A New Languidulane Diterpenoid from Salvia mexicana var. mexicana
Abstract
:1. Introduction
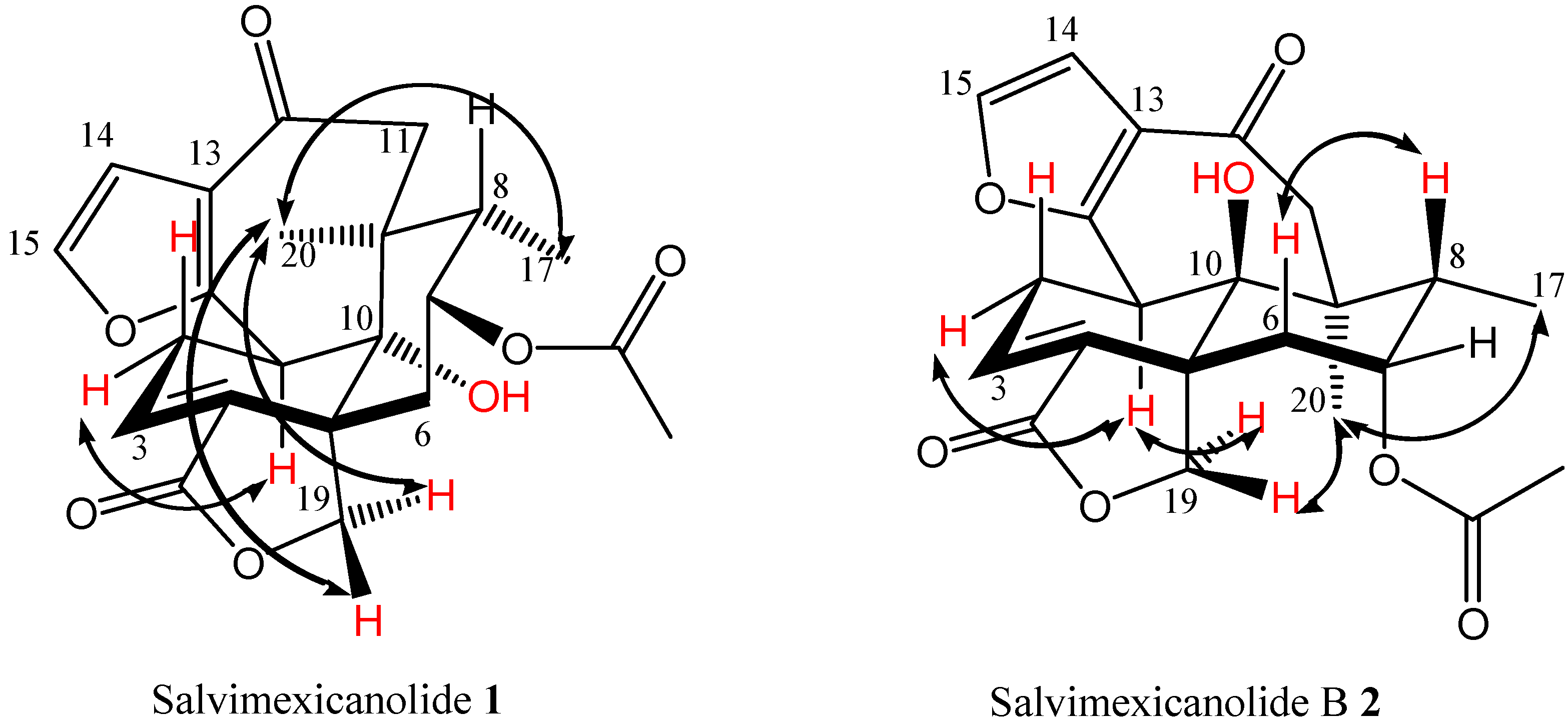
2. Results and Discussion
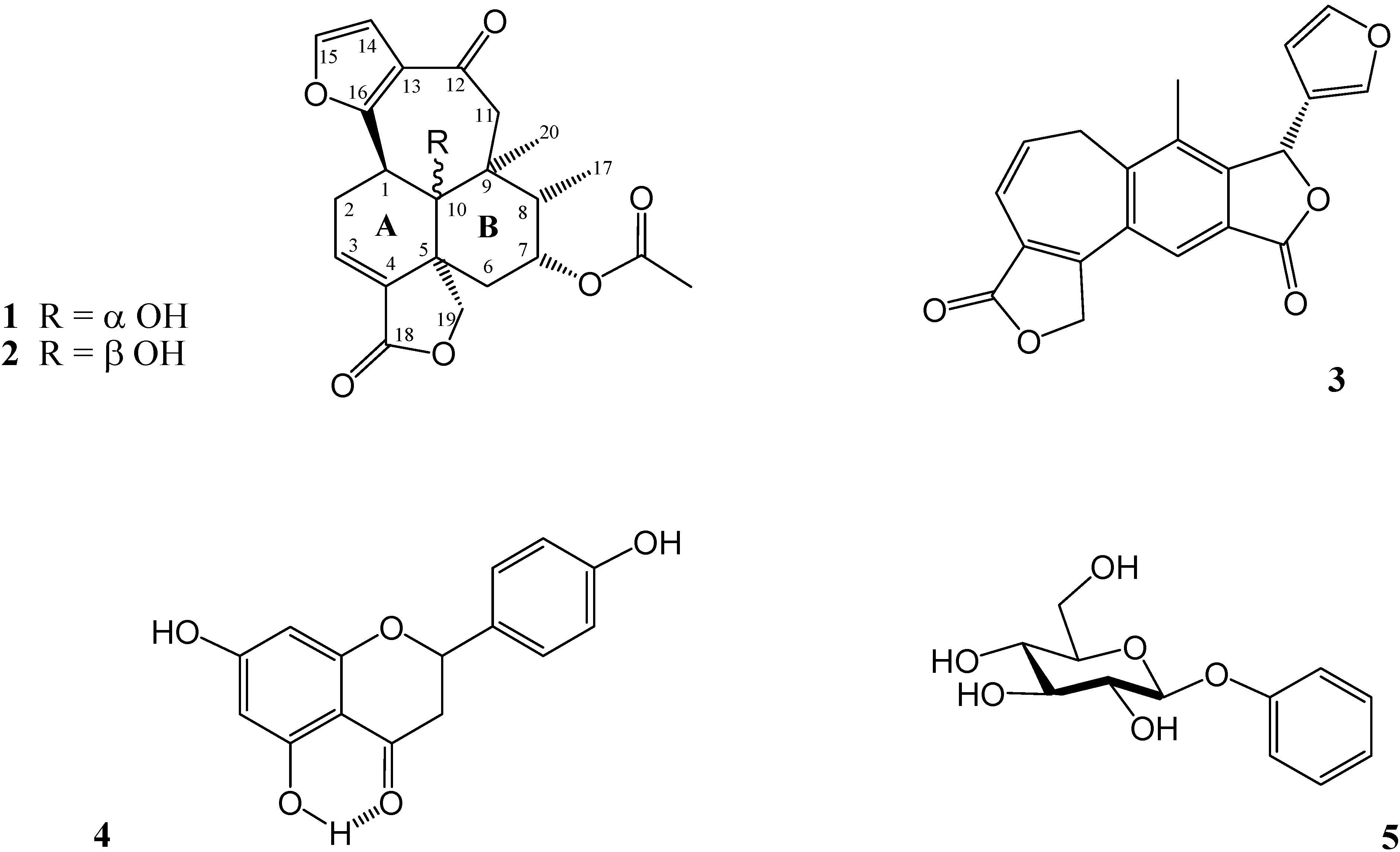
| H | 1 (δ ppm) b | 1 (δ ppm) c | 1 NOESY | 2 (δ ppm) c | 2 NOESY |
| 1 | 4.18 (1H) | 3.97 (1H) | H-2α, H-11α, | 3.33 (1H) | 2α, H-19 pro-S |
| dd, J = 9.3, 1.8 | dd, J = 9.9, 2.7 | H-11β | dd, J = 12.3, 5.7 | ||
| 2αeq | 2.97 (1H) | 2.93 (1H) | H-1, H-2β, H-3 | 2.71 (1H) | H-1α, H-2β, |
| ddd J = 21.0, 9.6, 4.2 | ddd, J = 21.0, 9.9, 4.8 | dt, J = 20.7, 5.7 | H-3 | ||
| 2βax | 3.28 (1H) | 3.42 (1H) | H-2α, H-3 | 2.38 (1H) | H-2α, H-3 |
| ddd, J = 21.0, 3.6, 2.1 | dt, J = 21.0, 3.0 | ddd, J = 20.7, 12.3, 3.0 | |||
| 3 | 6.93 (1H) dd, J = 4.2, | 7.09 (1H) | H-2α, H-2β | 7.18 (1H) | H-2α, H-2β |
| 3.6 | t, J = 3.6 | dd, J = 5.7, 3.0 | |||
| 6αeq | 1.97 (1H) | 2.08 (1H) | H-6β, H-7, | 1.86 (1H) | H-6β, H-7 |
| m | dd, J = 15.6, 3.0 | H-19 pro-R | dd, J = 15.6, 2.4 | ||
| 6βax | 1.06 (1H) | 1.10 (1H) | H-6α, H-7, H-8 | 1.79 (1H) | H-6α, H-7, H-8 |
| ddd,J = 15.6, 3.6, 2.1 | dt, J = 15.6, 3.0, 2.1 | ddd, J = 15. 6, 4.5, 1.5 | |||
| 7 | 4.83 (1H) | 4.9 (1H) | H-6α, H-6β, | 5.12 (1H) | H-6α, H-6β, |
| q, J = 3.9 | dt, J = 6.5, 3.3 | H-8, Me-17 | td, J = 4.2, 2.4 | H-8, Me-17 | |
| 8 | 1.97 (1H)m | 1.95 (1H)qd, J = 7.0, 4.2 | H-6β, H-7, Me-17 | 1.96 (1H)qd, J = 7.2, 4.2 | H-6β, H-7,Me-17 |
| 11α | 2.48 (1H) | 2.62 (1H) | H-1α, H-11β, | 2.48 (1H) | H-11β, Me-17, |
| d, J = 17.1 | d, J = 17.7 | Me-17, Me-20 | d, J = 15.6 | Me-20 | |
| 11β | 3.43 (1H) | 3.3 (1H) | H-1α, H-11α, | 3.52 (1H) | H-11α |
| d, J = 17.1 | d, J = 17.7 | d, J = 15.6 | |||
