X-Ray Supramolecular Structure, NMR Spectroscopy and Synthesis of 3-Methyl-1-phenyl-1H-chromeno[4,3-c]pyrazol-4-ones Formed by the Unexpected Cyclization of 3-[1-(Phenyl-hydrazono)ethyl]-chromen-2-ones
Abstract
:1. Introduction
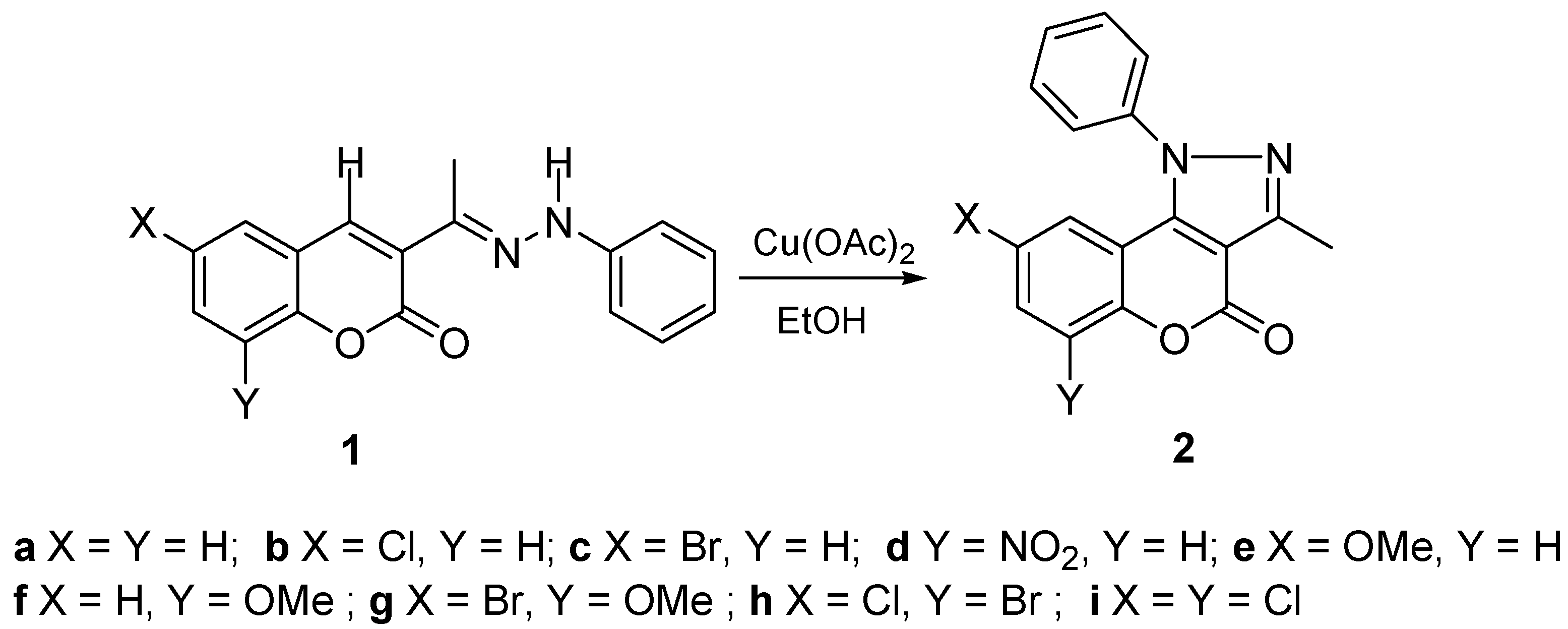
2. Results and Discussion
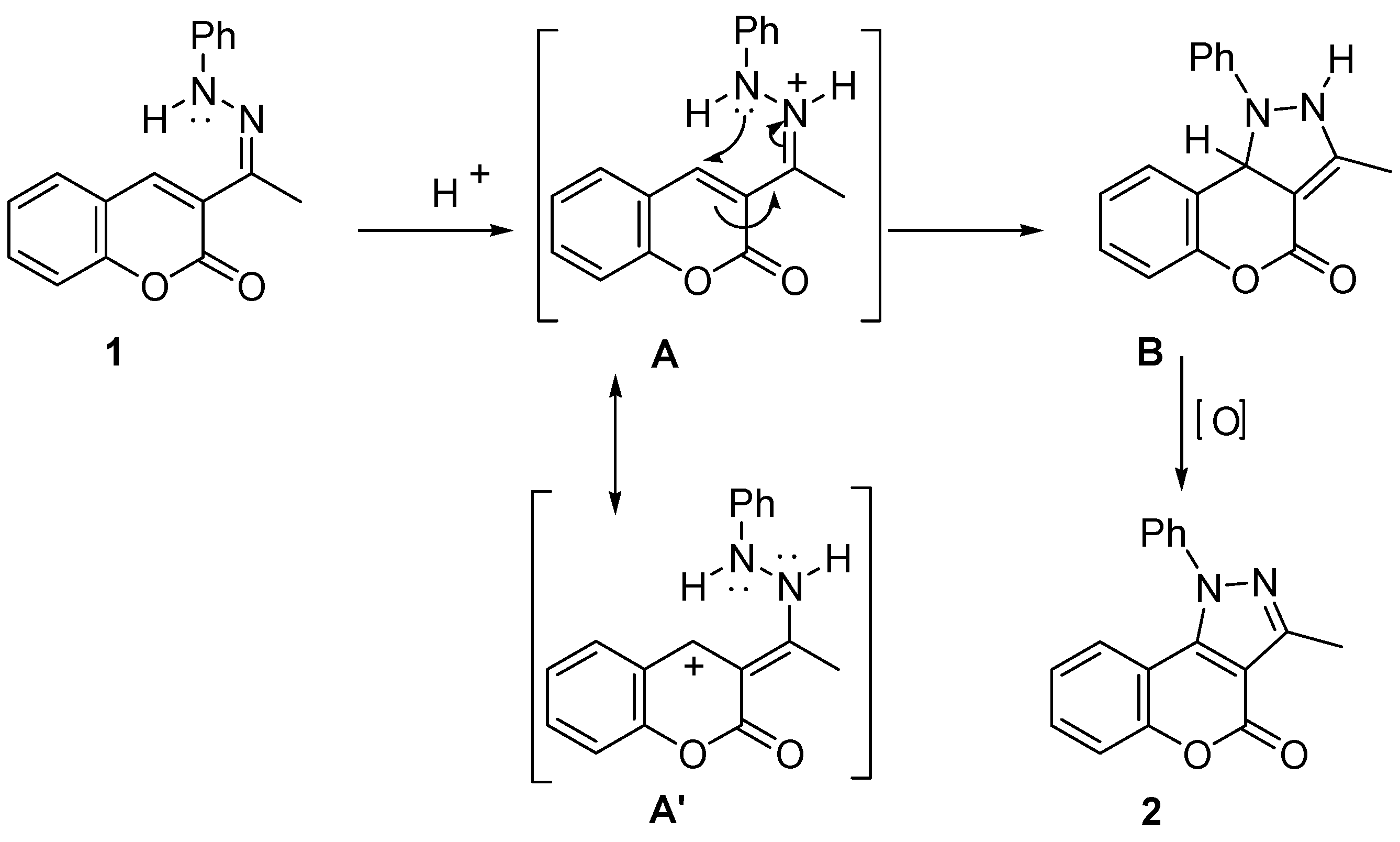
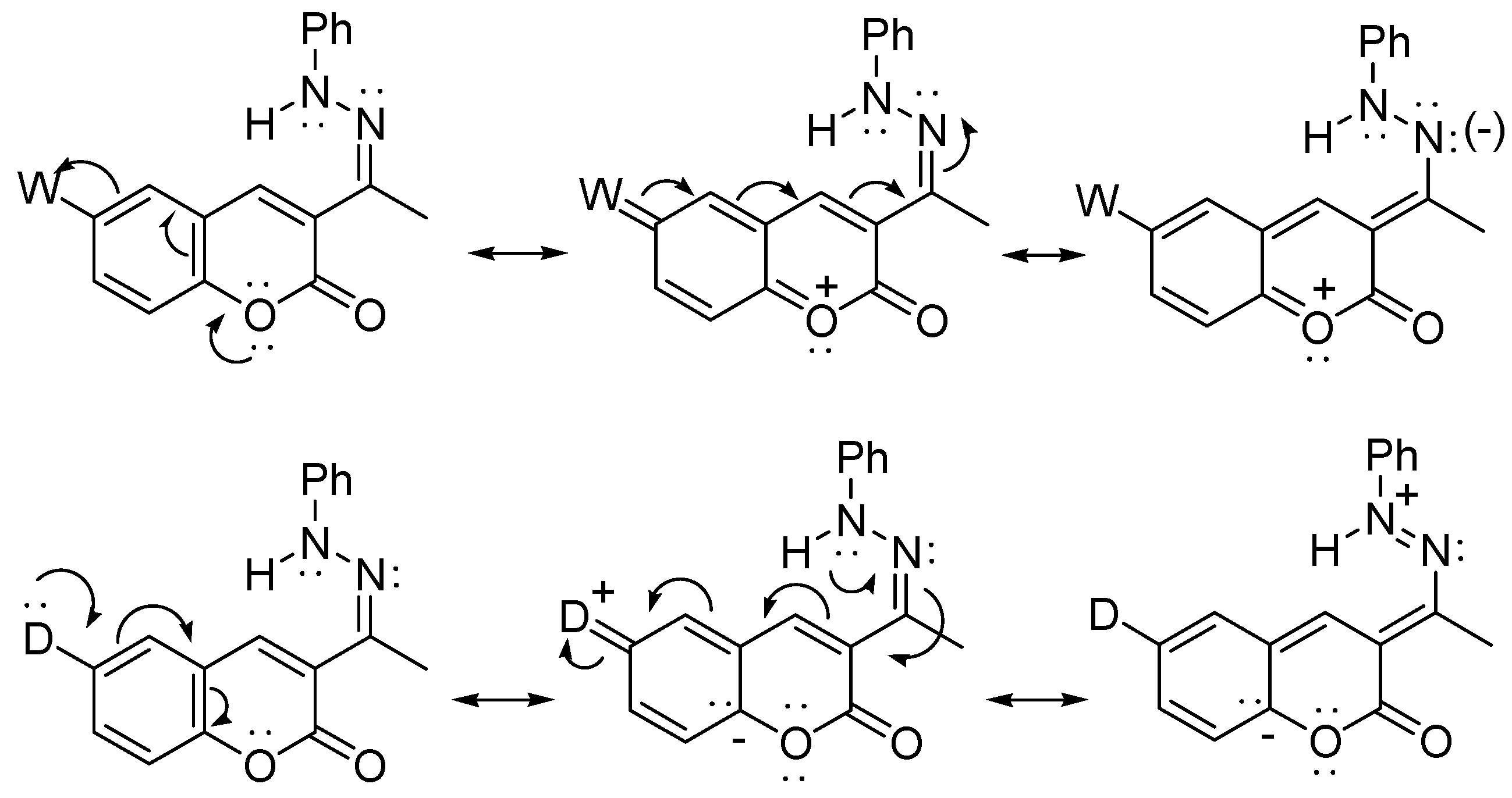
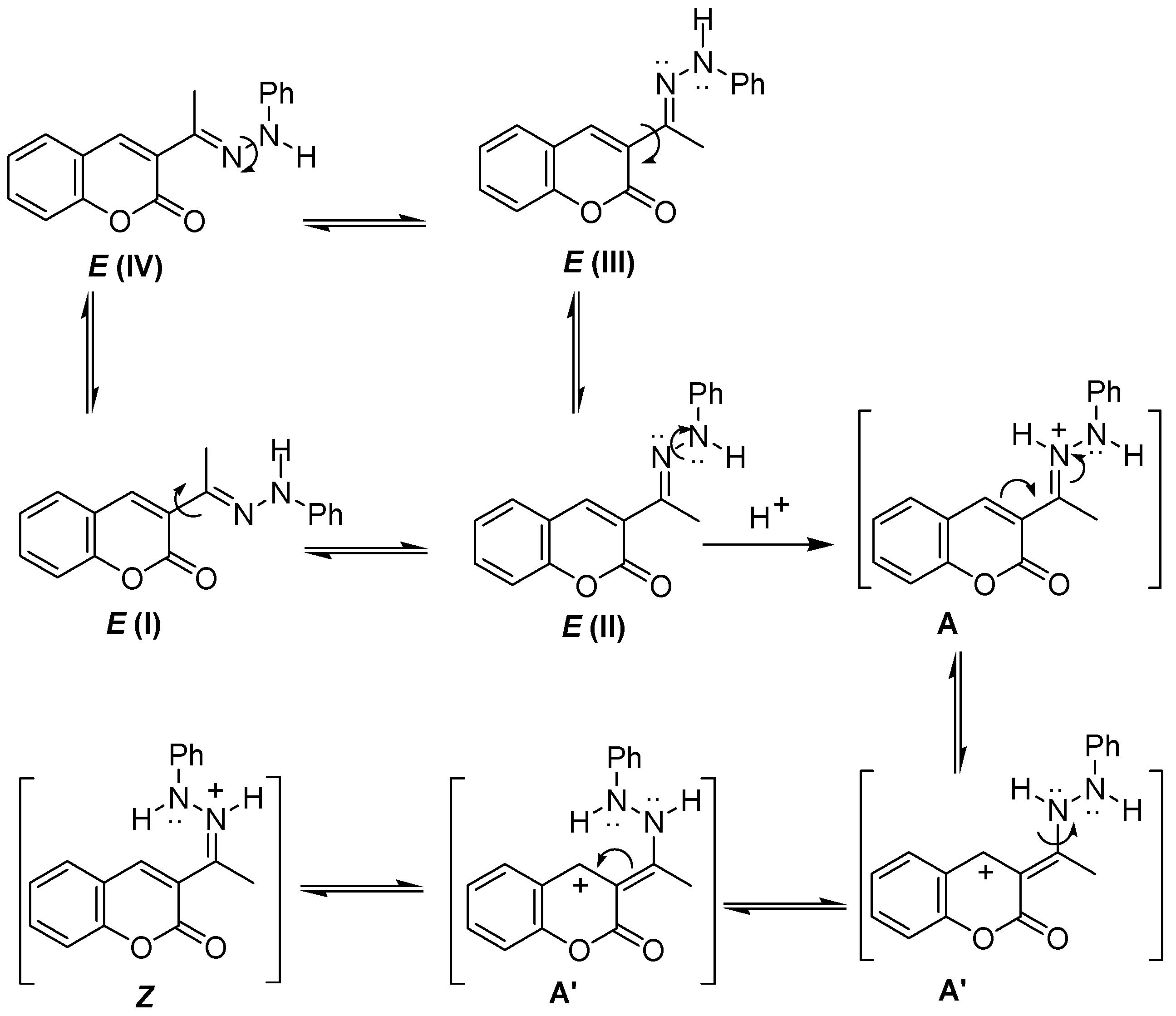
