Three New Steroidal Glycosides from the Roots of Cynanchum auriculatum
Abstract
:1. Introduction
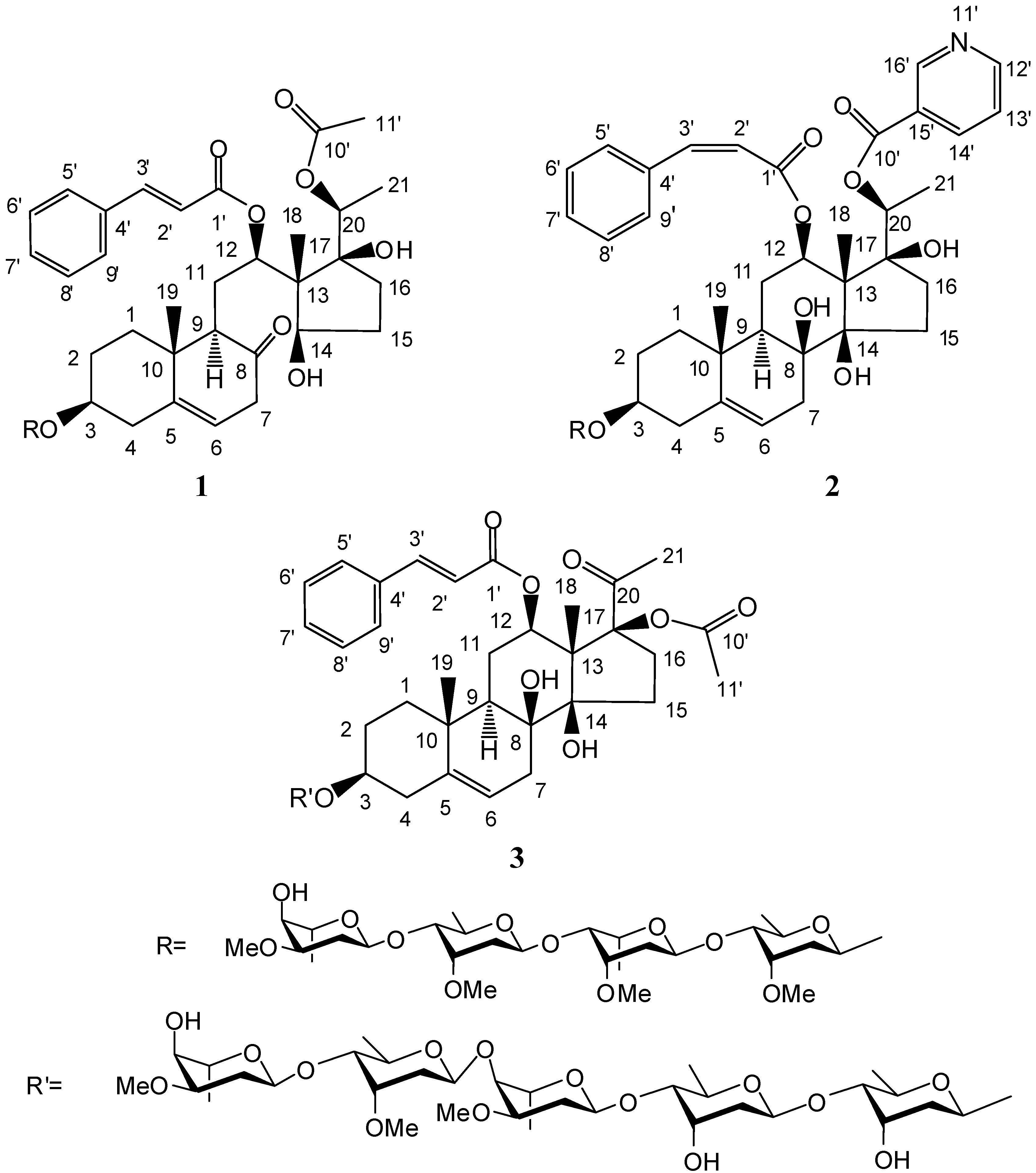
2. Results and Discussion
| No | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | |
| 1 | 39.5 | 1.09 (m), 1.82 (m) | 39.2 | 1.11 (m), 1.83 (m) | 39.0 | 1.10 (m), 1.82 (m) |
| 2 | 29.8 | 1.78 (m), 2.10 (m) | 29.8 | 1.75 (m), 2.03 (m) | 29.9 | 1.79 (m), 2.09 (m) |
| 3 | 76.9 | 3.85 (m) | 77.6 | 3.81 (m) | 77.7 | 3.84 (m) |
| 4 | 38.6 | 2.42 (m), 2.56 (m) | 38.2 | 2.40 (m), 2.52 (m) | 39.3 | 2.41 (m), 2.52 (m) |
| 5 | 140.5 | 139.1 | 139.3 | |||
| 6 | 118.9 | 5.26 (br s) | 119.3 | 5.31 (br s) | 119.2 | 5.30 (m) |
| 7 | 41.8 | 2.84 (m), 3.31 (m) | 33.6 | 2.30 (m), 2.45 (m) | 34.7 | 2.32 (m), 2.48 (m) |
| 8 | 209.8 | 74.1 | 74.4 | |||
| 9 | 57.2 | 2.21 (m) | 44.0 | 1.71 (m) | 44.6 | 1.72 (m) |
| 10 | 38.0 | 37.2 | 37.5 | |||
| 11 | 25.4 | 2.18 (m), 2.30(m) | 25.1 | 2.15 (m), 2.29 (m) | 25.1 | 2.16 (m), 2.32 (m) |
| 12 | 74.6 | 5.17 (m) | 74.5 | 5.18 (m) | 73.7 | 5.18 (m) |
| 13 | 57.2 | 56.9 | 58.2 | |||
| 14 | 81.5 | 4.95 (m) | 88.8 | 89.6 | ||
| 15 | 34.2 | 2.11 (m) | 34.7 | 2.09 (m) | 33.9 | 2.12 (m) |
| 16 | 33.4 | 2.02 (m), 3.26 (m) | 33.8 | 2.03 (m), 3.23 (m) | 33.1 | 2.05 (m), 3.24 (m) |
| 17 | 87.7 | 87.2 | 92.5 | |||
| 18 | 20.3 | 2.02 (s) | 11.0 | 2.02 (s) | 10.8 | 2.04 (s) |
| 19 | 18.9 | 1.31 (s) | 18.0 | 1.33 (s) | 18.3 | 1.32 (s) |
| 20 | 76.8 | 4.03 (m) | 76.3 | 3.93 (m) | 210.2 | |
| 21 | 14.9 | 1.57 (d, J = 5.8) | 15.3 | 1.54 (d, J = 6.0) | 27.9 | 2.49 (s) |
| 1′ | 167.8 | 166.6 | 166.0 | |||
| 2′ | 119.4 | 6.81 (d, J = 16.0) | 120.0 | 6.80 (d, J = 12.0) | 119.4 | 6.82 (d, J = 16.0) |
| 3′ | 145.7 | 8.02 (d, J = 16.0) | 144.6 | 7.96 (d, J = 12.0) | 145.1 | 7.99 (d, J = 16.0) |