| 14 | 6.63 (1H) | 6.72 (1H) | H-15 | 6.71 (1H) | H-15 |
| d, J = 1.4 | d, J = 1.8 | d, J = 2.0 | |||
| 15 | 7.57 (1H) | 7.35 (1H) | H-14 | 7.29 (1H) | H-14 |
| d, J = 1.4 | d, J = 1.8 | d, J = 2.0 | |||
| 17 | 0.81 (1H) | 0.83 (3H) | H-7, H-8, | 0.95 (3H) | H-7, H-8, |
| d, J = 6.9 | d, J = 7.0 | H-11α, Μe-20 | d, J = 7.2 | H-11α, Μe-20 | |
| 19 pro-R | 4.93 (1H) | 5.0 (1H) | H-6α, H-19 pro-S, | 4.7 (1H) | H-19 pro-S, |
| d, J = 8.1 | d, J = 8.7 | Me-20 | d, J = 8.4 | Me-20 | |
| 19 pro-S | 4.50 (1H) | 4.47, (1H) | H-19 pro-R, | 4.52, (1H) | H-1, H-19 pro-R |
| dd, J = 8.1, 2.1 | dd, J = 8.7, 2.1 | Me-20 | dd, J = 8.4, 1.5 | ||
| 20 | 1.15 (3H) | 1.15 (3H) | H-11α, Me-17, H-19 | 1.23 (3H) | H-11α, Me-17, |
| s | s | pro-S and pro-R | s | H-19 pro-R | |
| CH3COO | 2.10 (3H) | 2.10 (3H) | |||
| s | s | ||||
| -OH | 2.2 | 3.38 |
| C | 1 (δ ppm) | 2 (δ ppm) |
| 1 | 42.7 d | 39.4 d |
| 2 | 23.6 t | 22.4 t |
| 3 | 133. 6d | 136.1 d |
| 4 | 130.8 s | 132.5 s |
| 5 | 46.1 s | 48.6 s |
| 6 | 34.7 t | 29.4 t |
| 7 | 70.9 d | 72.0 d |
| 8 | 37.2 d | 37.3 d |
| 9 | 45.3 s | 46.4 s |
| 10 | 77.6 s | 77.8 s |
| 11 | 52.0 t | 52.2 t |
| 12 | 194.6 s | 194.2 s |
| 13 | 125.7 s | 122.1 s |
| 14 | 109.1 d | 110.1 d |
| 15 | 142.4 d | 142.4 d |
| 16 | 159.8 s | 154.6 s |
| 17 | 13.0 q | 11.6 q |
| 18 | 169.7 s | 175.7 s |
| 19 | 72.5 t | 73.2 t |
| 20 | 20.0 q | 21.4 q |
| CH3COO | 21.2 t | 21.2 q |
| CH3COO | 169.0 s | 169.9 s |
3. Experimental
3.1. General
3.1. Extraction and Isolation
4. Conclusions
Supplementary Materials
Supplementary Materials
Supplementary File 1Acknowledgments
Conflict of Interest
References and Notes