2.1. Synthesis and Molecular Structure in Solution
| δ 1H | δ 13C | ν/cm−1 | |||||
|---|---|---|---|---|---|---|---|
| Comp. | H-4 | H-5 | C-2 | C-3 | C-4 | C-10 | CO |
| 1a | 8.16 | 7.81 | 160.2 | 127.9 | 139.8 | 119.9 | 1695, 1596 |
| 1b | 8.17 | 7.97 | 159.2 | 128.2 | 137.8 | 119.5 | 1703, 1598 |
| 1c | 8.15 | 8.08 | 159.1 | 128.3 | 137.7 | 116.1 | 1704, 1597 |
| 1d | 8.40 | 8.84 | 159.2 | 130.6 | 137.7 | 119.9 | 1726, 1604 |
| 1e | 7.98 | 6.97 | 160.9 | 127.9 | 139.7 | 120.1 | 1698, 1574 |
| 1f | 8.02 | 7.06 | 160.2 | 127.9 | 140.0 | 120.4 | 1700, 1601 |
| 1g | 7.95 | 7.34 | 156.3 | 128.8 | 138.3 | 116.9 | 1713, 1599 |
| 1h | 7.95 | 7.51 | 159.2 | 129.2 | 137.6 | 121.6 | 1707, 1530 |
| 1i | 7.96 | 7.41 | 159.0 | 129.7 | 137.6 | 121.6 | 1709, 1533 |
| δ 1H | δ 13C | ν/cm−1 | ||||
|---|---|---|---|---|---|---|
| Comp. | H-9 | C-4 | C-3a | C-9a | C-9b | CO |
