Synthesis and Screening of Aromatase Inhibitory Activity of Substituted C19 Steroidal 17-Oxime Analogs
Abstract
:1. Introduction
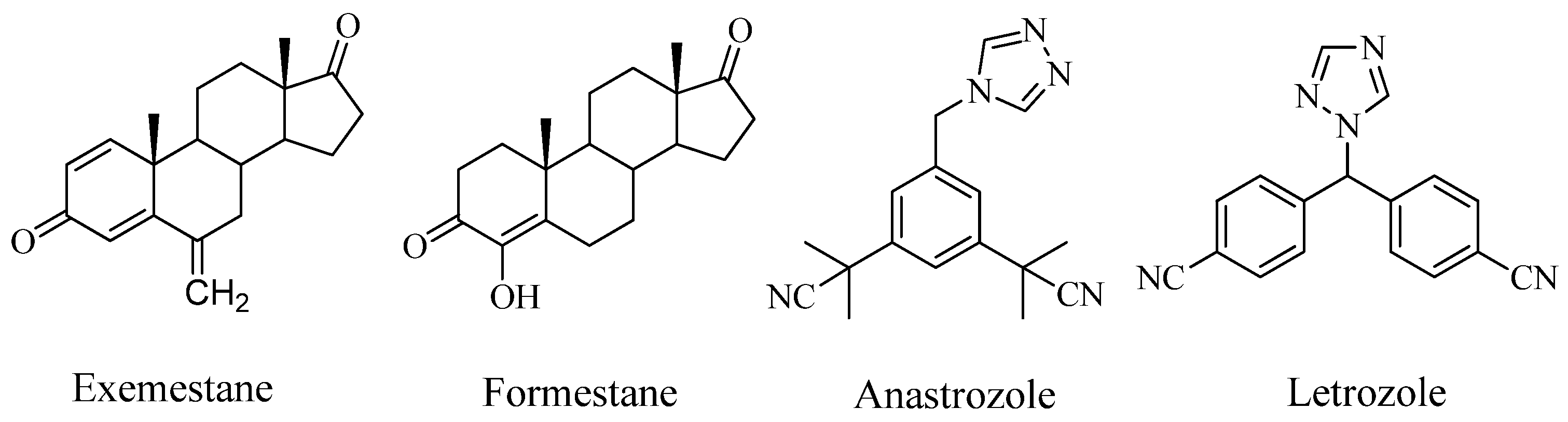
2. Results and Discussion
2.1. Synthesis
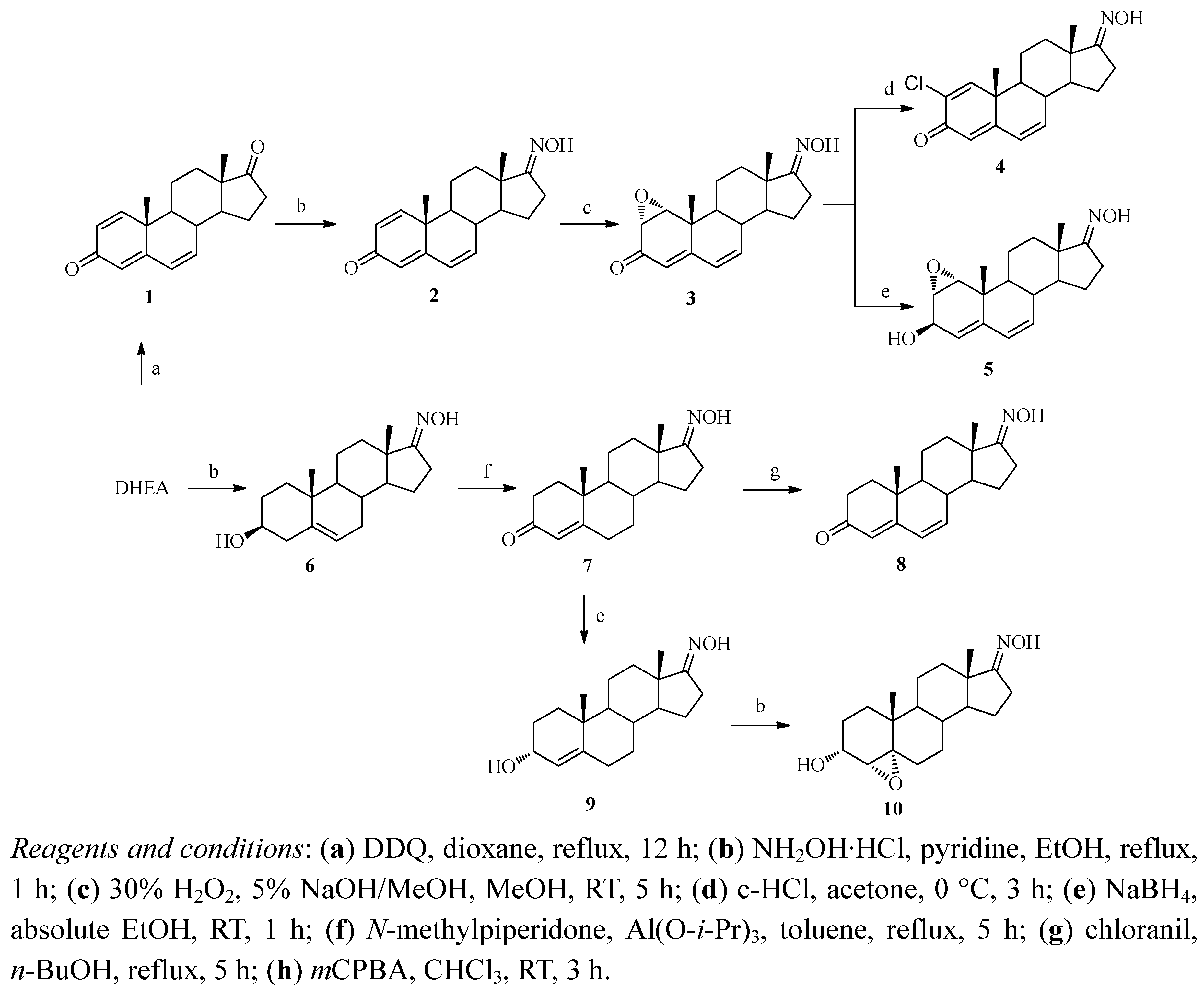
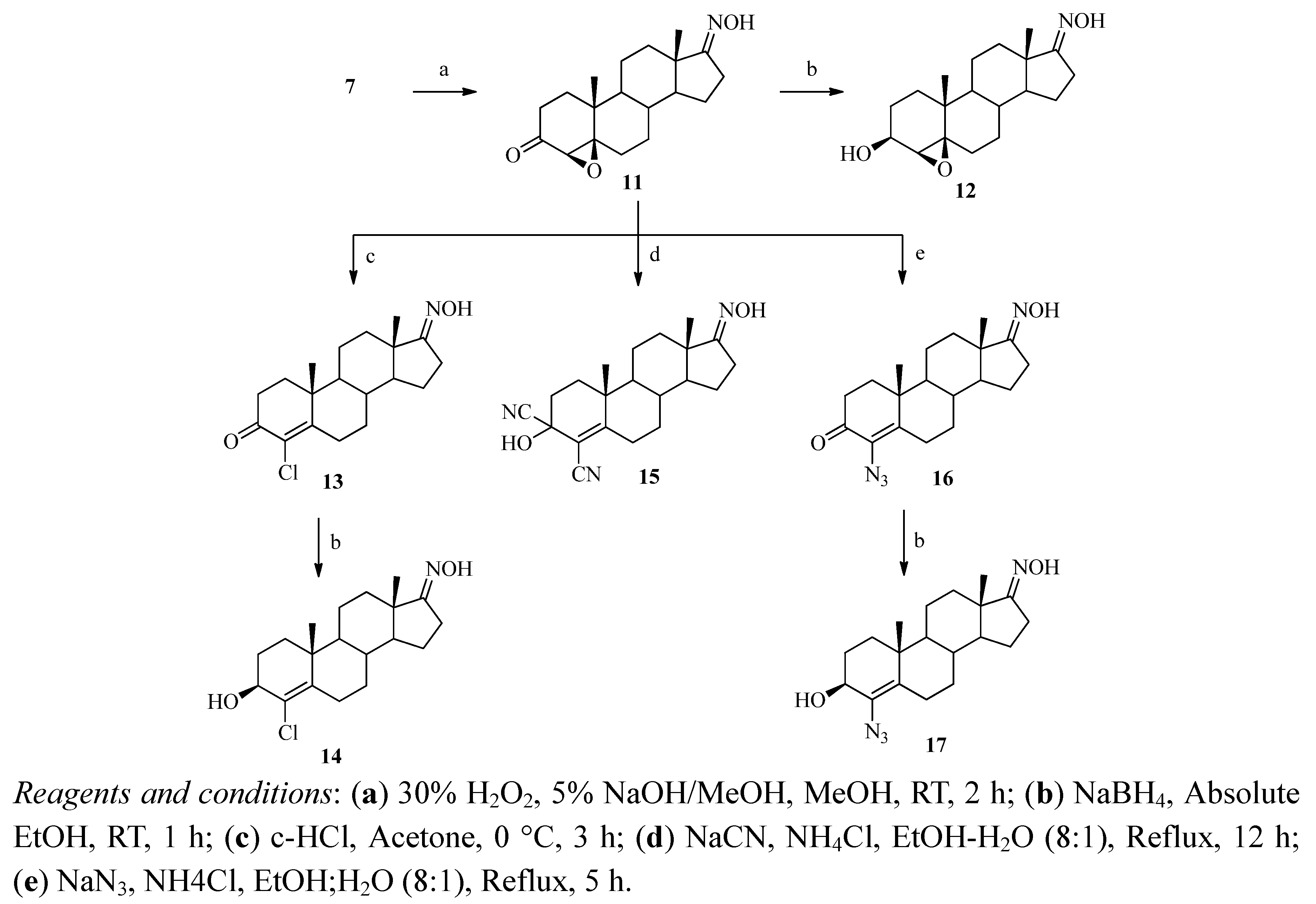
2.2. Aromatase Inhibitory Activity
| Compounds | % Inhibition * | Compounds | % Inhibition |
|---|---|---|---|
| 1 | 92.9 ± 5.4 | 10 | 79.9 ± 4.1 |
| 2 | 71.1 ± 4.8 | 11 | 81.2 ± 3.8 |
| 3 | 72.2 ± 4.1 | 12 | 88.1 ± 2.4 |
| 4 | 70.1 ± 3.9 | 13 | 75.4 ± 3.0 |
| 5 | 84.6 ± 2.8 | 14 | 93.8 ± 1.1 |
| 6 | 82.3 ± 3.5 | 15 | 85.2 ± 2.6 |
| 7 | 79.6 ± 4.6 | 16 | 76.7 ± 3.6 |
| 8 | 76.7 ± 2.7 | 17 | 32.8 ± 5.1 |
| 9 | 74.2 ± 3.3 | Formestane | 74.2 ± 2.2 |
3. Experimental
3.1. General
3.2. Synthesis
3.2.1. 1,4,6-Androstatriene-3,17-dione 17-oxime (2)
3.2.2. 1α,2α-Epoxy-4,6-androstadiene-3,17-dione 17-oxime (3)
3.2.3. 2-Chloro-1,4,6-androstatriene-3,17-dione 17-oxime (4)
3.2.4. 1α,2α-Epoxy-3β-hydroxy-2,4-androstadien-17-one Oxime (5)
3.2.5. 3β-Hydroxy-5-androsten-17-one Oxime (6)
3.2.6. 4-Androstene-3,17-dione 17-oxime (7)
3.2.7. 2,4-Androstadiene-3,17-dione 17-oxime (8)
3.2.8. 3α-Hydroxy-4-androsten-17-one Oxime (9)
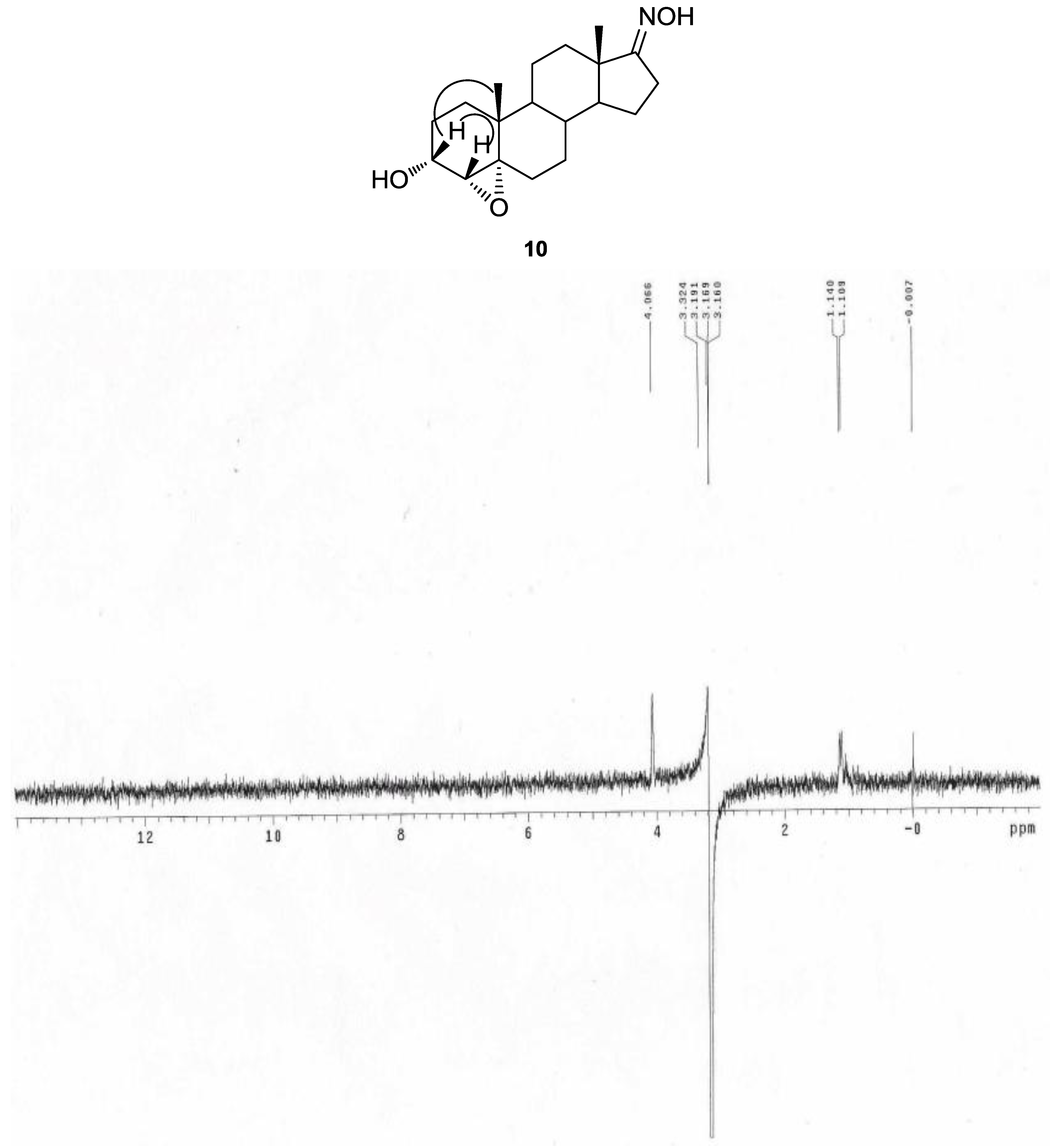
