The Influence of Comonomer on Ethylene/α-Olefin Copolymers Prepared Using [Bis(N-(3-tert butylsalicylidene)anilinato)] Titanium (IV) Dichloride Complex
Abstract
:1. Introduction
2. Results and Discussion
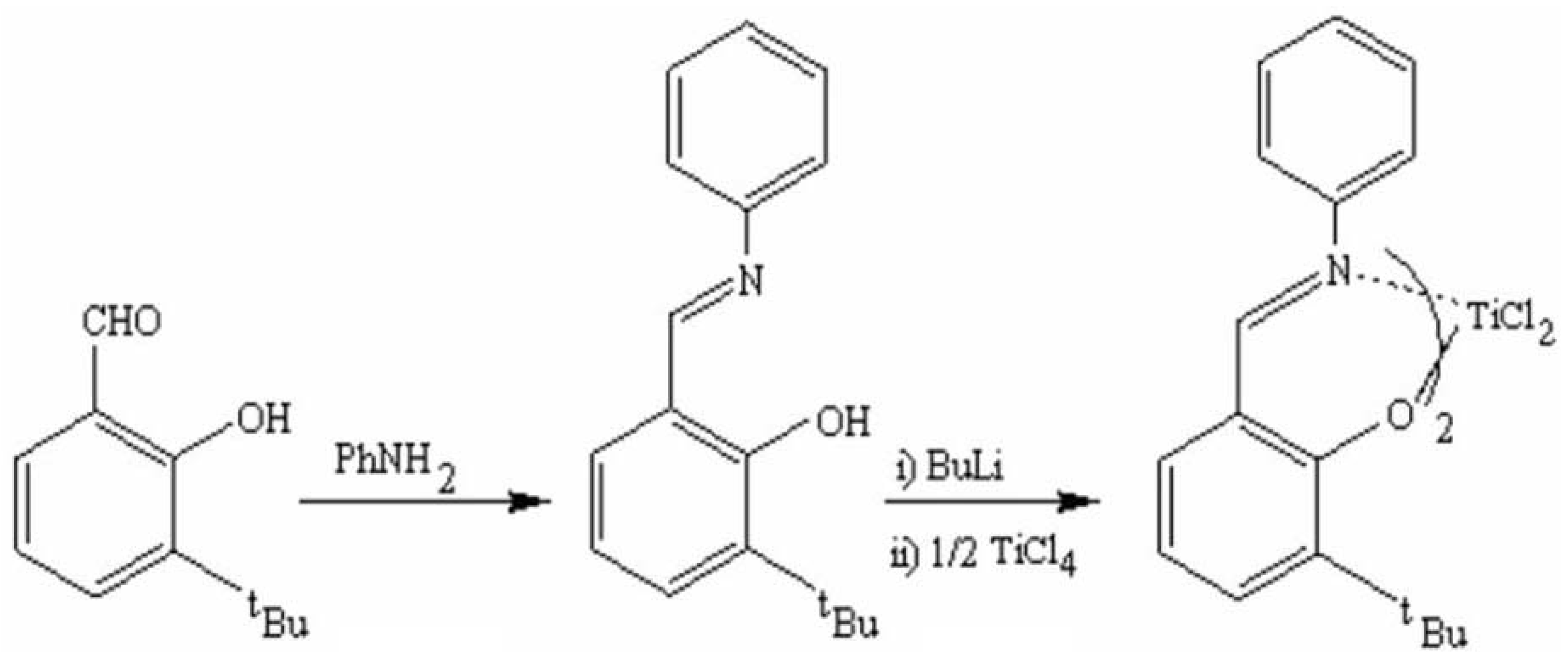
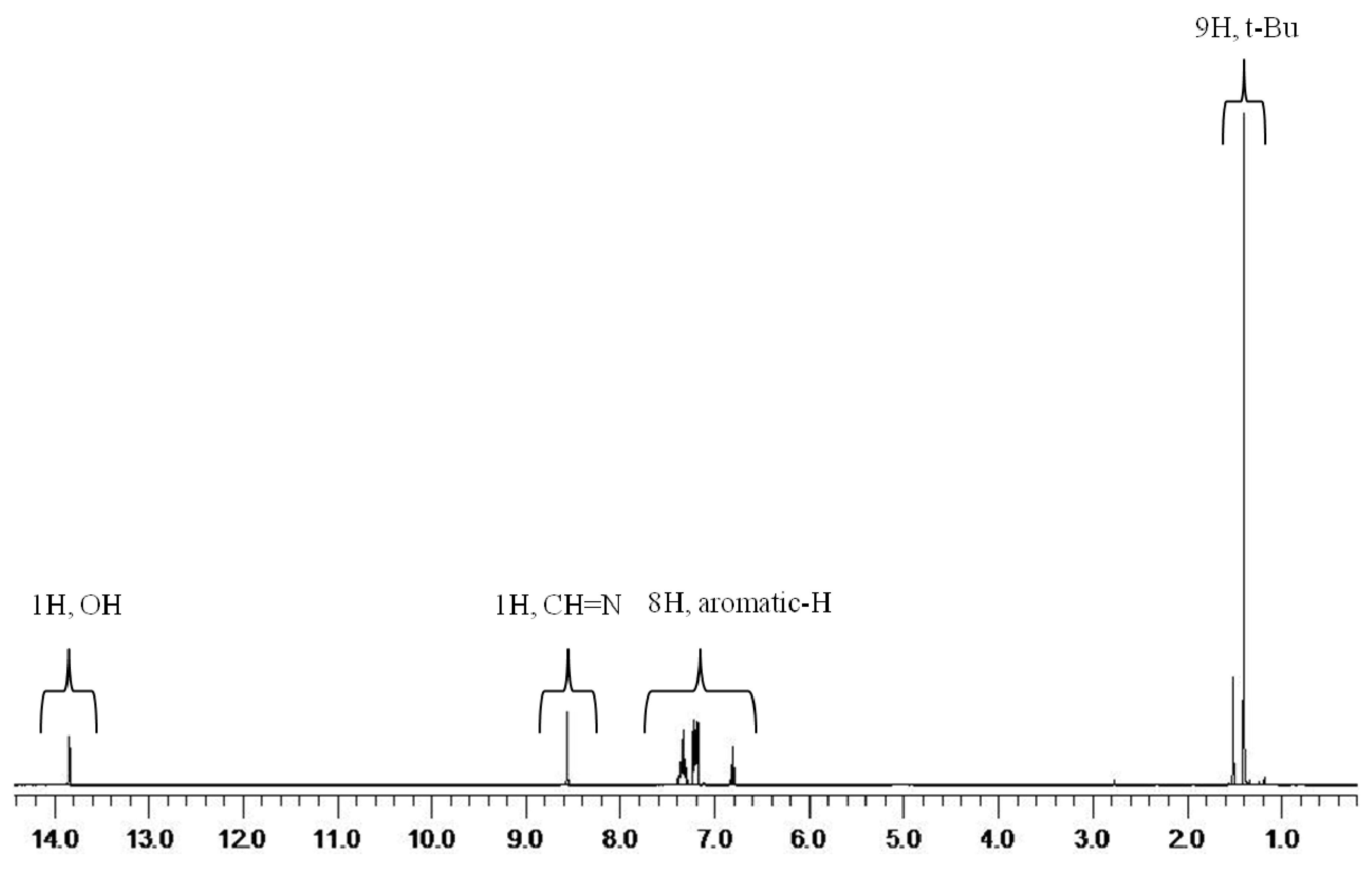
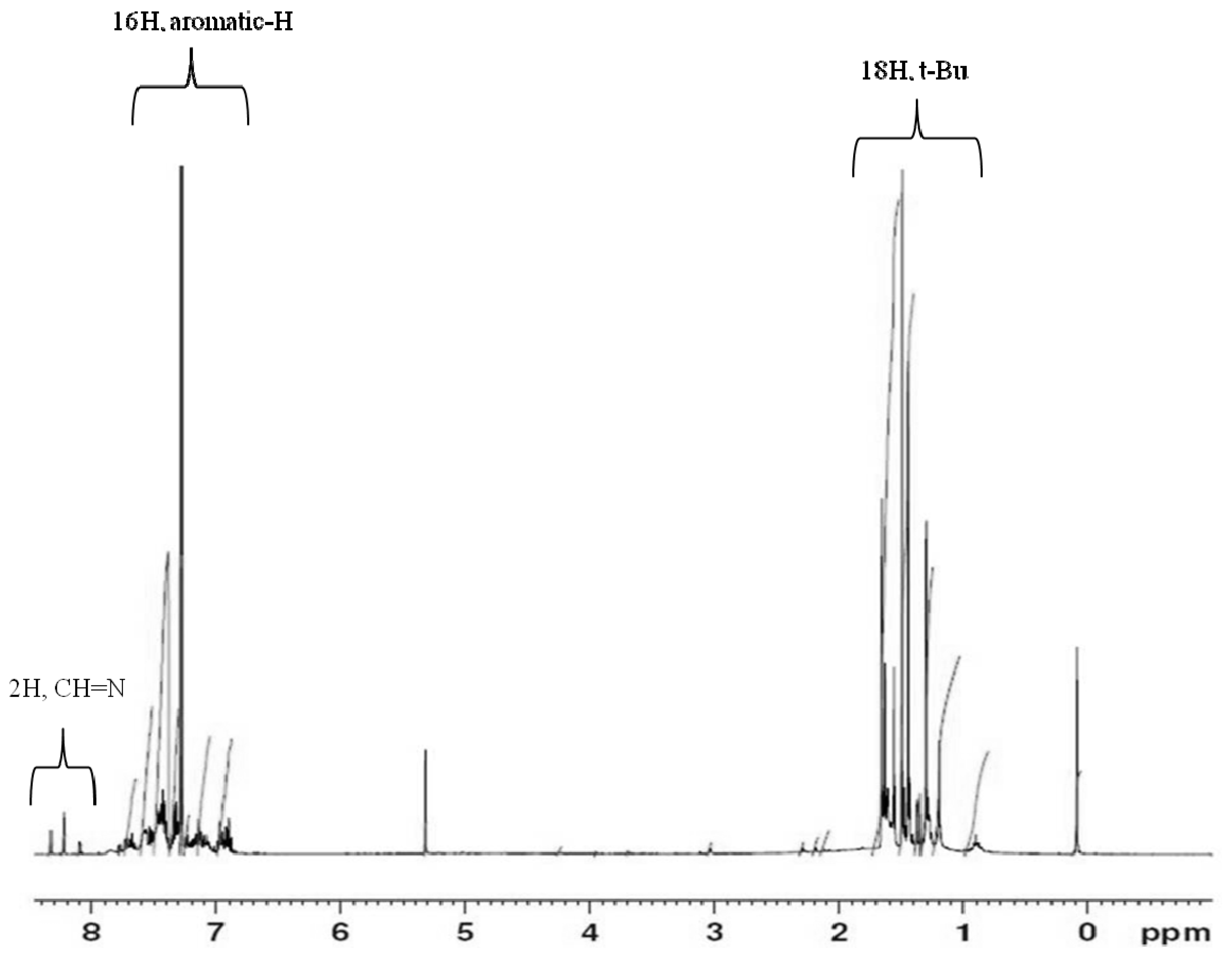
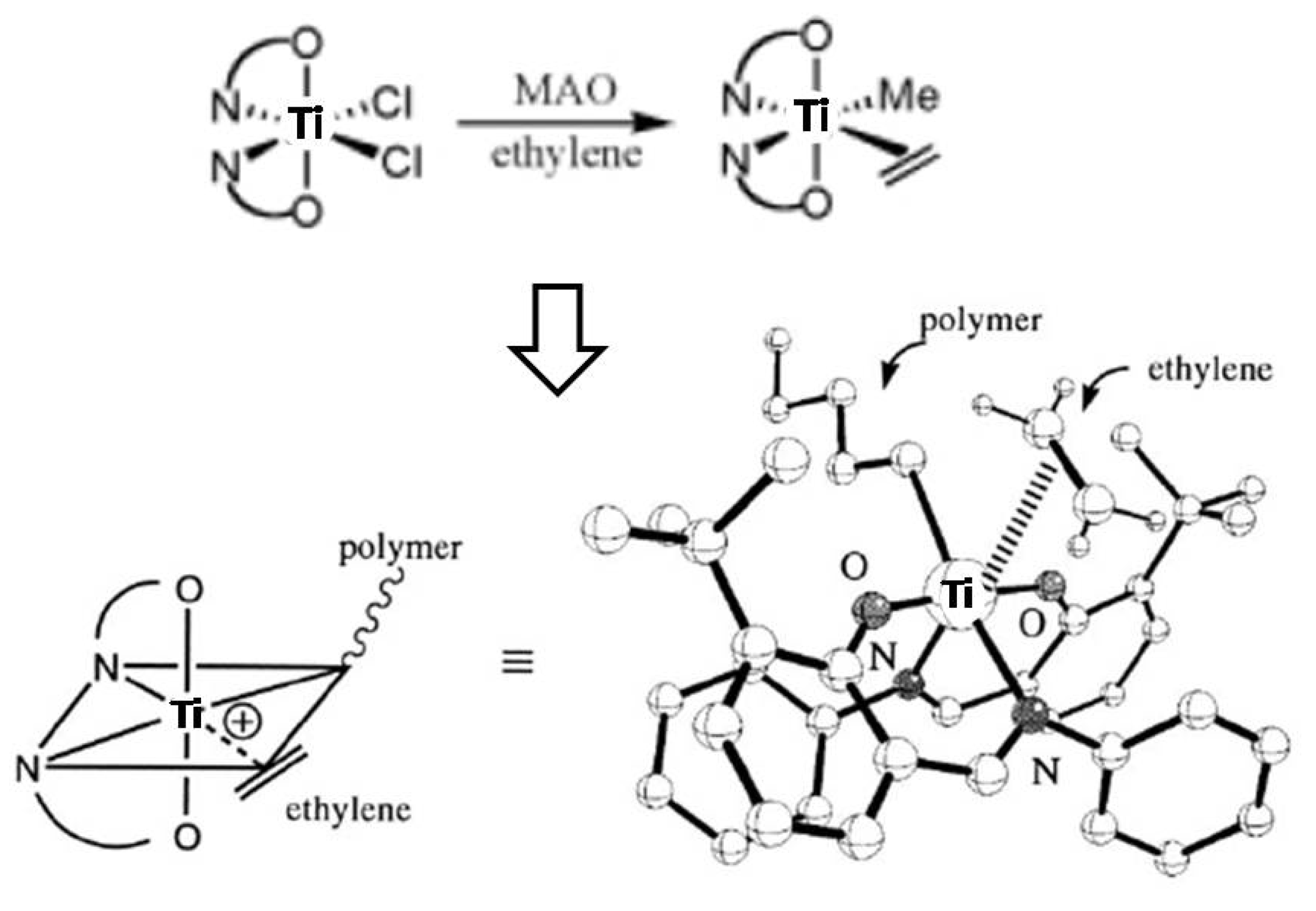
2.1. Synthesis and characterization of titanium complex (Ti-FI catalyst)
2.2. Catalytic properties of ethylene and ethylene/α-olefins copolymerization
2.3. Properties of polymers
2.4. Microstructure of the polymers
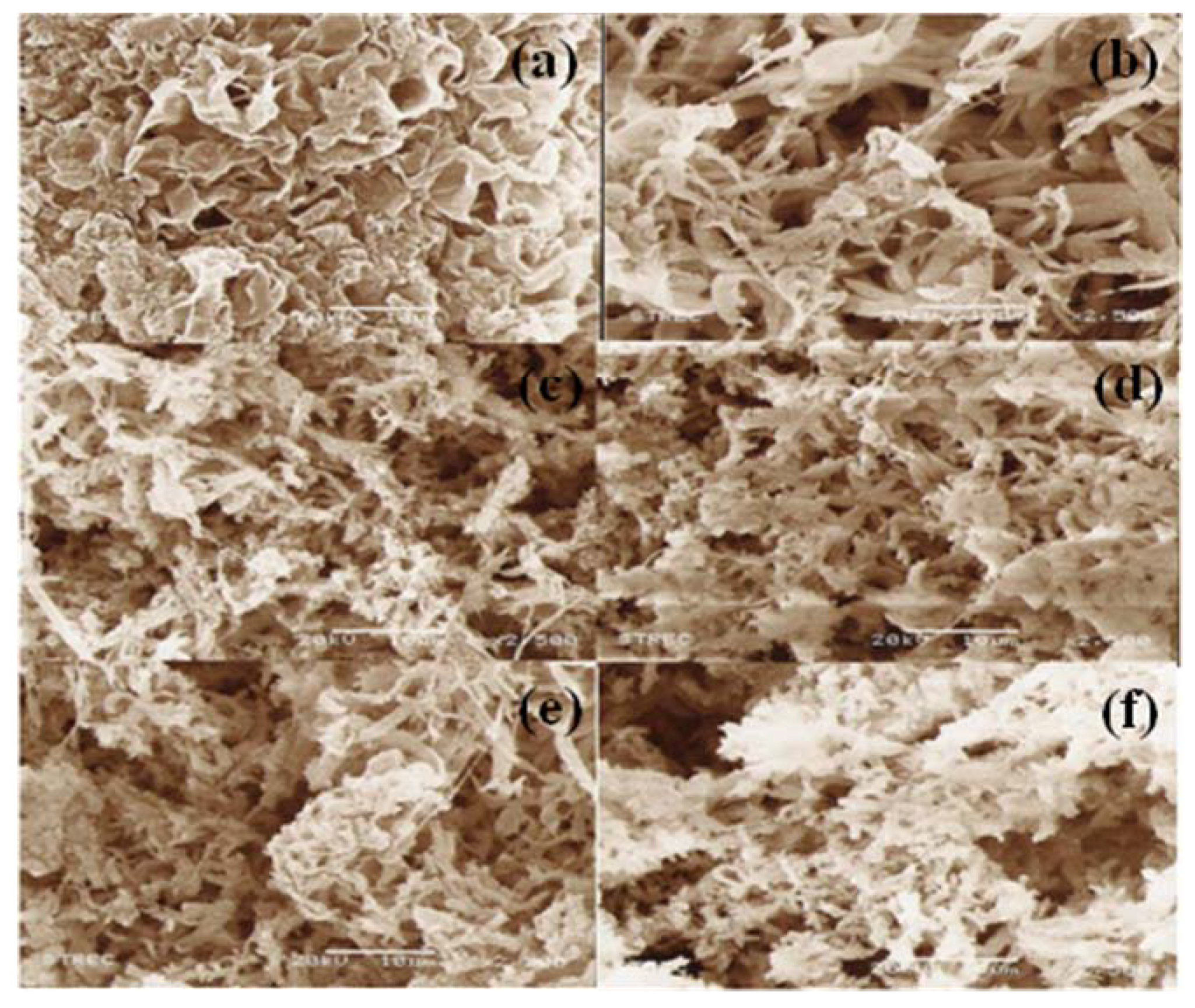
2.5. Morphology of polymers
3. Experimental
3.1. Materials
3.2. Titanium-FI complex synthesis
3.2.1. Synthesis of N-(3-tert-butylsalicylidene) aniline
3.2.2. Synthesis of Bis[N-(3-tert-butylsalicylidene)anilinato] titanium (IV) dichloride
3.3. Polymerization procedure
3.4. Characterization of polymers
4. Conclusions
Acknowledgements
References
