Chemical and Pharmacological Aspects of Capsaicin
Abstract
:1. Introduction
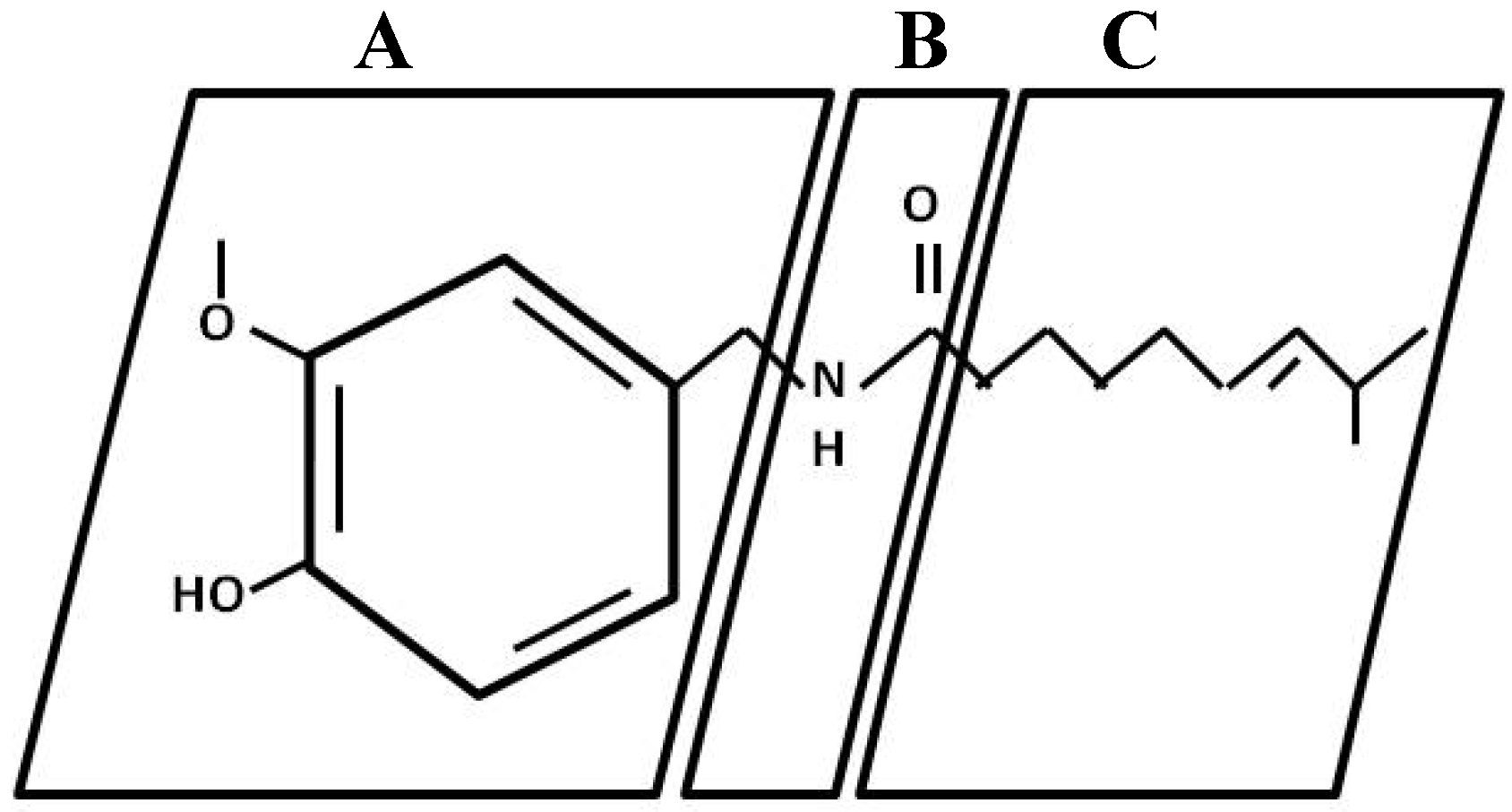
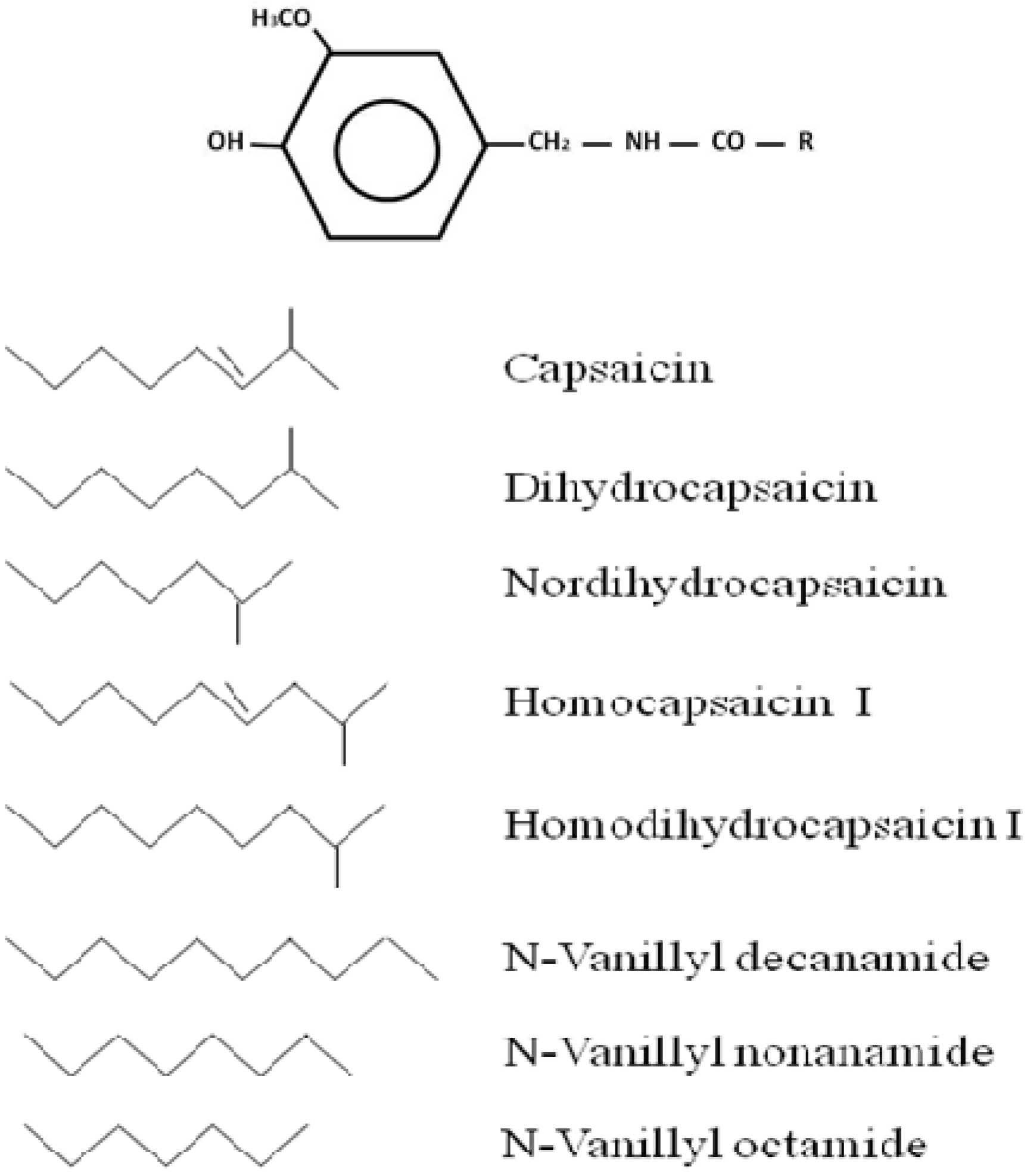
2. Capsaicin Production and Synthesis
2.1. Genetic Regulation of Capsaicin Synthesis
2.2. Capsaicin Biosynthesis in Plants
2.3. Chemical and Enzymatic Synthesis
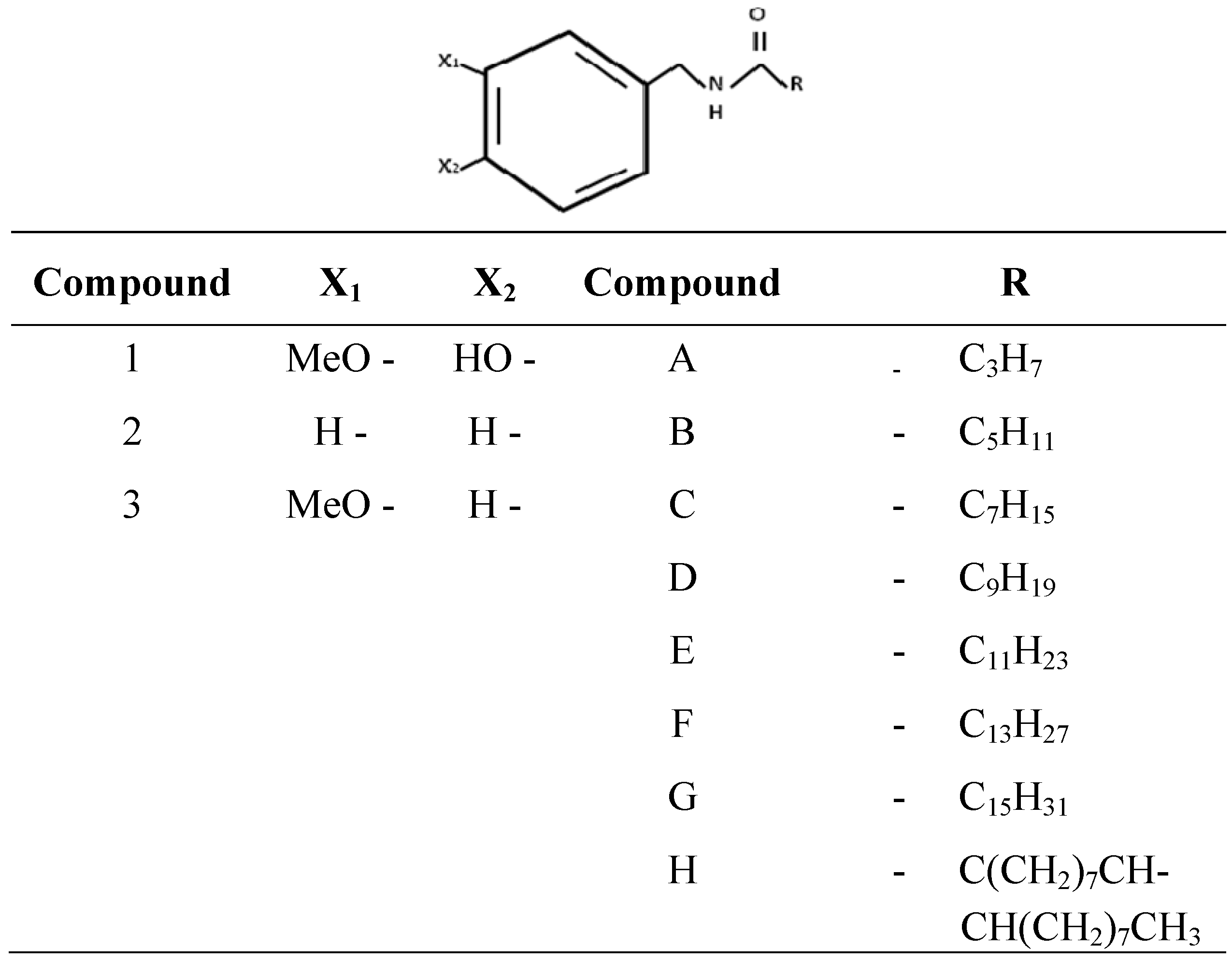
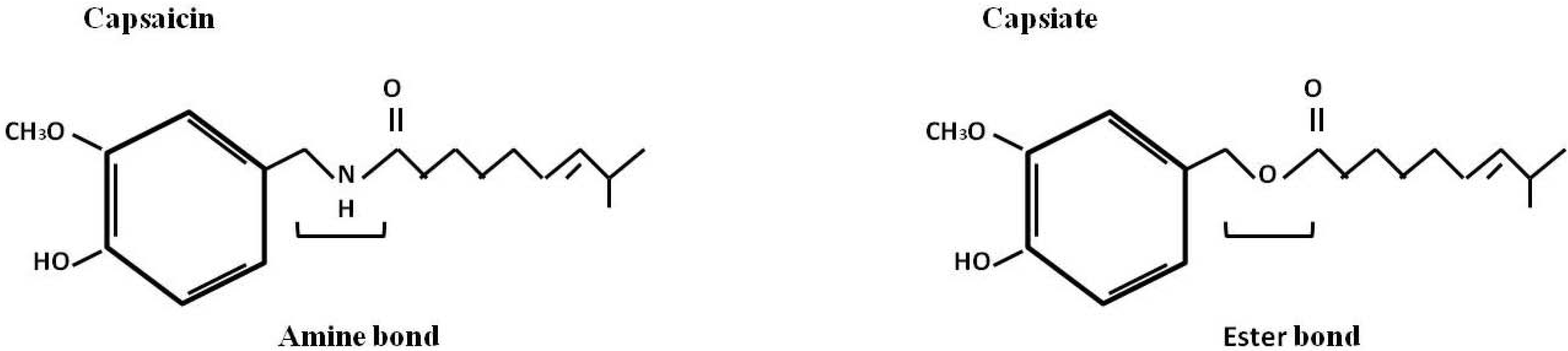
2.4. In Vitro Synthesis
3. Capsaicin Characterization
4. Capsaicin Pharmacology
4.1. Pharmacokinetics
4.2. Mechanism of Action
4.3. Clinical Applications in Humans
5. Conclusions
Acknowledgements
References
- Bernal, M.A.; Calderon, A.A.; Pedreno, M.A.; Muñoz, R.; Ros Barceló, A.; Merino de Caceres, F. Capsaicin oxidation by peroxidase from Capsicum annuum (variety Annuum) fruits. J. Agric. Food Chem. 1993, 41, 1041–1044. [Google Scholar] [CrossRef]
- Walpole, C.S.; Bevan, S.; Bloomfield, G.; Breckenridge, R.; James, I.F.; Ritchie, T.; Szallasi, A.; Winter, J.; Wrigglesworth, R. Similarities and differences in the structure-activity relationships of capsaicin and resiniferatoxin analogues. J. Med. Chem. 1996, 39, 2939–2952. [Google Scholar]
- Kobata, K.; Kawamura, M.; Toyoshima, M.; Tamura, Y.; Ogawa, S.; Watanabe, T. Lipase-catalyzed synthesis of capsaicin analogs by amidation of vanillylamine with fatty acid derivatives. Biotechnol. Lett. 1998, 20, 451–454. [Google Scholar]