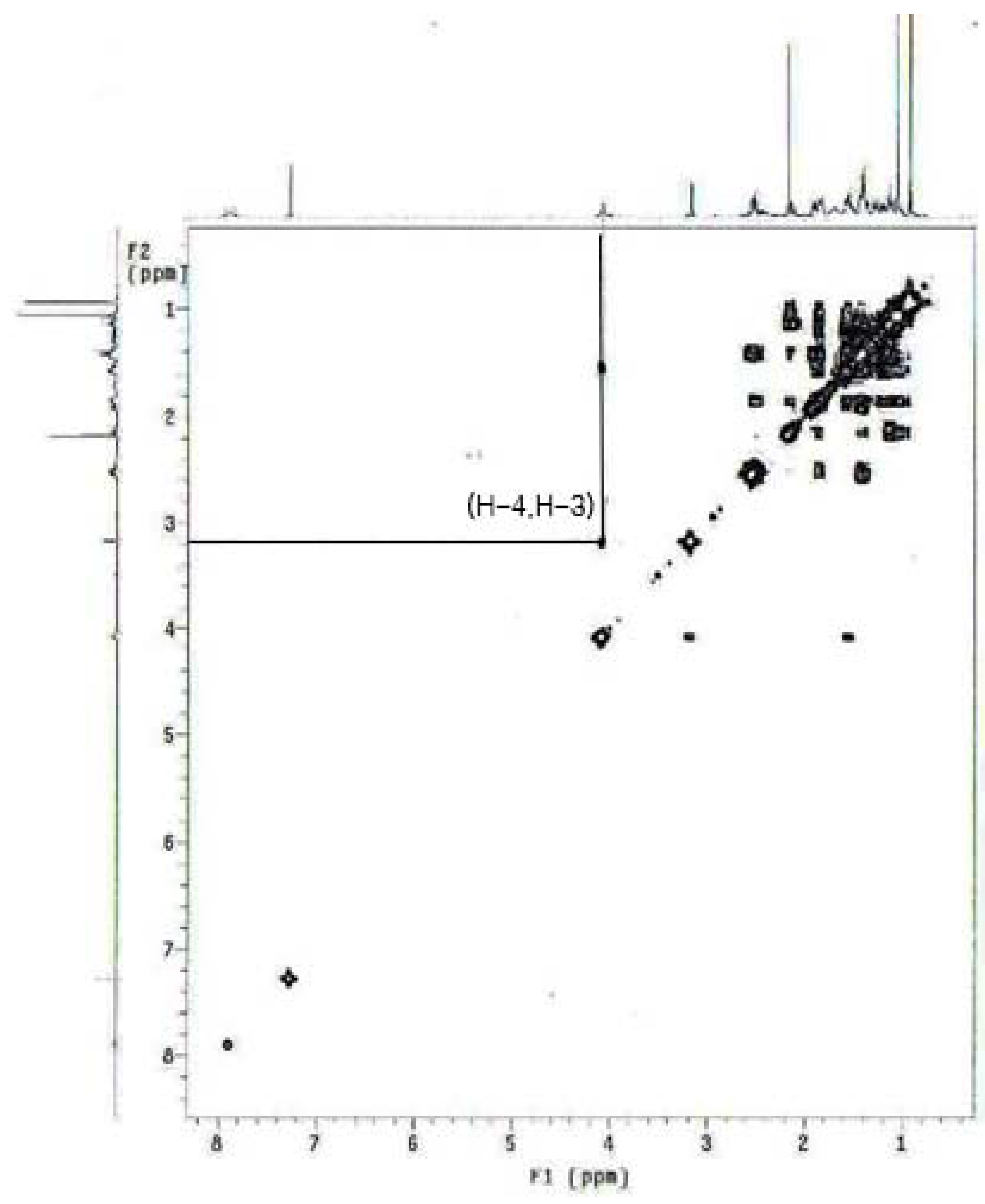
3.2.9. 4α,5α-Epoxy-3α-hydroxyandrostan-17-one Oxime (10)
3.2.10. 4β,5β-Epoxyandrostane-3,17-dione 17-oxime (11)
3.2.11. 4β,5β-Epoxy-3β-hydroxyandrostan-17-one oxime (12)
3.2.12. 4-Chloro-4-androstene-3,17-dione 17-oxime (13)
3.2.13. 4-Chloro-3β-hydroxy-4-androsten-17-one Oxime (14)
3.2.14. 3,4-Dicyano-3-hydroxy-4-androsten-17-one Oxime (15)
3.2.15. 4-Azido-4-androstene-3,17-dione 17-oxime (16)
3.2.16. 4-Azido-3β-hydroxy-4-androsten-17-one Oxime (17)
3.3. Aromatase Inhibition Assay
3.3.1. Enzyme Preparation
3.3.2. Inhibition Study
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
References and Notes
- Kellis, J., Jr.; Vickery, L.E. Purification and characterization of human placental aromatase cytochrome P-450. J. Biol. Chem. 1987, 262, 4413–4420. [Google Scholar]
- Clarke, L.H.; Olivio, S.; Kerr, L.; Bouker, K.B.; Clarke, R. Do estrogens always increase breast cancer risk? J. Steroid Biochem. Mol. Biol. 2002, 80, 163–174. [Google Scholar] [CrossRef]
- Woo, P.M.; Woo, L.W.L.; Humphreys, A.; Chander, S.K. A letrozole-based dual aromatase-sulphatase inhibitor with in vivo activity. J. Steroid Biochem. Molec. Biol. 2005, 94, 123–130. [Google Scholar] [CrossRef]