| 4′ | 135.0 | 134.2 | 135.1 | |||
| 5′ | 128.8 | 7.65 (m) | 128.1 | 7.62 (m) | 128.7 | 7.64 (m) |
| 6′ | 129.3 | 7.33 (m) | 130.0 | 7.35 (m) | 129.4 | 7.37 (m) |
| 7′ | 130.7 | 7.33 (m) | 129.0 | 7.35 (m) | 130.8 | 7.37 (m) |
| 8′ | 129.3 | 7.33 (m) | 130.0 | 7.35 (m) | 129.4 | 7.37 (m) |
| 9′ | 128.8 | 7.65 (m) | 128.1 | 7.62 (m) | 128.7 | 7.64 (m) |
| 10′ | 170.5 | 164.6 | 170.6 | |||
| 11′ | 21.6 | 1.96 (s) | 21.2 | 2.06 (s) | ||
| 12′ | 151.6 | 9.59 (d, J = 1.6) | ||||
| 13′ | 123.9 | 7.28 (d, J = 7.2) | ||||
| 14′ | 137.4 | 8.40 (d, J = 7.8) | ||||
| 15′ | 127.1 | |||||
| 16′ | 153.7 | 8.79 (dd, J = 5.6, 1.6) | ||||
| No | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | |
| β-D-cym | β-D-cym | β-D-digit | ||||
| 1I | 96.5 | 5.10 (overlap) | 96.0 | 5.23 (overlap) | 96.4 | 4.89 (d, J = 9.5) |
| 2I | 35.2 | 1.76 (m), 2.37 (m) | 35.2 | 1.73 (m), 2.30 (m) | 39.2 | 1.79 (m), 2.06 (m) |
| 3I | 77.5 | 3.88 (m) | 77.6 | 3.88 (m) | 67.2 | 4.64 (m) |
| 4I | 82.4 | 3.44 (m) | 82.3 | 3.46 (m) | 83.5 | 3.51 (m) |
| 5I | 69.2 | 4.18 (m) | 69.3 | 4.19 (m) | 68.6 | 4.27 (m) |
| 6I | 18.8 | 1.49 (d, J = 6.1) | 18.5 | 1.50 (m) | 18.6 | 1.51 (d, J = 6.0) |
| -OMe | 57.2 | 3.50 (s) | 57.2 | 3.46 (s) | ||
| α-L-digin | α-L-digin | β-D-digit | ||||
| 1II | 101.0 | 5.10 (overlap) | 100.9 | 5.14 (overlap) | 99.7 | 5.12 (d, J = 10.0) |
| 2II | 32.5 | 2.00 (m), 2.33 (m) | 32.4 | 1.98 (m), 2.32 (m) | 36.6 | 1.62 (m), 2.32 (m) |
| 3II | 73.8 | 4.01 (m) | 73.8 | 3.98 (m) | 70.7 | 3.85 (m) |
| 4II | 74.6 | 3.82 (m) | 74.7 | 3.79 (m) | 80.0 | 3.23 (m) |
| 5II | 67.5 | 4.23 (m) | 67.4 | 4.11 (m) | 69.8 | 3.64 (m) |
| 6II | 17.9 | 1.40 (d, J = 6.3) | 17.8 | 1.40 (m) | 18.7 | 1.33 (d, J = 7.5) |