- Nelson, E.K.; Dawson, L.E. The constitution of capsaicin, the pungent principle of Capsicum. III. J. Am. Chem. Soc. 1923, 45, 2179–2181. [Google Scholar]
- Available online: http://www.3dchem.com/molecules.asp?ID=105 accessed on 28 January 2011.
- Katritzky, A.R.; Xu, Y.J.; Vakulenko, A.V.; Wilcox, A.L.; Bley, K.R. Model compounds of caged capsaicin: design, synthesis, and photoreactivity. J. Org. Chem. 2003, 68, 9100–9104. [Google Scholar]
- Barbero, G.F.; Molinillo, J.M.G.; Varela, R.M.; Palma, M.; Macias, F.A.; Barroso, C.G. Application of Hansch´s model to capsaicinoids and capsinoids: a study using the quantitative structure-activity relationship. A novel method for the synthesis of capsinoids. J. Agric. Food Chem. 2010, 58, 3342–3349. [Google Scholar]
- Sung, Y.; Chang, Y.Y.; Ting, N.L. Capsaicin biosynthesis in water-stressed hot pepper fruits. Bot. Bull. Acad. Sin. 2005, 46, 35–42. [Google Scholar]
- Kaga, H.; Miura, M.; Orito, K. A facile procedure for synthesis of capsaicin. J. Org. Chem. 1989, 54, 3477–3478. [Google Scholar]
- Castillo, E.; Lopez-Gonzalez, I.; De Regil-Hernandez, R.; Reyes-Duarte, D.; Sánchez-Herrera, D.; López-Munguía, A.; Darszon, A. Enzymatic synthesis of capsaicin analogs and their effect on the T-type Ca2+ channels. Biochem. Biophys. Res. Comm. 2007, 356, 424–430. [Google Scholar] [CrossRef]
- Castillo, E.; Torres-Gavilán, A.; Severiano, P.; Arturo, N.; López-Munguía, A. Lipase-catalyzed synthesis of pungent capsaicin analogues. Food Chem. 2007, 100, 1202–1208. [Google Scholar] [CrossRef]