- Chetrite, G.S.; Prieto, J.C.C.; Philippe, J.C.; Pasqualini, J.R. Estradiol inhibits the estrone sulfatase activity in normal and cancerous human breast tissues. J. Steroid Biochem. Mol. Biol. 2007, 104, 289–292. [Google Scholar] [CrossRef]
- Kendall, A.; Folkerd, E.J.; Dowsett, M. Influences on circulating oestrogens in postmenopausal women: Relationship with breast cancer. J. Steroid Biochem. Mol. Biol. 2007, 103, 99–109. [Google Scholar] [CrossRef]
- Brueggemeier, R.W.; Hackett, J.C.; Diaz-Cruz, E.S. Aromatase inhibitors in the treatment of breast cancer. Endocr. Rev. 2005, 26, 331–345. [Google Scholar] [CrossRef]
- Sikora, M.J.; Condero, K.E.; Larios, J.M.; Johnson, M.D.; Lippman, M.E.; Rae, J.M. The androgen metabolic 5-androstane-3,17-diol (3-Adiol) induces breast cancer growth via estrogen receptor; implication for aromatase inhibitor resistance. Breast Cancer Res. Treat. 2009, 115, 289–296. [Google Scholar] [CrossRef]
- Schwarzel, W.C.; Kruggel, W.G.; Brodie, H.J. Studies on the mechanism of estrogen biosynthesis. VII. The development of inhibitors of the enzyme system in human placenta. Endocrinology 1973, 92, 866–880. [Google Scholar] [CrossRef]