- Rodríguez-Hahn, L.; Cárdenas, J. Comparative chemotaxonomy in labiatae. Curr. Top. Phytochem. 1999, 2, 91–102. [Google Scholar]
- Epling, C. A Revision of Salvia Subgenus Salosphace. In Repertorium Specierum Novarum Regni Vegetabilis; Verlag des Repertoriums: Berlin, Germany, 1939. [Google Scholar]
- Rodríguez-Hahn, L.; Esquivel, B.; Cárdenas, J. New diterpenoid skeletons of clerodanic origin from Mexican Salvia species. Trends Org. Chem. 1992, 3, 99–111. [Google Scholar]
- Rodríguez-Hahn, L.; Esquivel, B.; Cárdenas, J. Clerodane diterpenes in Labiatae. Prog. Chem. Org. Nat. Prod. 1994, 63, 141–157. [Google Scholar]
- Cárdenas, J.; Esquivel, B.; Toscano, R.A.; Rodríguez-Hahn, L. Languiduline, a diterpenoid with an unusual structure from Salvia languidula. Heterocycles 1988, 27, 1809–1812. [Google Scholar] [CrossRef]
- Maldonado, E.; Ortega, A. Languidulane, clerodane and secoclerodane diterpenes from Salvia tonalensis. Phytochemistry 1997, 45, 1461–1464. [Google Scholar]
- Esquivel, B.; Ramírez-Dávalos, N.; Espinosa-Pérez, G. A cis-Languidulane diterpenoid from Salvia mexicana var. major (labiatae). Heterocycles 1999, 51, 1647–1651. [Google Scholar] [CrossRef]
- The mixture was identified by comparition with a real sample of the triterpenic acids
- Xu, G.; Peng, L.; Niu, X.; Zhao, Q.; Li, R.; Sun, H. Novel diterpenoids from Salvia dugesii. Helv. Chim. Acta 2004, 87, 949–955. [Google Scholar] [CrossRef]
- Aoyagi, Y.; Yamazaki, A.; Kato, R.; Tobe, F.; Fukaya, H.; Nishikawa, T.; Nakahashi, A.; Miura, N.; Monde, K.; Takeya, K. Salvileucalin C, a novel rearranged neoclerodane diterpene from Salvia leucantha. Tetrahedron Lett. 2011, 52, 1851–1853. [Google Scholar]