- Ochoa-Alejo, N.; Ramírez-Malagón, R. In vitro chili pepper biotechnology. In Vitro Cell. Dev. Biol. Plant. 2001, 37, 701–729. [Google Scholar] [CrossRef]
- Materska, M.; Perucka, I. Antioxidant activity of the main phenolic compounds isolated from hot pepper fruit (Capsicum annuum L.). J. Agric. Food Chem. 2005, 53, 1750–1756. [Google Scholar] [CrossRef]
- Macho, A.; Lucena, C.; Sancho, R.; Daddario, N.; Minassi, A.; Munoz, E.; Appendino, G. Non-pungent capsaicinoids from sweet pepper synthesis and evaluation of the chemopreventive and anticancer potential. Eur. J. Nutr. 2003, 42, 2–9. [Google Scholar] [CrossRef]
- Ohnuki, K.; Niwa, S.; Maeda, S.; Inoue, N.; Yazawa, S.; Fushiki, T. CH-19 sweet, a non-pungent cultivar of red pepper, increased body temperature and oxygen consumption in humans. Biosci. Biotechnol. Biochem. 2001, 65, 2033–2036. [Google Scholar] [CrossRef]
- Sancho, R.; Lucena, C.; Macho, A.; Calzado, M.A.; Blanco-Molina, M.; Minassi, A.; Appendino, G.; Muñoz, E. Immunosuppressive activity of capsaicinoids: capsiate derived from sweet peppers inhibits NF-κB activation and is a potent antiinflammatory compound in vivo. Eur. J. Immunol. 2002, 32, 1753–1763. [Google Scholar] [CrossRef]
- González Molinillo, J.M.; Macias Domínguez, F.A.; Varela Montoya, R.M.; Palma Lovillo, M.; García Barroso, C.; Fernández Barbero, G. Method for the chemical synthesis of capsinoids. US Patent 2010/0256413 A1.
