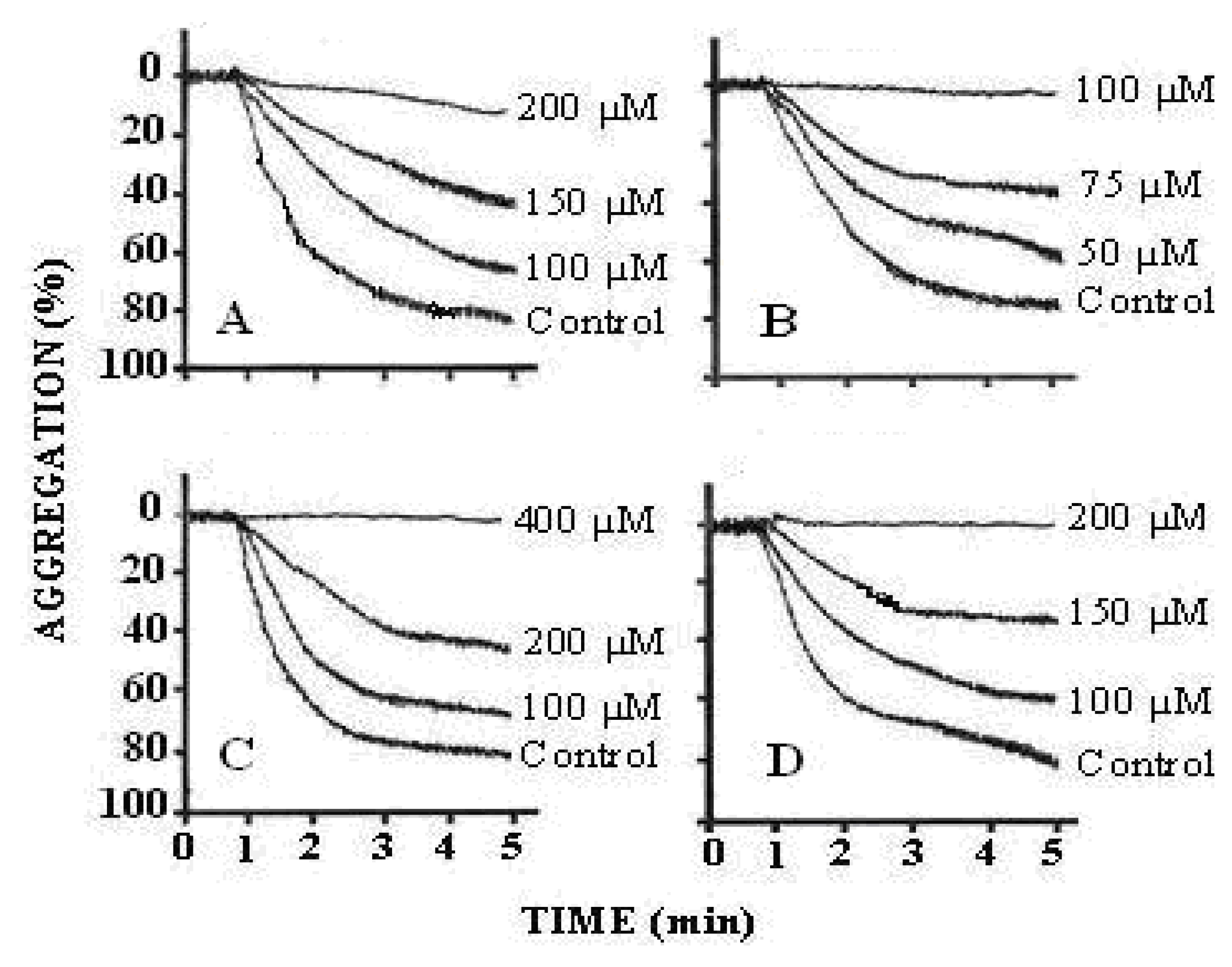
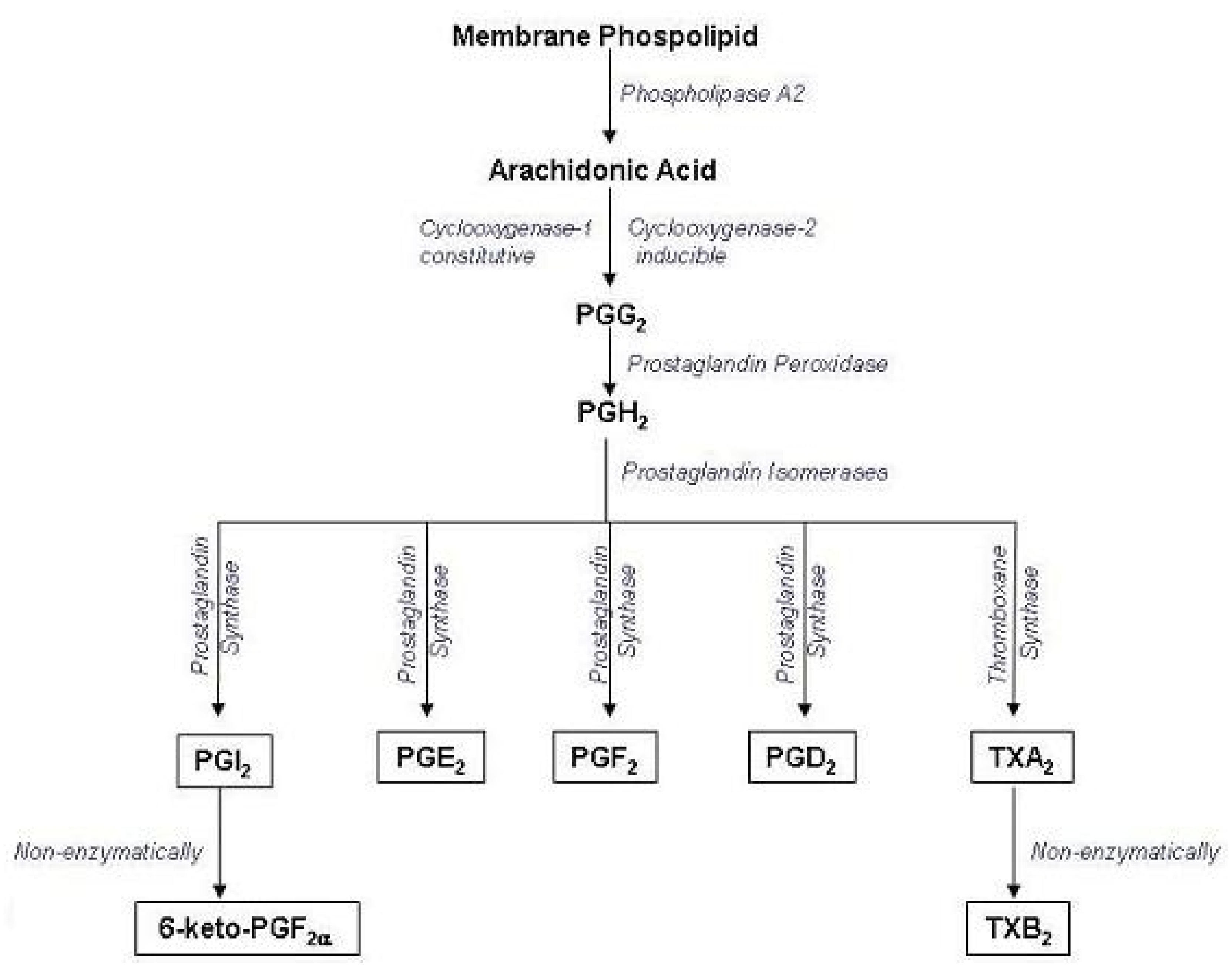
| Position | 1H | 13C | HMBC (correlation with 1H) |
|---|---|---|---|
| 1 | - | - | - |
| 2 | - | 159.0 | - |
| 3 | - | - | - |
| 4 | - | 164.5 | - |
| 5 | 8.16 (dd, 8.0, 1.5) | 127.1 | 4, 6, 7, 9, 10 |
| 6 | 7.48 (ddd, 8.0, 7.0, 1.5) | 127.9 | 5, 7, 8 |
| 7 | 7.79 (ddd, 8.0, 7.0, 1.5) | 136.0 | 5, 6, 8, 9 |
| 8 | 7.76 (dd, 8.0, 1.5) | 127.5 | 6, 7, 9, 10 |
| 9 | - | 150.0 | - |
| 10 | - | 121.8 | - |
| 1′ | - | 137.1 | - |
| 2′/6′ | 7.30 (m) | 129.9 | 1′,3′,4′,5′ |
| 3′/5′ | 7.35 (m) | 129.8 | 1′, 2′, 4′, 6′ |
| 4′ | 7.23 (m) | 128.3 | 2′,3′,5′,6′ |
| 7′ | 4.00 (s) | 42.3 | 2,1′,2′,6′ |
| N-Me | 1.88 (s) | 28.2 | 2, 4 |
| Position | 1H | 13C | HMBC (correlation with 1H) |
|---|---|---|---|
| 1 | - | 141.7 | - |
| 2 | - | 131.3 | - |
| 3 | 8.43 (dd, 8.5, 1.5) | 122.0 | 1, 2, 4, 5, 1′ |
| 4 | 7.52 (ddd, 8.5, 7.0, 1.5) | 135.2 | 2, 3, 5, 6 |
| 5 | 7.12 (ddd, 8.0, 7.0, 1.5) | 124.2 | 1, 3, 4, 6 |
| 6 | 8.43 (dd, 8.0, 1.5) | 131.9 | 1, 2, 4, 5 |
| 1′ | - | 171.4 | - |
| 2′ | 3.91 (s) | 52.9 | 1′ |
| 1″ | - | 169.7 | - |
| 2″ | 2.19 (s) | 24.8 | 1″ |