- Barros, D.A.D.; de Alvarenga, M.A.; Gottlieb, O.R.; Gottlieb, H.E. The chemistry of Brazilian euphorbiaceae. Part 4. naringenin coumaroylglucosides from Mabea caudata. Phytochemistry 1982, 21, 2107–2109. [Google Scholar]
- Pawlowska, A.M.; de Leo, M.; Braca, A. Phenolics of Arbutus unedo L. (Ericaceae) fruits: Identification of anthocyanins and gallic acid derivatives. J. Agric. Food Chem. 2006, 54, 10234–10238. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compound 1–4 are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Frontana-Uribe, B.A.; Escárcega-Bobadilla, M.V.; Estrada-Reyes, R.; Morales-Serna, J.A.; Salmón, M.; Cárdenas, J. A New Languidulane Diterpenoid from Salvia mexicana var. mexicana. Molecules 2011, 16, 8866-8873. https://doi.org/10.3390/molecules16108866
Frontana-Uribe BA, Escárcega-Bobadilla MV, Estrada-Reyes R, Morales-Serna JA, Salmón M, Cárdenas J. A New Languidulane Diterpenoid from Salvia mexicana var. mexicana. Molecules. 2011; 16(10):8866-8873. https://doi.org/10.3390/molecules16108866
Chicago/Turabian StyleFrontana-Uribe, Bernardo Antonio, Martha Verónica Escárcega-Bobadilla, Rosa Estrada-Reyes, José Antonio Morales-Serna, Manuel Salmón, and Jorge Cárdenas. 2011. "A New Languidulane Diterpenoid from Salvia mexicana var. mexicana" Molecules 16, no. 10: 8866-8873. https://doi.org/10.3390/molecules16108866
APA StyleFrontana-Uribe, B. A., Escárcega-Bobadilla, M. V., Estrada-Reyes, R., Morales-Serna, J. A., Salmón, M., & Cárdenas, J. (2011). A New Languidulane Diterpenoid from Salvia mexicana var. mexicana. Molecules, 16(10), 8866-8873. https://doi.org/10.3390/molecules16108866