| -OMe | 55.4 | 3.40 (s) | 55.3 | 3.41 (s) | ||
| β-D-cym | β-D-cym | α-L-cym | ||||
| 1III | 99.5 | 4.95 (overlap) | 99.3 | 5.05 (overlap) | 99.4 | 4.98 (d, J = 2.5) |
| 2III | 36.4 | 1.86 (m), 2.41 (m) | 36.3 | 1.86 (m), 2.41 (m) | 32.3 | 1.82 (m), 2.05 (m) |
| 3III | 77.9 | 3.87 (m) | 77.6 | 3.86 (m) | 77.9 | 3.85 (m) |
| 4III | 82.4 | 3.44 (m) | 82.3 | 3.42 (m) | 73.4 | 3.62 (m) |
| 5III | 69.5 | 4.21 (m) | 69.3 | 4.21 (m) | 65.1 | 4.48 (m) |
| 6III | 18.7 | 1.25 (d, J = 6.1) | 18.3 | 1.26 (m) | 18.7 | 1.50 (d, J = 6.0) |
| -OMe | 58.3 | 3.51 (s) | 58.2 | 3.46 (s) | 56.8 | 3.37 (s) |
| α-L-cym | α-L-cym | β-D-cym | ||||
| 1IV | 99.1 | 5.05 (overlap) | 98.9 | 5.16 (m) | 95.5 | 5.19 (d, J = 10.0) |
| 2IV | 32.2 | 1.89 (m), 2.33 (m) | 32.1 | 1.89 (m), 2.33 (m) | 36.6 | 3.37 (m) |
| 3IV | 76.5 | 3.70 (m) | 76.1 | 3.69 (m) | 77.8 | 3.41 (m) |
| 4IV | 73.3 | 3.59 (m) | 73.2 | 3.61 (m) | 82.4 | 3.31 (m) |
| 5IV | 66.4 | 4.55 (m) | 66.3 | 4.54 (m) | 69.5 | 3.56 (m) |
| 6IV | 18.5 | 1.37 (d, J = 6.3) | 18.1 | 1.37 (m) | 18.7 | 1.35 (d, J = 6.5) |
| -OMe | 56.7 | 3.37 (s) | 56.5 | 3.37 (m) | 57.1 | 3.39 (s) |
| α-L-cym | ||||||
| 1V | 99.1 | 4.96 (d, J = 3.0) | ||||
| 2V | 32.3 | 1.93 (m), 2.37 (m) | ||||
| 3V | 76.6 | 3.72 (m) | ||||
| 4V | 73.1 | 3.57 (m) | ||||
| 5V | 66.4 | 4.53 (m) | ||||
| 6V | 18.8 | 1.49 (d, J = 6.0) | ||||
| -OMe | 58.4 | 3.42 (s) | ||||
3. Experimental
3.1. General
3.2. Plant material
3.3. Extraction and isolation procedures
3.4. Acid hydrolysis
3.5. Physical data of new compounds
4. Conclusions
Acknowledgements
References
- Jiangsu College of New Medicine, A Dictionary of the Traditional Chinese Medicines; People’sHygiene Publisher: Shanghai, China, 2004; p. 732.
