Antibacterial Characteristics and Activity of Water-Soluble Chitosan Derivatives Prepared by the Maillard Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antibacterial Activity of Various Chitosans
| Water-soluble | Antibacterial activity (%) ** | |||||
|---|---|---|---|---|---|---|
| chitosan | S. aureus | L. monocytogenes | B. cereus | E. coli | S. dysenteriae | S. tphimurium |
| derivative * | ||||||
| pH 5.0 | ||||||
| GN50-5 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | >99 ± 0 | >99 ± 0 |
| GN70-3 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | >100 ± 0 |
| Chitosan | >99 ± 0 | >99 ± 0 | 90 ± 0 | >99 ± 0 | >99 ± 0 | >99 ± 0 |
| pH 7.0 | ||||||
| GN50-5 | 100 ± 0 | >99 ± 0 | >99 ± 0 | >99 ± 0 | >99 ± 0 | >99 ± 0 |
| GN70-3 | 100 ± 0 | >99 ± 0 | >99 ± 0 | >99 ± 0 | >99 ± 0 | >99 ± 0 |
| Chitosan | 14 ± 2 | 11 ± 5 | 4 ± 2 | 11 ± 5 | 5 ± 2 | 4 ± 1 |
2.2. Concentration Effect
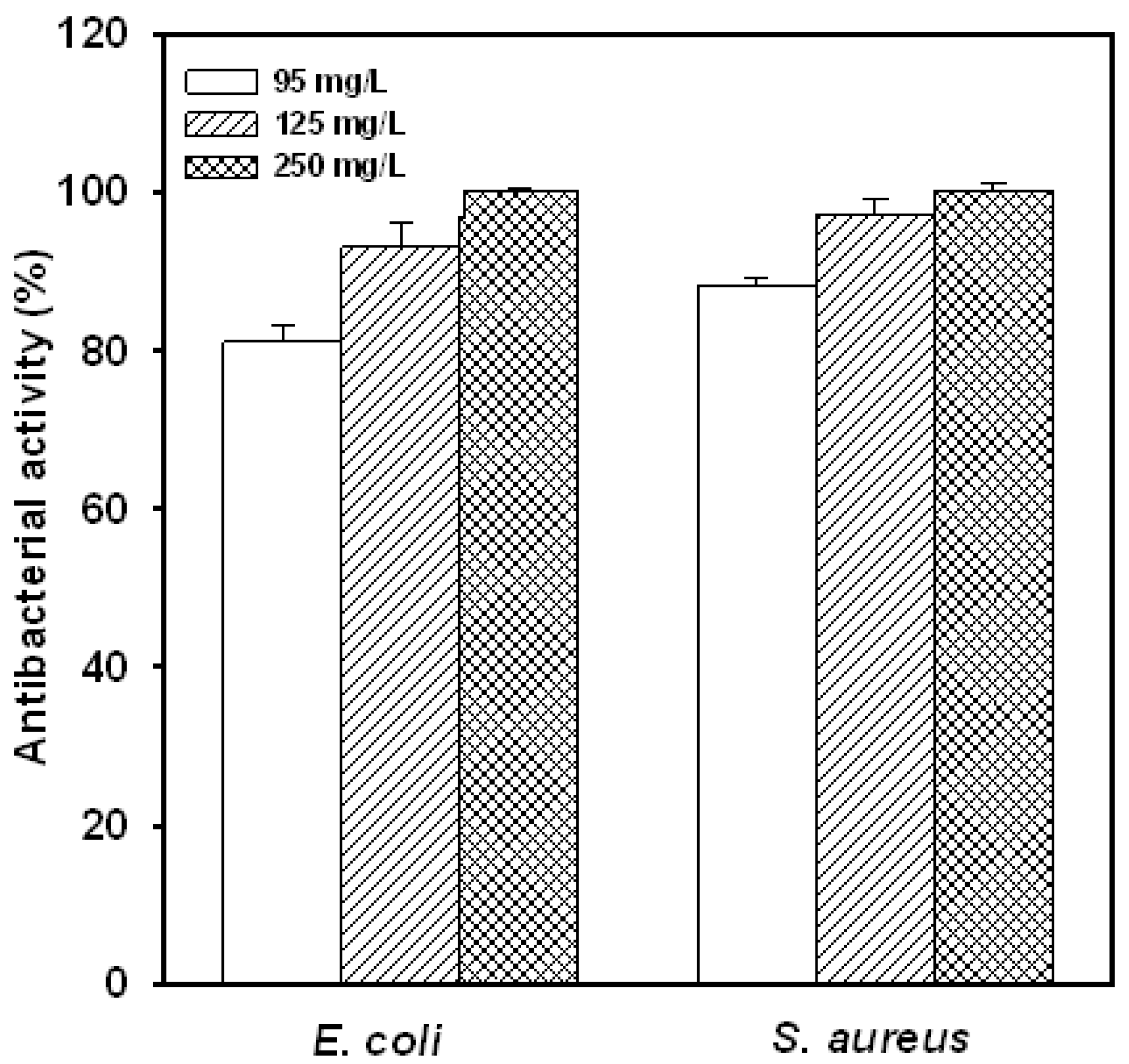
2.3. Suspension Medium Influences the Antibacterial Activity of Water-Soluble Chitosan Derivative
2.4. Effect of Metal Ions on the Antibacterial Activity of Water-Soluble Chitosan Derivative

| Viable population (log CFU/mL) * | Water-soluble | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | GN70-3 | chitosan derivative | |||||||
| activity (%) ** | |||||||||
| Salt concentration | Salt concentration | Salt concentration | |||||||
| Salt | 0 mM | 15 mM | 30 mM | 0 mM | 15 mM | 30 mM | 0 | 15 | 30 |
| mM | mM | mM | |||||||
| MgCl2 | 7.59 ± 0.06 | 7.51 ± 0.06B | 7.50 ± 0.08B | 4.45 ± 0.10 | 6.28 ± 0.03C | 6.53 ± 0.04C | 100 | 39.2 | 22.9 |
| NaCl | 7.59 ± 0.06 | 7.60 ± 0.04B | 7.63 ± 0.02B | 4.45 ± 0.10 | 6.95 ± 0.02A | 7.10 ± 0.02A | 100 | 20.7 | 16.9 |
| CaCl2 | 7.59 ± 0.06 | 7.55 ± 0.03B | 7.57 ± 0.01B | 4.45 ± 0.10 | 6.55 ± 0.04B | 6.94 ± 0.05B | 100 | 31.8 | 20.1 |
| BaCl2 | 7.59 ± 0.06 | 7.69 ± 0.05A | 7.71 ± 0.04A | 4.45 ± 0.10 | 7.09 ± 0.06A | 7.23 ± 0.08A | 100 | 19.1 | 15.3 |