- Hung, J.; Cole, A.P.; Waymouth, R.M. Control of sequence distribution of ethylene copolymers: Influence of comonomer sequence on the melting behavior of ethylene copolymers. Macromolecules 2003, 36, 2454–2463. [Google Scholar] [CrossRef]
- Makio, H.; Kashiwa, N.; Fujita, T. FI Catalysts: A New family of high performance catalysts for olefin polymerization. Adv. Syn. Catal. 2002, 344, 477–493. [Google Scholar] [CrossRef]
- Grieken, R.V.; Carrero, A.; Suarez, I.; Paredes, B. Effect of 1-hexene comonomer on polyethylene particle growth and kinetic profiles. Macromol. Sym. 2007, 259, 243–252. [Google Scholar] [CrossRef]
- Ishii, S.; Saito, J.; Mitani, M.; Mohr, J.; Matsukawa, N.; Tohi, Y.; Matsui, S.; Kashiwa, N.; Fujita, T. Highly active ethylene polymerization catalysts based on titanium complexes having two phenoxy-imine chelate ligands. J. Mol. Catal. A Chem. 2002, 179, 11–16. [Google Scholar] [CrossRef]
- Chaichana, E.; Khaubunsongserm, S.; Prasethdam, P.; Jongsomjit, B. Ethylene-hexene copolymer derived from [t-butylfluorenylsilyl-amido] dimethyl titanium complex. Express Polym. Lett. 2010, 4, 94–100. [Google Scholar] [CrossRef]