- Teng, H.L.; Lu, Y.; Li, J.; Yang, G.Z.; Mei, Z.N. Two new steroidal glycosides from the root of Cynanchum auriculatum. Chin. Chem. Lett. 2011, 22, 77–80. [Google Scholar] [CrossRef]
- Peng, Y.R.; Li, Y.B.; Liu, X.D.; Zhang, J.F.; Duan, J.A. Antitumor activity of C-21 steroidal glycosides from Cynanchum auriculatum Royle ex Wight. Phytomedicine 2008, 15, 1016–1020. [Google Scholar] [CrossRef]
- Zhang, R.S.; Ye, Y.P.; Shen, Y.M.; Liang, H.L. Two New Cytotoxic C-21 Steroidal Glycosides from the Root of Cynanchum auriculatum. Tetrahedron 2000, 56, 3875–3879. [Google Scholar] [CrossRef]
- Li, X.Y.; Sun, H.X.; Ye, Y.P.; Chen, F.Y.; Pan, Y.J. C-21 steroidal glycosides from the roots of Cynanchum chekiangense and their immunosuppressive activities. Steroids 2006, 71, 61–66. [Google Scholar] [CrossRef]
- Gan, H.; Xiang, W.J.; Ma, L.; Hu, L.H. Six New C21 Steroidal Glycosides from Cynanchum bungei Decne. Helv. Chim. Acta 2008, 91, 2222–2234. [Google Scholar]
- Abe, F.; Mori, Y.; Okabe, H.; Yamauchi, T. Steroidal Constituents from the Roots and Stems of Asclepias fruticosa. Chem. Pharm. Bull. 1994, 42, 1777–1783. [Google Scholar] [CrossRef]
- Rubio, S.; Quintana, J.; Estévez, F. Pregnane steroidal glycosides and their cytostatic activities. Glycobiology 2011, 21, 1–22. [Google Scholar] [CrossRef]
- Gu, X.J.; Yao, N.; Qian, S.H.; Li, Y.B.; Li, P. Four New C21 Steroidal Glycosides from the Roots of Cynanchum auriculatum. Helv. Chim. Acta 2009, 92, 88–97. [Google Scholar] [CrossRef]
- Khan, I.A. New oxypregnane glycosides from appetite supresant herbal supplement Hoodia gordonii. Steroids 2007, 72, 524–534. [Google Scholar] [CrossRef]
- Sachiko, T.; Koji, H.; Hiroshi, M. Studies on the Constituents of Asclepiadaceae Plants. LX. Further Studies on Glycosides with a Novel Sugar Chain Containing a Pair of Optically Isomeric Sugars, D- and L-Cymarose, from Cynanchum wilfordi. Chem. Pharm. Bull. 1985, 33, 2294–2304. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lu, Y.; Teng, H.-L.; Yang, G.-Z.; Mei, Z.-N. Three New Steroidal Glycosides from the Roots of Cynanchum auriculatum. Molecules 2011, 16, 1901-1909. https://doi.org/10.3390/molecules16021901
Lu Y, Teng H-L, Yang G-Z, Mei Z-N. Three New Steroidal Glycosides from the Roots of Cynanchum auriculatum. Molecules. 2011; 16(2):1901-1909. https://doi.org/10.3390/molecules16021901
Chicago/Turabian StyleLu, Yu, Hong-Li Teng, Guang-Zhong Yang, and Zhi-Nan Mei. 2011. "Three New Steroidal Glycosides from the Roots of Cynanchum auriculatum" Molecules 16, no. 2: 1901-1909. https://doi.org/10.3390/molecules16021901