| Positions | 1H | 13C | HMBC (correlation with 1H) |
|---|---|---|---|
| 1 | - | 132.7 | - |
| 2 | 6.74 (d, 1.5) | 114.8 | 1, 3, 4, 6, 7 |
| 3 | - | 150.7 | - |
| 4 | - | 149.2 | - |
| 5 | 6.81 (d, 8.0) | 113.1 | 1, 3, 4, 6 |
| 6 | 6.59 (br s) | 122.1 | 1, 2, 4, 5, 7 |
| 7 | 2.53 (m) | 38.9 | 8′, 1, 2, 6, 8, 9 |
| 8 | 2.48 (m) | 42.5 | 7′, 8′, 9′, 1, 7, 9 |
| 9 | 4.18 (dd, 7.5, 9.0) 3.93 (dd, 7.5, 9.0) | 72.9 | 8′, 9′, 7, 8 |
| 1′ | - | 134.3 | - |
| 2′ | 6.61 (d, 1.5) | 113.6 | 1′, 3′, 4′, 6′, 7′ |
| 3′ | - | 150.4 | - |
| 4′ | - | 146.9 | - |
| 5′ | 7.03 (d, 8.5) | 117.8 | 1′, 3′, 4′, 6′ |
| 6′ | 6.63 (dd, 8.0, 1.5) | 123.0 | 1′, 2′, 4′, 5′, 7′ |
| 7′ | 2.98 (dd, 5.0, 13.5) 2.80 (dd, 7.5, 13.5) | 35.4 | 1′, 2′, 6′, 8′, 9′, 8 |
| 8′ | 2.67 (m) | 47.6 | 1′, 7′, 9′, 7, 8, 9 |
| 9′ | - | 181.3 | - |
| 1″ | 4.88 (d, 7.5) | 102.9 | 4′, 2″, 3″ |
| 2″ | 3.47 (m) | 74.9 | 1″, 3″, 4″ |
| 3″ | 3.93 (m) | 78.1 | 1″, 2″, 4″, 5″ |
| 4″ | 3.38 (m) | 71.3 | 2″, 3″, 5″, 6″ |
| 5″ | 3.46 (m) | 77.8 | 3″, 4″, 6″ |
| 6″ | 3.66 (br d, 12.5) 3.84 (br d, 12.5) | 62.5 | 4″, 5″ |
| 3-OMe | 3.79 (s) | 56.5 | 3 |
| 4-OMe | 3.79 (s) | 56.5 | 4 |
| 3′-OMe | 3.74 (s) | 56.7 | 3′ |