- Lida, T.; Moriyama, T.; Kobata, K.; Morita, A.; Murayama, N.; Hashizume, S.; Fushiki, T.; Yazawa, S.; Watanabe, T.; Tominaga, M. TRPV1 activation and induction of nociceptive response by a non-pungent capsaicin-like compound, capsiate. Neuropharmacology 2003, 44, 958–967. [Google Scholar] [CrossRef]
- Harvell, K.P.; Bosland, P.W. The environment produces a significant effect on pungency of chiles. Hort. Sci. 1997, 32, 1292. [Google Scholar]
- Blum, E.; Mazourek, M.; O'Connell, M.; Curry, J.; Thorup, T.; Liu, K.; Jahn, M.; Paran, I. Molecular mapping of capsaicinoid biosynthesis genes and quantitative trait loci analysis for capsaicinoid content in Capsicum. Theor. Appl. Genet. 2003, 108, 79–86. [Google Scholar] [CrossRef]
- Curry, J.; Aluru, M.; Mendoza, M.; Nevarez, J.; Melendrez, M.; O'Connell, M.A. Transcripts for possible capsaicinoid biosynthetic genes are differentially accumulated in pungent and non-pungent Capsicum spp. Plant Sci. 1999, 148, 47–57. [Google Scholar] [CrossRef]
- Aluru, M.R.; Mazourek, M.; Landry, L.G.; Curry, J.; Jahn, M.; O´Conell, M.A. Differential expression of fatty acid synthase genes, Acl, Fat and Kas, in Capsicum fruit. J. Exp. Bot. 2003, 54, 1655–1664. [Google Scholar] [CrossRef]
- Andrews, J. Peppers: The Domesticated Capsicums; University of Texas Press: Austin, TX, USA, 1995; p. 274. [Google Scholar]
- Stewart, C.; Kang, B.C.; Liu, K.; Mazourek, M.; Moore, S.L.; Yoo, E.Y.; Kim, B.D.; Paran, I.; Jahn, M.M. The pun1 gene for pungency in pepper encodes a putative acyltransferase. Plant J. 2005, 42, 675–688. [Google Scholar]
- Prasad, B.C.; Kumar, V.; Gururaj, H.B.; Parimalan, R.; Giridhar, P.; Ravishankar, G.A. Characterization of capsaicin synthase and identification of its gene (csy1) for pungency factor capsaicin in pepper (Capsicum sp.). Proc. Natl. Acad. Sci. USA 2006, 103, 13315–13320. [Google Scholar]
- Stewart, C.; Mazourek, M.; Stellari, G.M.; O'Connell, M.; Jahn, M. Genetic control of pungency in C. chinense via the Pun1 locus. J. Exp. Bot. 2007, 58, 979–991. [Google Scholar] [CrossRef]
- Ochoa-Alejo, N.; Gomez-Peralta, J.E. Activity of enzymes involved in capsaicin biosynthesis in callus tissue and fruits of chili pepper (Capsicum annuum L.). J. Plant Physiol. 1993, 141, 147–152. [Google Scholar]
- Contreras-Padilla, M.; Yahia, E.M. Changes in capsaicinoids during development, maturation, and senescence of chile peppers and relation with peroxidase activity. J. Agric. Food Chem. 1998, 46, 2075–2079. [Google Scholar]
- Bernal, M.A.; Ros Barceló, A. 5,5´- dicapsaicin, 4´-O-5-dicapsaicin ether, and dehydrogenation polymers with high molecular weights are the main products of the oxidation of capsaicin by peroxidase from hot pepper. J. Agric. Food Chem. 1996, 43, 352–355. [Google Scholar]
- Estrada, B.; Pomar, F.; Díaz, J.; Merino, F.; Bernal, M.A. Pungency levels in fruits of the padron pepper with different water supply. Hort. Sci. 1999, 81, 385–396. [Google Scholar] [CrossRef]
- Prasad, N.C.; Gururaj, H.B.; Kumar, V.; Giridhar, P.; Parimalan, R.; Sharma, A.; Ravishankar, G.A. Influence of 8-methyl-nonenoic acid on capsaicin biosynthesis in in-vivo and in-vitro cell cultures of Capsicum spp. J. Agric. Food Chem. 2006, 54, 1854–1859. [Google Scholar]
- Burch, R.M.; Carter, R.B.; Lazar, J. Injectable capsaicin. Patent US 2005/0019436 A1, 2005. [Google Scholar]
- Iwai, K.; Watanabe, T.; Tamura, Y.; Ogawa, S. Method of producing capsaicin analogues. US Patent 6,022,718.