- Brodie, A.M.H.; Garrett, W.M.; Hendrickson, J.R.; Marcotte, P.A.; Robinson, C.H. Inactivation of aromatase activity in placental and ovarian microsomes by 4-hydroxyandrostene-3,17-dione and 4-acetoxyandrostenedione-3,17-dione. Steroids 1981, 38, 693–702. [Google Scholar] [CrossRef]
- Disalle, E.; Giudici, D.; Ornati, G.; Briatico, G.; D’Alessio, R.; Villa, V.; Lombardi, P. 4-Aminoandrostenedione derivatives: A novel class of irreversible aromatase inhibitors. Comparison with FCE24304 and 4-hydroxyandrostenedione. J. Steroid Biochem. Mol. Biol. 1990, 37, 369–374. [Google Scholar] [CrossRef]
- Lesuisse, D.; Gourvest, J.F.; Hartman, C.; Tric, B.; Benslimane, O.; Philibert, D.; Vevert, J.P. Synthesis and evaluation of a new series of mechanism-based aromatase inhibitors. J. Med. Chem. 1992, 35, 1588–1597. [Google Scholar]
- Abul-Hajj, Y.J.; Liu, X.-P.; Hedge, M. Synthesis and evaluation of substituted-4-androstene-3,17-dione derivatives as aromatase inhibitors. J. Steroid Biochem. Mol. Chem. 1995, 54, 111–119. [Google Scholar] [CrossRef]
- Rodríguez, J.; Nunez, L.; Peixinbo, S.; Jimenez, C. Isolation and synthesis of the first natural 6-hydroxyimino-4-en-3-one steroids from the sponges Cinachyrella spp. Tetrahedron Lett. 1997, 38, 1833–1836. [Google Scholar] [CrossRef]