- Hong, H.; Zhang, Z.; Chung, T.C.M.; Lee, R.W. Synthesis of new 1-decene-based LLDPE resins and comparison with the corresponding 1-octene- and 1-hexene-based LLDPE resins. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 639–649. [Google Scholar] [CrossRef]
- Forlini, F.; Fan, Z.Q.; Tritto, I.; Locatelli, P.; Sacchi, M.C. Metallocene-catalyzed propene/1-hexene copolymerization: Influence of amount and bulkiness of cocatalyst and of solvent polarity. Macromol. Chem. Phys. 1997, 198, 2397–2408. [Google Scholar] [CrossRef]
- Matsui, S.; Fujita, T. FI Catalysts: Super active new ethylene polymerization catalysts. Catal. Today 2001, 66, 63–73. [Google Scholar] [CrossRef]
- Galland, G.B.; Seferin, M.; Mauler, R.S.; Santos, J.H.Z. Linear low-density polyethylene synthesis promoted by homogeneous and supported catalysts. Polym. Inter. 1999, 48, 660–664. [Google Scholar] [CrossRef]
- Desharun, C.; Jongsomjit, B.; Praserthdam, P. Study of LLDPE/alumina nanocomposites synthesized by in situ polymerization with zirconocene/d-MMAO catalyst. Catal. Commun. 2008, 9, 522–528. [Google Scholar] [CrossRef]