2.5. Cell Leakage Induced by the Water-Soluble Chitosan Derivative
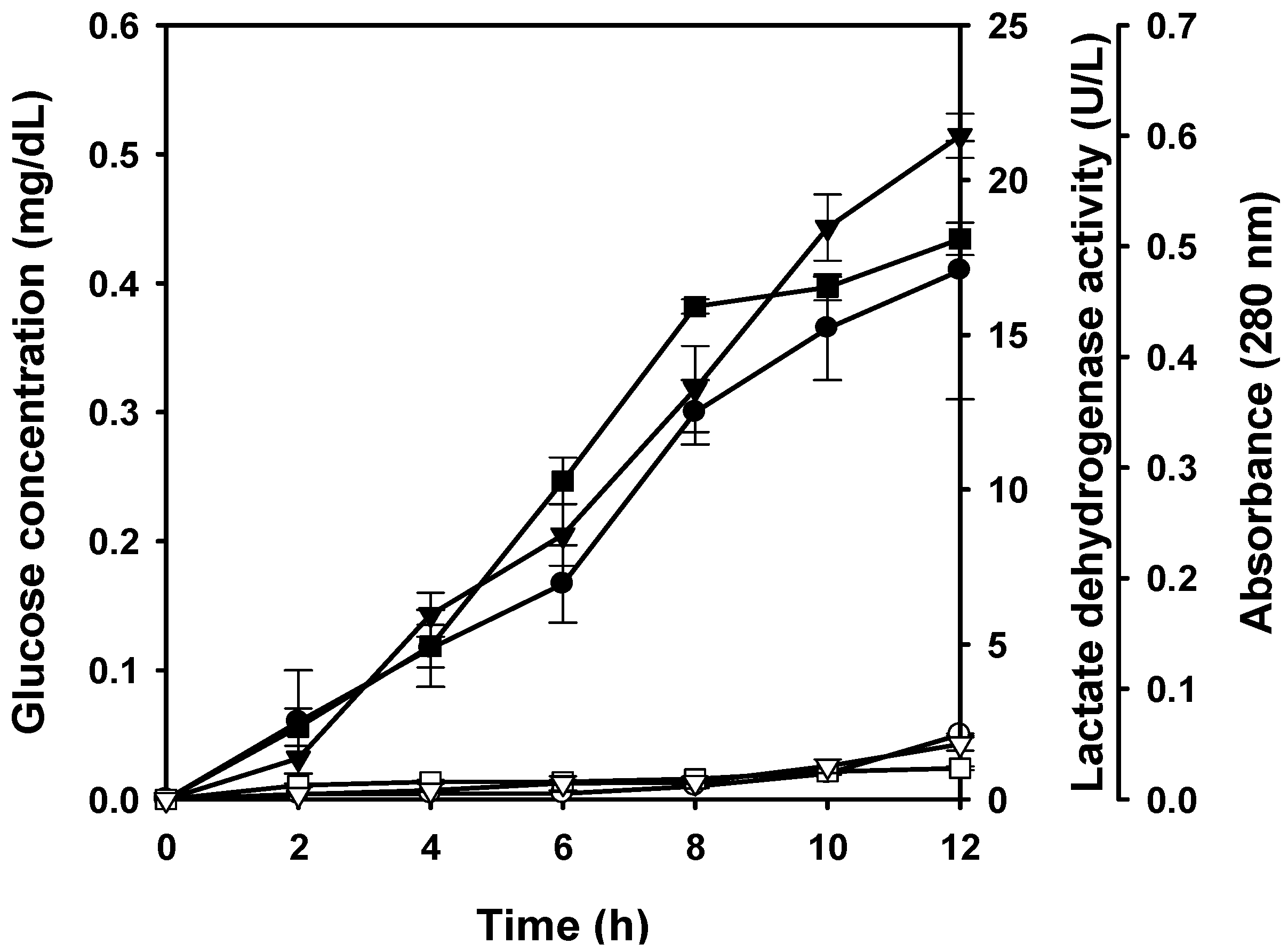
3. Experimental
3.1. Chitosan
3.2. Preparation of Water-Soluble Chitosan Derivatives
3.3. Assay for Antimicrobial Activity
3.4. Effect of Metal Ions
3.5. Leakage of Glucose, Lactate Dehydrogenase and Protein from Cells
3.6. Statistical Analysis
4. Conclusions
Acknowledgment
References
- Fernandes, J.C.; Tavaria, F.K.; Soares, J.C.; Ramos, O.S.; Monteiro, M.J.; Pintado, M.E.; Malcata, F.X. Antimicrobial effects of chitosans and chitooligosaccharides, upon Staphylococcus aureus and Escherichia coli, in food model systems. Food Microbiol. 2009, 25, 922–928. [Google Scholar]
- Yen, M.T.; Yang, J.H.; Mau, J.L. Physicochemical characterization of chitin and chitosan from crab shells. Carbohydr. Polym. 2009, 75, 15–21. [Google Scholar] [CrossRef]
- Chen, K.J.; Chiu, Y.L.; Chen, Y.M.; Ho, Y.C.; Sung, H.W. Intracellularly monitoring/imaging the release of doxorubicin from pH-responsive nanoparticles using Förster resonance energy transfer. Biomaterials 2011, 32, 2586–2592. [Google Scholar]
- Hu, Y.; Yang, J.; Tang, Y.; Li, J.; Wang, X. Self-aggregation and antibacterial activity of N-acylated chitosan. Polymer 2007, 48, 3098–3106. [Google Scholar] [CrossRef]
- Andres, Y.; Giraud, L.; Gerente, C.; Le Cloirec, P. Antibacterial effects of chitosan powder: Mechanisms of action. Environ. Technol. 2007, 28, 1357–1363. [Google Scholar] [CrossRef]
- Alves, N.M.; Mano, J.F. Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int. J. Biol. Macromol. 2008, 43, 401–414. [Google Scholar] [CrossRef]
- Campaniello, D.; Bevilacqua, A.; Sinigaglia, M.; Corbo, M.R. Chitosan: Antimicrobial activity and potential applications for preserving minimally processed strawberries. Food Microbiol. 2008, 25, 992–1000. [Google Scholar] [CrossRef]