- Deive, N.; Rodriguez, J.; Jimenez, C. Synthesis of cytotoxic 6E-hydroxyimino-4-ene steroids: Structure/activity studies. J. Med. Chem. 2001, 44, 2612–2618. [Google Scholar] [CrossRef]
- Cui, J.G.; Fan, I.; Huang, L.I.; Liu, H.L.; Zhou, A.M. Synthesis and evaluation of some steroid oximes as cytotoxic agents: Structure/activity studies (I). Steroids 2009, 74, 62–72. [Google Scholar] [CrossRef]
- Poza, J.; Rega, M.; Paz, V.; Alonso, B.; Rodriguez, J.; Salvador, N. Synthesis and evaluation of new 6-hydroxyiminosteroid analogues as cytotoxic agents. Bioorg. Med. Chem. 2007, 15, 4722–4740. [Google Scholar]
- Hollands, H.L.; Kumaresan, S.; Tan, L.; Nzar, V.C.O. Synthesis of 6-hydroximino-3-oxo steroids, a new class of aromatase inhibitor. J. Chem. Soc. Perkin Trans. 1 1992, 13, 585–587. [Google Scholar]
- Jindal, D.P.; Chattopadhaya, R.; Guleria, S.; Gupta, R. Synthesis and antineoplastic activity of 2-alkylaminoethyl derivatives of various steroidal oximes. Eur. J. Med. Chem. 2003, 38, 1025–1034. [Google Scholar] [CrossRef]
- Covey, D.F.; Hood, H.F. Enzyme-generated intermediates derived from 4-androstene-3,6,17-trione and 1,4,6-androstatriene-3,17-dione. Endocrinology 1981, 108, 1597–1599. [Google Scholar] [CrossRef]