- Wongwaiwattanakul, P.; Jongsomjit, B. Copolymerization of ethylene/1-octene via different pore sized silica-based-supported zirconocene/dMMAO catalysts. Catal. Commun. 2008, 10, 118–122. [Google Scholar] [CrossRef]
- Bunchongturakarn, S.; Jongsomjit, B.; Praserthdam, P. Impact of bimodal pore MCM-41-supported zirconocene/dMMAO catalyst on copolymerization of ethylene/1-octene. Catal. Commun. 2008, 9, 789–795. [Google Scholar] [CrossRef]
- Jongsomjit, B.; Kaewkrajang, P.; Shiono, T.; Praserthdam, P. Supporting effects of silica-supported methylaliminoxane (MAO) with zirconocene catalyst on ethylene/1-olefin copolymerization behaviors for linear low-density polyethylene (LLDPE). Ind. Eng. Chem. Res. 2004, 43, 7959–7963. [Google Scholar] [CrossRef]
- Jongsomjit, B.; Ngamposri, S.; Praserthdam, P. Catalytic activity during copolymerization of ethylene and 1-hexene via mixed TiO2/SiO2-supported MAO with rac-Et[Ind]2ZrCl2 metallocene catalysts. Molecules 2005, 10, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Saito, J.; Mitani, M.; Matsui, S.; Tohi, Y.; Makio, H.; Nakano, T.; Tanaka, H.; Kashiwa, N.; Fujita, T. A new titanium complex having two phenoxy-imine chelate ligands for ethylene polymerization. Macromol. Chem. Phys. 2002, 203, 59–65. [Google Scholar] [CrossRef]
- Randall, J.C. A review of high resolution liquid 13carbon nuclear magnetic resonance characterizations of ethylene-based polymers. J. Macromol. Sci. Rev. Macromol. Chem. Phys. 1989, 29, 201–317. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| Entrya | Ethyleneb | Comonomer | Time | Weight | Activityc |
|---|---|---|---|---|---|
| [mol/L] | [mol/L] | [s] | [g] | [kg polymer/mol Ti·h] | |
| E-1 | 0.6 | 0 | 146.4 | 0.2881 | 2833 |
| E-2 | 0.6 | 0 | 133.2 | 0.3230 | 3491 |
| H-1 | 0.6 | 0.3 | 91.2 | 0.3496 | 5520 |
| H-2 | 0.6 | 0.45 | 91.8 | 0.3647 | 5720 |
| H-3 | 0.6 | 0.6 | 83.4 | 0.3766 | 6502 |
| H-4 | 0 | 0.6 | n.a.d | n.a.d | n.a.d |
| O-1 | 0.6 | 0.3 | 180 | 0.4405 | 3524 |
| O-2 | 0.6 | 0.45 | 135.6 | 0.3748 | 3980 |