- Tikhonov, V.E.; Stepnova, E.A.; Babak, V.G.; Yamskov, I.A.; Guerrero, J.P.; Jansson, H.B.; Llorca, L.V.; Salinas, J.; Gerasimenko, D.V.; Avdienko, I.D.; Varlamov, V.P. Bactericidal and antifungal activities of a low molecular weight chitosan and its N-/2(3)-(dodec-2-enyl)succinoyl/-derivatives. Carbohydr. Polym. 2006, 64, 66–72. [Google Scholar] [CrossRef]
- Chen, C.S.; Liau, W.Y.; Tsai, G.J. Antibacterial effects of N-sulfonated and N-sulfobenzoyl chitosan and application to oyster preservation. J. Food Prot. 1998, 61, 1124–1128. [Google Scholar]
- Young, D.H.; Kohle, H.; Kauss, H. Effect of chitosan on membrane permeability of suspension-cultured Glycine max and Phaseolus vulgaris cells. Plant Physiol. 1982, 70, 1449–1454. [Google Scholar] [CrossRef]
- Hadwiger, L.A.; Kendra, D.F.; Fristensky, B.W.; Wagoner, W. Chitosan both activates genes in plants and inhibits RNA synthesis in fungi. In Chitin in Nature and Technology; Muzzarelli, R., Jeuniaux, C., Gooday, G.W., Eds.; Plenum Press: New York, NY, USA, 1986; pp. 209–214. [Google Scholar]
- Sudarshan, N.R.; Hoover, D.G.; Knorr, D. Antibacterial action of chitosan. Food Biotechnol. 1990, 6, 257–272. [Google Scholar]
- Vishu Kumar, A.B.; Varadaraj, M.C.; Gowda, L.R.; Tharanathan, R.N. Characterization of chito-oligosaccharides prepared by chitosanolysis with the aid of papain and Pronase, and their bactericidal action against Bacillus cereus and Escherichia coli. Biochem. J. 2005, 391, 167–175. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.G.; Liu, N.; Liu, C.S.; Liu, C.G.; Meng, X.H.; Yu, L.J.; Kenendy, J.F. Physicochemical characterization and antibacterial property of chitosan acetates. Carbohydr. Polym. 2007, 67, 227–232. [Google Scholar] [CrossRef]
- Chung, Y.C.; Tsai, C.F.; Li, C.F. Enhanced solubility and characterization of chitosan in aqueous solutions prepared by Maillard reaction. Fish. Sci. 2006, 72, 1096–1103. [Google Scholar] [CrossRef]
- Liu, X.F.; Guan, Y.L.; Yang, D.Z.; Li, Z.; Yao, K.D. Antibacterial action of chitosan and carboxymethylated chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar] [CrossRef]