- Kobata, K.; Kobayashi, M.; Tamura, Y.; Miyoshi, S.; Ogawa, S.; Watanabe, T. Lipase-catalyzed synthesis of capsaicin analogs by transacylation of capsaicin with natural oils or fatty acid derivatives in n-hexane. Biotechnol. Lett. 1999, 21, 547–550. [Google Scholar] [CrossRef]
- Kobata, K.; Toyoshima, M.; Kawamura, M.; Watanabe, T. Lipase-catalyzed synthesis of capsaicin analogs using natural oils as an acyl donor. Biotechnol. Lett. 1998, 20, 781–783. [Google Scholar]
- Kaga, H.; Goto, K.; Takahashi, T.; Hino, M.; Tokuhashi, T.; Orito, K. A general and stereoselective synthesis of the capsaicinoids via the orthoester Claisen rearrangement. Tetrahedron 1996, 52, 8451–8470. [Google Scholar] [CrossRef]
- Choi, H.Y.; Yoon, S.H. Bioisoster of capsaicin: Synthesis of 1-hydroxy-2-pyridone analogue. Bull. Kor. Chem. Soc. 1999, 20, 857–859. [Google Scholar]
- Appendino, G.; Minassi, A.; Morello, A.S.; De Petrocellis, L.; Di Marzo, V. N-acylvanillamides: Development of an expeditious synthesis and discovery of new acyl templates for powerful activation of the vanilloid receptor. J. Med. Chem. 2002, 45, 3739–3745. [Google Scholar] [CrossRef]
- Torregiani, E.; Seu, G.; Minassi, A.; Appendino, G. Cerium (III) chloride promoted chemoselective esterification of phenolic alcohols. Tetrahedron Lett. 2005, 46, 2193–2196. [Google Scholar] [CrossRef]
- Sultana, I.; Shimamoto, M.; Obata, R.; Nishiyama, S.; Sugai, T. An expeditious chemo-enzymatic synthesis of dihydronorcapsaicin β-D-glucopyranoside. Sci. Technol. Adv. Mater. 2006, 7, 197–201. [Google Scholar] [CrossRef]
- Sutoh, K.; Kobata, K.; Yazawa, S.; Watanabe, T. Capsinoid is biosynthesized from phenylalanine and valine in a non-pungent pepper, Capsicum annuum L. cv. CH-19 sweet. Biosci. Biotechnol. Biochem. 2006, 70, 1513–1516. [Google Scholar]
- Amino, Y.; Kurosawa, W.; Nakano, T.; Hirasawa, K. Production method of capsinoid by dehydratation condensation, stabilizing method of capsinoid, and capsinoid composition. US Patent.
- Johnson, T.S.; Ravishankar, G.A.; Venkataraman, L.V. Biotransformation of ferulic acid and vanillylamine to capsaicin and vanillin in immobilized cell cultures of Capsicum frutescens. Plant Cell. Tiss. Organ. Cult. 1996, 44, 117–121. [Google Scholar] [CrossRef]
- Nuñez-Palenius, H.; Ochoa-Alejo, N. Effect of phenylalanine and phenylpropanoids on the accumulation of capsaicinoids and lignin in cell cultures of chili pepper (Capsicum annuum L.). In Vitro Cell. Dev. Biol. Plant. 2005, 41, 801–805. [Google Scholar] [CrossRef]
- Pandhair, V.; Gosal, S.S. Capsaicin production in cell suspension cultures derived from placenta of Capsicum annuum L. fruit. Indian J. Agric. Biochem. 2009, 22, 78–82. [Google Scholar]
- Gutiérrez-Carbajal, M.; Monforte-González, M.; Miranda-Ham, M.; Godoy-Hernández, G.; Vázquez-Flota, F. Induction of capsaicinoid synthesis in Capsicum chinense cell cultures by salicylic acid or methyl jasmonate. Biol. Plant. 2010, 54, 430–434. [Google Scholar] [CrossRef]
- Veeresham, C.; Kokate, C.K.; Apte, S.S.; Venkateshwarly, V. Effect of precursors on capsaicin Capsicum annuum. Plant Tiss. Cult. 1993, 3, 67–70. [Google Scholar]
- Johnson, T.; Sarada, R.; Ravishankart, G. Capsaicin formation in p-fluorophenylalanine resistant and normal cell cultures of Capsicum frutescens and activity of phenylalanine ammonia lyase. J. Biosci. 1998, 23, 209–212. [Google Scholar] [CrossRef]
- Sudha, G.; Ravishankar, G.A. Putrescine facilitated enhancement of capsaicin production in cell suspension cultures of Capsicum frutescens. J. Plant Physiol. 2003, 160, 339–346. [Google Scholar]
- Lindsey, K. Incorporation of [14C]phenylalanine and [14C]cinnamic acid into capsaicin in cultured cells of Capsicum frutescens. Phytochemistry 1986, 25, 2793–2801. [Google Scholar] [CrossRef]
- Johnson, T.S.; Ravishankar, G.A.; Venkataraman, L.V. In vitro capsaicin production by immobilized cells and placental tissues of Capsicum annuum L. grown in liquid medium. Plant Sci. 1990, 70, 223–229. [Google Scholar] [CrossRef]
- Spanyar, P.; Blazovich, M. A thin-layer chromatographic method for the determination of capsaicin in ground paprika. Analyst 1969, 94, 1084–1089. [Google Scholar] [CrossRef]
- Pankar, D.S.; Magar, N.G. New method for the determination of capsaicin by using multi-band thin-layer chromatography. J. Chromatogr. 1977, 144, 149–152. [Google Scholar] [CrossRef]
- Sato, K.; Sasaki, S.S.; Goda, Y.; Yamada, T.; Nunomura, O.; Ishikawa, K.; Maitani, T. Direct connection of supercritical fluid extraction and supercritical fluid chromatography as a rapid quantitative method for capsaicinoids in placentas of Capsicum. J. Agric. Food Chem. 1999, 47, 4665–4668. [Google Scholar] [CrossRef]