| O-3 | 0.6 | 0.6 | 142.2 | 0.4470 | 4526 |
| O-4 | 0 | 0.6 | n.a.d | n.a.d | n.a.d |
| Entrya | Feed ratio [E]/[α-olefin] [mol/mol] | Melting pointc (Tm)[°C] | Crystallinityd (Xc)[%] | Molecular weighte (Mw)[kg/mol] | PDIe (Mw/Mn) |
|---|---|---|---|---|---|
| E-1 | 1/0 | 134.5 | 38.78 | 432 | 2.5 |
| E-2 | 1/0 | 134.3 | 64.01 | 920 | 1.60 |
| H-1 | 1/0.5 | 128.7 | 41.94 | 729 | 1.66 |
| H-2 | 1/0.75 | 126.3 | 44.93 | 768 | 1.69 |
| H-3 | 1/1 | 127.0 | 43.21 | 675 | 1.67 |
| H-4 | 0/1 | n.a.b | n.a.b | n.a.b | n.a.b |
| O-1 | 1/0.5 | 128.0 | 42.21 | 668 | 1.64 |
| O-2 | 1/0.75 | 127.2 | 48.13 | 641 | 1.63 |
| O-3 | 1/1 | 128.3 | 50.37 | 633 | 1.65 |
| O-4 | 0/1 | n.a.b | n.a.b | n.a.b | n.a.b |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kaivalchatchawal, P.; Suttipitakwong, P.; Samingprai, S.; Praserthdam, P.; Jongsomjit, B. The Influence of Comonomer on Ethylene/α-Olefin Copolymers Prepared Using [Bis(N-(3-tert butylsalicylidene)anilinato)] Titanium (IV) Dichloride Complex. Molecules 2011, 16, 1655-1666. https://doi.org/10.3390/molecules16021655