- Chung, Y.C.; Wang, H.L.; Chen, Y.M.; Li, S.L. Effect of abiotic factors on the antibacterial activity of chitosan against waterborne pathogens. Bioresour. Technol. 2003, 88, 179–184. [Google Scholar] [CrossRef]
- Fujimoto, T.; Tsuchiya, Y.; Terao, M.; Nakamura, K.; Yamamoto, M. Antibacterial effects of chitosan solution® against Legionella pneumophila, Escherichia coli, and Staphylococcus aureus. Int. J. Food Microbiol. 2006, 112, 96–101. [Google Scholar] [CrossRef]
- Kumar, S.; Thippareddi, H.; Subbiah, J.; Zivanovic, S.; Davidson, P.M.; Harte, F. Inactivation of Escherichia coli K-12 in apple juice using combination of high-pressure homogenization and chitosan. J. Food Sci. 2009, 74, M8–M14. [Google Scholar] [CrossRef]
- Popper, L.; Knorr, D. Applications of high-pressure homogenization for food preservation. Food Technol. 1990, 44, 84–89. [Google Scholar]
- Wang, G.H. Inhibition and inactivation of five species of foodborne pathogens by chitosan. J. Food Prot. 1992, 55, 916–919. [Google Scholar]
- Bassi, R.; Prasher, S.O.; Simpson, B.K. Efects of organic acids on the adsorption of heavy metal ions by chitosan flakes. J. Environ. Sci. Health A 1999, 34, 289–294. [Google Scholar]
- Tsai, G.J.; Su, W.H. Antibacterial activity of shrimp chitosan against Escherichia coli. J. Food Prot. 1999, 62, 239–243. [Google Scholar]
- Goldberg, S.; Doyle, R.J.; Rosenberg, M. Mechanism of enhancement of microbial cell hydrophobicity by cationic polymers. J. Bacteriol. 1990, 172, 5650–5654. [Google Scholar]
- Yang, T.C.; Li, C.F.; Chou, C.C. Cell age, suspending medium and metal ion influence the susceptibility of Escherichia coli O157:H7 to water-soluble maltose chitosan derivative. Int. J. Food Microbiol. 2007, 113, 258–262. [Google Scholar] [CrossRef]
- Leuba, J.L.; Stossel, P. Chitosan and other polyamines: Antifungal activity and interaction with biologicalmembranes. In Chitin in Nature and Technology; Muzzarelli, R., Jeuniaux, C., Gooday, G.W., Eds.; Plenum Press: New York, NY, USA, 1986; pp. 215–222. [Google Scholar]