- Gamoh, K.; Hirayama, M.; Ikekawa, N. Stereocontrolled synthesis of Withanolide D and related compounds. J. Chem. Soc. Perkin Trans. 1 1984, 440–454. [Google Scholar]
- Sharpless, K.B.; Verhoeven, T.R. Metal-catalysed, highly selective oxygenations of olefins and acetylenes with tert-butyl hydroperoxide. Practical considerations and mechanisms. Aldrichim. Acta 1979, 12, 63–75. [Google Scholar]
- Ryan, K.J. Biological aromatization of steroids. J. Biol. Chem. 1959, 234, 268–272. [Google Scholar]
- Thompson, A.E., Jr.; Siiteri, P.K. The involvement of human microsomal cytochrome P-450 in aromatization. J. Biol. Chem. 1975, 249, 5373–5378. [Google Scholar]
- Reed, K.C.; Ohno, S. Kinetic properties of human placental aromatase. J. Biol. Chem. 1976, 251, 1625–1631. [Google Scholar]
- Numazawa, M.; Mutsumi, A.; Tachibana, M.; Hoshi, K. Synthesis of androst-5-en-7-ones and androst-3,5-diene-7-ones and their related 7-deoxyanalogues as conformational and catalytic probes for the active site on aromatase. J. Med. Chem. 1994, 37, 2198–2205. [Google Scholar] [CrossRef]
- Numazawa, M.; Yamaguchi, S. Synthesis and structure-activity relationships of 6-phenylaliphatic-substituted C19 steroids having a 1,4-diene, 4,6-diene or 1,4,6-triene structure as aromatase inhibitors. Steroids 2001, 64, 187–196. [Google Scholar] [CrossRef]