Kaivalchatchawal P, Suttipitakwong P, Samingprai S, Praserthdam P, Jongsomjit B. The Influence of Comonomer on Ethylene/α-Olefin Copolymers Prepared Using [Bis(N-(3-tert butylsalicylidene)anilinato)] Titanium (IV) Dichloride Complex. Molecules. 2011; 16(2):1655-1666. https://doi.org/10.3390/molecules16021655
Chicago/Turabian StyleKaivalchatchawal, Patcharaporn, Pattiya Suttipitakwong, Sutheerawat Samingprai, Piyasan Praserthdam, and Bunjerd Jongsomjit. 2011. "The Influence of Comonomer on Ethylene/α-Olefin Copolymers Prepared Using [Bis(N-(3-tert butylsalicylidene)anilinato)] Titanium (IV) Dichloride Complex" Molecules 16, no. 2: 1655-1666. https://doi.org/10.3390/molecules16021655
APA StyleKaivalchatchawal, P., Suttipitakwong, P., Samingprai, S., Praserthdam, P., & Jongsomjit, B. (2011). The Influence of Comonomer on Ethylene/α-Olefin Copolymers Prepared Using [Bis(N-(3-tert butylsalicylidene)anilinato)] Titanium (IV) Dichloride Complex. Molecules, 16(2), 1655-1666. https://doi.org/10.3390/molecules16021655