- Tortora, J.; Funke, B.R.; Case, C.L. Microbiology: An Introduction; Pearson Benjamin Cummings: New York, NY, USA, 2010; pp. 85–88. [Google Scholar]
- Raafat, D.; von Bargen, K.; Haas, A.; Sahl, H.G. Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.; Wang, X.; Sun, L. Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol. 2004, 95, 147–155. [Google Scholar] [CrossRef]
- Toei, K.; Kohara, T. A conductometric method for colloid titrations. Anal. Chim. Acta 1976, 83, 59–65. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Park, P.J.; Kim, S.K. Antimicrobial effect of chitooligosaccharides produced by bioreactor. Carbohydr. Polym. 2001, 44, 71–76. [Google Scholar] [CrossRef]
- SAS, SAS User's Guide: Statistics, Version 6; SAS Institute: Cary, NC, USA; p. 1989.
- Sample Availability: Not Available.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chung, Y.-C.; Yeh, J.-Y.; Tsai, C.-F. Antibacterial Characteristics and Activity of Water-Soluble Chitosan Derivatives Prepared by the Maillard Reaction. Molecules 2011, 16, 8504-8514. https://doi.org/10.3390/molecules16108504
Chung Y-C, Yeh J-Y, Tsai C-F. Antibacterial Characteristics and Activity of Water-Soluble Chitosan Derivatives Prepared by the Maillard Reaction. Molecules. 2011; 16(10):8504-8514. https://doi.org/10.3390/molecules16108504
Chicago/Turabian StyleChung, Ying-Chien, Jan-Ying Yeh, and Cheng-Fang Tsai. 2011. "Antibacterial Characteristics and Activity of Water-Soluble Chitosan Derivatives Prepared by the Maillard Reaction" Molecules 16, no. 10: 8504-8514. https://doi.org/10.3390/molecules16108504
APA StyleChung, Y.-C., Yeh, J.-Y., & Tsai, C.-F. (2011). Antibacterial Characteristics and Activity of Water-Soluble Chitosan Derivatives Prepared by the Maillard Reaction. Molecules, 16(10), 8504-8514. https://doi.org/10.3390/molecules16108504