- Choi, S.H.; Suh, B.S.; Kozukue, E.; Kozukue, N.; Levin, C.E.; Friedman, M. Analysis of the contents of pungent compounds in fresh korean red peppers and in pepper-containing foods. J. Agric. Food Chem. 2006, 54, 9024–9031. [Google Scholar]
- Barbero, G.F.; Palma, M.; Barroso, C.G. Pressurized liquid extraction of capsaicinoids from peppers. J. Agric. Food Chem. 2006, 54, 3231–3236. [Google Scholar]
- Barbero, G.F.; Palma, M.; Barroso, C.G. Determination of capsaicinoids in peppers by microwave-assisted extraction-high-performance liquid chromatography with fluorescence detection. Anal. Chim. Acta. 2006, 578, 227–233. [Google Scholar] [CrossRef]
- Barbero, G.F.; Liazid, A.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of capsaicinoids from peppers. Talanta 2008, 75, 1332–1337. [Google Scholar] [CrossRef]
- Thapa, B.; Skalko-Basnet, N.; Takano, A.; Masuda, K.; Basnet, P. High-performance liquid chromatography analysis of capsaicin content in 16 Capsicum fruits from Nepal. J. Med. Food 2009, 12, 908–913. [Google Scholar] [CrossRef]
- Mueller-Seitz, E.; Hiepler, C.; Petz, M. Chili pepper fruits: content and pattern of capsaicinoids in single fruits of different ages. J. Agric. Food Chem. 2008, 56, 12114–12121. [Google Scholar] [CrossRef]
- Lu, J.; Cwik, M. Determination of capsaicin and zucapsaicin in human serum by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B Biomed. Sci. Appl. 1997, 701, 135–139. [Google Scholar] [CrossRef]
- Saria, A.; Lembeck, F.; Skofitsch, G. Determination of capsaicin in tissues and separation of capsaicin analogues by high-performance liquid chromatography. J. Chromatogr. 1981, 208, 41–46. [Google Scholar]
- Tucker, S.P. Determination of capsaicin and dihydrocapsaicin in air in a pickle and pepper processing plant. AIHAJ 2001, 62, 45–48. [Google Scholar]
- Kopec, S.E.; DeBellis, R.J.; Irwin, R.S. Chemical analysis of freshly prepared and stored capsaicin solutions: implications for tussigenic challenges. Pulm. Pharmacol. Ther. 2002, 15, 529–534. [Google Scholar] [CrossRef]
- Weaver, K.M.; Awde, D.B. Rapid high-performance liquid chromatographic method for the determination of very low capsaicin levels. J. Chromatogr. 1986, 367, 438–442. [Google Scholar]
- Henderson, D.E.; Slickman, A.M.; Henderson, S.K. Quantitative HPLC determination of the antioxidant activity of capsaicin on the formation of lipid hydroperoxides of linoleic acid: a comparative study against BHT and melatonin. J. Agric. Food Chem. 1999, 47, 2563–2570. [Google Scholar] [CrossRef]
- Kozukue, N.; Han, J.S.; Kozukue, E.; Lee, S.J.; Kim, J.A.; Lee, K.R.; Levin, C.E.; Friedman, M. Analysis of eight capsaicinoids in peppers and pepper-containing foods by high-performance liquid chromatography and liquid chromatography−mass spectrometry. J. Agric. Food Chem. 2005, 53, 9172–9181. [Google Scholar] [CrossRef]
- Friedman, M.; Levin, C.E.; Lee, S.U.; Lee, J.S.; Ohnisi-Kameyama, M.; Kozukue, N. Analysis by HPLC and LC/MS of pungent piperamides in commercial black, white, green, and red whole and ground peppercorns. J. Agric. Food Chem. 2008, 56, 3028–3036. [Google Scholar] [CrossRef]
- Kobata, K.; Saito, K.; Tate, H.; Nashimoto, A.; Okuda, H.; Takemura, I.; Miyakawa, K.; Takahashi, M.; Iwai, K.; Watanabe, T. Long-chain N-vanillyl-acylamides from Capsicum oleoresin. J. Agric. Food Chem. 2010, 58, 3627–3631. [Google Scholar]
- Srinivas, N.R. LC/MS/MS analysis of capsaicin using multiple transition pairs-some view points to ponder. Biomed. Chromatogr. 2009, 23, 1129–1130. [Google Scholar]
- Reilly, C.A.; Crouch, D.J.; Yost, G.S.; Fatah, A.A. Determination of capsaicin, dihydrocapsaicin, and nonivamide in self-defense weapons by liquid chromatography-mass spectrometry and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2001, 912, 259–267. [Google Scholar] [CrossRef]
- Reilly, C.A.; Crouch, D.J.; Yost, G.S.; Fatah, A.A. Determination of capsaicin, nonivamide, and dihydrocapsaicin in blood and tissue by liquid chromatography-tandem mass spectrometry. J. Anal. Toxicol. 2002, 26, 313–319. [Google Scholar]
- Garcés-Claver, A.; Arnedo-Andrés, M.S.; Abadía, J.; Gil-Ortega, R.; Alvarez-Fernandez, A. Determination of capsaicin and dihydrocapsaicin in Capsicum fruits by liquid chromatography-electrospray/time-of-flight mass spectrometry. J. Agric. Food Chem. 2006, 54, 9303–9311. [Google Scholar]
- Garcés-Claver, A.; Gil-Ortega, R.; Alvarez-Fernandez, A.; Arnedo-Andrés, M.S. Inheritance of capsaicin and dihydrocapsaicin, determined by HPLC-ESI/MS, in an intraspecific cross of Capsicum annuum L. J. Agric. Food Chem. 2007, 55, 6951–6957. [Google Scholar]