- Abul-Hajj, Y.J. Aromatase inhibition by 4-thiosubstituted-4-androstene-3,17-dione derivatives. J. Steroid Biochem. 1990, 35, 139–143. [Google Scholar] [CrossRef]
- Ma, E.; Kim, E. Epoxidation and reduction of DHEA, 1,4,6-androstatrien-3-one and 4,6-androstadien-3β,17β-diol. Molecules 2005, 10, 572–582. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pokhrel, M.; Ma, E. Synthesis and Screening of Aromatase Inhibitory Activity of Substituted C19 Steroidal 17-Oxime Analogs. Molecules 2011, 16, 9868-9885. https://doi.org/10.3390/molecules16129868
Pokhrel M, Ma E. Synthesis and Screening of Aromatase Inhibitory Activity of Substituted C19 Steroidal 17-Oxime Analogs. Molecules. 2011; 16(12):9868-9885. https://doi.org/10.3390/molecules16129868
Chicago/Turabian StylePokhrel, Muna, and Eunsook Ma. 2011. "Synthesis and Screening of Aromatase Inhibitory Activity of Substituted C19 Steroidal 17-Oxime Analogs" Molecules 16, no. 12: 9868-9885. https://doi.org/10.3390/molecules16129868
APA StylePokhrel, M., & Ma, E. (2011). Synthesis and Screening of Aromatase Inhibitory Activity of Substituted C19 Steroidal 17-Oxime Analogs. Molecules, 16(12), 9868-9885. https://doi.org/10.3390/molecules16129868