| Inhibitors | Mean IC50 μM ± SEM |
|---|---|
| Compound 1 Compound 2 Compound 3 Aspirin | 160 ± 3.5 75 ± 2.1 45 ± 2.8 150 ± 4.4 |
© 2011 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Khan, A.N.; Fatima, I.; Khaliq, U.A.; Malik, A.; Miana, G.A.; Qureshi, Z.-u.-R.; Rasheed, H. Potent Anti-Platelet Constituents from Centaurea iberica. Molecules 2011, 16, 2053-2064. https://doi.org/10.3390/molecules16032053
Khan AN, Fatima I, Khaliq UA, Malik A, Miana GA, Qureshi Z-u-R, Rasheed H. Potent Anti-Platelet Constituents from Centaurea iberica. Molecules. 2011; 16(3):2053-2064. https://doi.org/10.3390/molecules16032053
Chicago/Turabian StyleKhan, Amna Nisar, Itrat Fatima, Urooj Abdul Khaliq, Abdul Malik, Ghulam Abbas Miana, Zia-ur-Rehman Qureshi, and Huma Rasheed. 2011. "Potent Anti-Platelet Constituents from Centaurea iberica" Molecules 16, no. 3: 2053-2064. https://doi.org/10.3390/molecules16032053
APA StyleKhan, A. N., Fatima, I., Khaliq, U. A., Malik, A., Miana, G. A., Qureshi, Z.-u.-R., & Rasheed, H. (2011). Potent Anti-Platelet Constituents from Centaurea iberica. Molecules, 16(3), 2053-2064. https://doi.org/10.3390/molecules16032053