- Beaudry, F.; Vachon, P. Quantitative determination of capsaicin, a transient receptor potential channel vanilloid 1 agonist, by liquid chromatography quadrupole ion trap mass spectrometry: evaluation of in vitro metabolic stability. Biomed. Chromatogr. 2009, 23, 204–211. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Sheng, L.; Li, Y. Simultaneous quantification of capsaicin and dihydrocapsaicin in rat plasma using HPLC coupled with tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010, 878, 2292–2297. [Google Scholar] [CrossRef]
- Muller-Stock, F.A.; Joshi, R.K.; Buchi, J. Study of the components of capsaicin. Quantitative gas chromatographic determination of individual homologs and analogs of capsaicin in mixtures from a natural source and of vanillyl pelargonic amide as adulteration. J. Chromatogr. 1971, 63, 281–287. [Google Scholar]
- Ha, J.; Han, K.J.; Kim, K.J.; Jeong, S.W. Gas chromatographic analysis of capsaicin in Gochujang. J. Assoc. Anal. Chem. Int. 2008, 91, 387–391. [Google Scholar]
- Todd, P.H.J., Jr.; Bensinger, G.; Biftu, T. Determination of pungency due to Capsicum by gas-liquid chromatography. J. Food Sci. 1977, 42, 660–665. [Google Scholar]
- DiCecco, J.J. Gas-liquid chromatographic determination of capsaicin. J. Assoc. Anal. Chem. 1976, 59, 1–4. [Google Scholar]
- Thiele, R.; Mueller-Seitz, E.; Petz, M. Chili pepper fruits: presumed precursors of fatty acids characteristic for capsaicinoids. J. Agric. Food Chem. 2008, 56, 4219–4224. [Google Scholar] [CrossRef]
- Peña-Alvarez, A.; Ramirez-Maya, E.; Alvarado-Suarez, L.A. Analysis of capsaicin and dihydrocapsaicin in peppers and pepper sauces by solid phase microextraction-gas chromatography-mass spectrometry. J. Chromatogr. A 2009, 1216, 2843–2847. [Google Scholar] [CrossRef]
- Laskaridou-Monnerville, A. Determination of capsaicin and dihydrocapsaicin by micellar electrokinetic capillary chromatography and its application to various species of Capsicum, Solanaceae. J. Chromatogr. A 1999, 838, 293–302. [Google Scholar]
- Aranda, F.J.; Villalain, J.; Gomez-Fernandez, J.C. Capsaicin affects the structure and phase organization of phospholipid membranes. Biochim. Biophys. Acta 1995, 1234, 225–234. [Google Scholar] [CrossRef]
- Peng, A.; Ye, H.; Li, X.; Chen, L. Preparative separation of capsaicin and dihydrocapsaicin from Capsicum frutescens by high-speed counter-current chromatography. J. Sep. Sci. 2009, 32, 2967–2973. [Google Scholar] [CrossRef]
- Nazari, F.; Ebrahimi, S.N.; Talebi, M.; Rassouli, A.; Bijanzadeh, H.R. Multivariate optimisation of microwave-assisted extraction of capsaicin from Capsicum frutescens L. and quantitative analysis by 1H-NMR. Phytochem. Anal. 2007, 18, 333–340. [Google Scholar] [CrossRef]
- Higashiguchi, F.; Nakamura, H.; Hayashi, H.; Kometani, T. Purification and structure determination of glucosides of capsaicin and dihydrocapsaicin from various Capsicum fruits. J. Agric. Food Chem. 2006, 54, 5948–5953. [Google Scholar] [CrossRef]
- Davis, C.B.; Markey, C.E.; Busch, M.A.; Busch, K.W. Determination of capsaicinoids in habanero peppers by chemometric analysis of UV spectral data. J. Agric. Food Chem. 2007, 55, 5925–5933. [Google Scholar]
- Kachoosangi, R.T.; Wildgoose, G.G.; Compton, R.G. Carbon nanotube-based electrochemical sensors for quantifying the 'heat' of chilli peppers: the adsorptive stripping voltammetric determination of capsaicin. Analyst 2008, 133, 888–895. [Google Scholar]
- Alberti, A.; Galasso, V.; Kovac, B.; Modelli, A.; Pichierri, F. Probing the molecular and electronic structure of capsaicin: a spectroscopic and quantum mechanical study. J. Phys. Chem. A 2008, 112, 5700–5711. [Google Scholar] [CrossRef]
- Pershing, L.K.; Reilly, C.A.; Corlett, J.L.; Crouch, D.J. Effects of vehicle on the uptake and elimination kinetics of capsaicinoids in human skin in vivo. Toxicol. Appl. Pharmacol. 2004, 200, 73–81. [Google Scholar] [CrossRef]
- Suresh, D.; Srinivasan, K. Tissue distribution and elimination of capsaicin, piperine and curcumin following oral intake in rats. Indian J. Med. Res. 2010, 131, 682–691. [Google Scholar]
- Chanda, S.; Bashir, M.; Babbar, S.; Koganti, A.; Bley, K. In vitro hepatic and skin metabolism of capsaicin. Drug Metab. Dispos. 2008, 36, 670–675. [Google Scholar]
- Kawada, T.; Iwai, K. In vivo and in vitro metabolism of dihydrocapsaicin, a pungent principle of hot pepper, in rats. Agric. Biol. Chem. 1985, 49, 441–448. [Google Scholar] [CrossRef]
- Cortright, D.N.; Szallasi, A. Biochemical pharmacology of the vanilloid receptor TRPV1. Eur. J. Biochem. 2004, 271, 1814–1819. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Tominaga, M.; Tominaga, T. Structure and function of TRPV1. Pflugers Arch. 2005, 451, 143–150. [Google Scholar] [CrossRef]
- Liu, M.; Liu, M.C.; Magoulas, C.; Priestley, J.V.; Willmott, N.J. Versatile regulation of cytosolic Ca2+ by vanilloid receptor I in rat dorsal root ganglion neurons. J. Biol. Chem. 2003, 278, 5462–5472. [Google Scholar]
- Kárai, L.J.; Russell, J.T.; Iadarola, M.J.; Oláh, Z. Vanilloid receptor 1 regulates multiple calcium compartments and contributes to Ca2+-induced Ca2+ release in sensory neurons. J. Biol. Chem. 2004, 279, 16377–16387. [Google Scholar]
- Morita, A.; Iwasaki, Y.; Kobata, K.; Lida, T.; Higashi, T.; Oda, K.; Susuki, A.; Narukawa, M.; Sasakuma, S.; Yokogoshi, H.; Yazawa, S.; Tominaga, M.; Watanabe, T. Lipophilicity of capsaicinoids and capsinoids influences the multiple activation process of rat TRPV1. Life Sci. 2006, 79, 2303–2310. [Google Scholar]
- Pingle, S.C.; Matta, J.A.; Ahern, G.P. Capsaicin receptor: TRPV1 a promiscuous TRP channel. Handb. Exp. Pharmacol. 2007, 179, 155–171. [Google Scholar] [CrossRef]
- Bevan, S.; Szolcsanyi, J. Sensory neuron-specific actions of capsaicin: mechanisms and applications. Trends Pharmacol. Sci. 1990, 11, 330–333. [Google Scholar]
- Saria, A.; Lundberg, J.M.; Hua, X.; Lembeck, F. Capsaicin-induced substance P release and sensory control of vascular permeability in the guinea-pig ureter. Neurosci. Lett. 1983, 41, 167–172. [Google Scholar] [CrossRef]
- Jhamandas, K.; Yaksh, T.L.; Harty, G.; Szolcsanyi, J.; Go, V.L. Action of intrathecal capsaicin and its structural analogues on the content and release of spinal substance P: selectivity of action and relationship to analgesia. Brain Res. 1984, 306, 215–225. [Google Scholar] [CrossRef]
- Purkiss, J.; Welch, M.; Doward, S.; Foster, K. Capsaicin-stimulated release of substance P from cultured dorsal root ganglion neurons: involvement of two distinct mechanisms. Biochem. Pharmacol. 2000, 59, 1403–1406. [Google Scholar] [CrossRef]
- Szolcsanyi, J.; Oroszi, G.; Nemeth, J.; Szilvassy, Z.; Tosaki, A. Endothelin release by capsaicin in isolated working rat heart. Eur. J. Pharmacol. 1999, 376, 247–250. [Google Scholar] [CrossRef]
- Dutta, A.; Deshpande, S.B. Mechanisms underlying the hypertensive response induced by capsaicin. Int. J. Cardiol. 2010, 145, 358–359. [Google Scholar] [CrossRef]
- Holzer, P.; Lippe, I.T. Stimulation of afferent nerve endings by intragastric capsaicin protects against ethanol-induced damage of gastric mucosa. Neuroscience 1988, 27, 981–987. [Google Scholar] [CrossRef]
- Szolcsanyi, J. Effect of capsaicin, resiniferatoxin and piperine on ethanol-induced gastric ulcer of the rat. Acta Physiol. Hung. 1990, 75 (Suppl.), 267–268. [Google Scholar]
- Mozsik, G.; Szolcsanyi, J.; Racz, I. Gastroprotection induced by capsaicin in healthy human subjects. World J. Gastroenterol. 2005, 11, 5180–5184. [Google Scholar]
- Kawada, T.; Hagihara, K.; Iwai, K. Effects of capsaicin on lipid metabolism in rats fed a high fat diet. J. Nutr. 1986, 116, 1272–1278. [Google Scholar]
- Watanabe, T.; Kawada, T.; Yamamoto, M.; Iwai, K. Capsaicin, a pungent principle of hot red pepper, evokes catecholamine secretion from the adrenal medulla of anesthetized rats. Biochem. Biophys. Res. Commun. 1987, 142, 259–264. [Google Scholar] [CrossRef]
- Backonja, M.M.; Malan, T.P.; Vanhove, G.F.; Tobias, J.K. NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomized, double-blind, controlled study with an open-label extension. Pain Med. 2010, 11, 600–608. [Google Scholar]
- Tesfaye, S. Advances in the management of diabetic peripheral neuropathy. Curr. Opin. Support. Palliat. Care. 2009, 3, 136–143. [Google Scholar] [CrossRef]
- Sawynok, J. Topical analgesics in neuropathic pain. Curr. Pharm. Des. 2005, 11, 2995–3004. [Google Scholar] [CrossRef]
- Derry, S.; Lloyd, R.; Moore, R.A.; McQuay, H.J. Topical capsaicin for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2009. CD007393. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Reyes-Escogido, M.D.L.; Gonzalez-Mondragon, E.G.; Vazquez-Tzompantzi, E. Chemical and Pharmacological Aspects of Capsaicin. Molecules 2011, 16, 1253-1270. https://doi.org/10.3390/molecules16021253
Reyes-Escogido MDL, Gonzalez-Mondragon EG, Vazquez-Tzompantzi E. Chemical and Pharmacological Aspects of Capsaicin. Molecules. 2011; 16(2):1253-1270. https://doi.org/10.3390/molecules16021253
Chicago/Turabian StyleReyes-Escogido, Maria De Lourdes, Edith G. Gonzalez-Mondragon, and Erika Vazquez-Tzompantzi. 2011. "Chemical and Pharmacological Aspects of Capsaicin" Molecules 16, no. 2: 1253-1270. https://doi.org/10.3390/molecules16021253
APA StyleReyes-Escogido, M. D. L., Gonzalez-Mondragon, E. G., & Vazquez-Tzompantzi, E. (2011). Chemical and Pharmacological Aspects of Capsaicin. Molecules, 16(2), 1253-1270. https://doi.org/10.3390/molecules16021253