| 2a | 7.09 | 158.3 | 106.5 | 112.0 | 141.9 | 1726 |
| 2b | 7.03 | 157.6 | 106.8 | 113.1 | 140.7 | 1743 |
| 2c | 7.16 | 157.6 | 106.8 | 113.7 | 140.6 | 1742 |
| 2d | 8.02 | 156.9 | 106.8 | 112.4 | 143.6 | 1756 |
| 2e | 6.50 | 158.4 | 106.7 | 112.1 | 141.9 | 1734 |
| 2f | 6.65 | 157.6 | 106.6 | 112.7 | 142.1 | 1743 |
| 2g | 6.72 | 156.7 | 106.8 | 113.7 | 140.1 | 1744 |
| 2h | 6.90 | 156.2 | 106.8 | 112.6 | 140.2 | 1749 |
| 2i | 6.90 | 156.2 | 106.8 | 114.0 | 140.2 | 1750 |
2.2. Molecular and Supramolecular Structure in Solid State
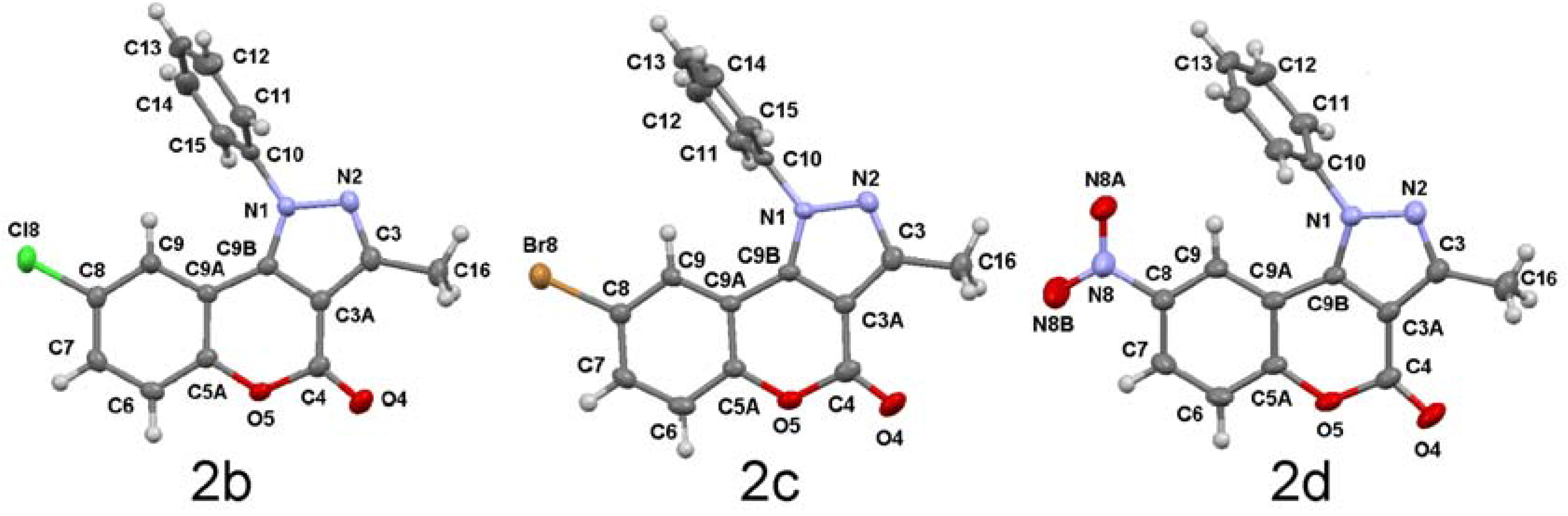
| 2b X = Cl | 2c X = Br | 2d X = NO2 | |
|---|---|---|---|
| Atoms | Bond lengths (Å) | ||
| X(8)―C(8) | 1.732(2) | 1.894(4) | 1.466(2) |
| O(4)―C(4) | 1.200(2) | 1.197(6) | 1.189(2) |
| O(5)―C(4) | 1.385(2) | 1.385(6) | 1.404(2) |
| O(5)―C(5A) | 1.382(2) | 1.379(5) | 1.374(2) |
| N(1)―N(2) | 1.376(2) | 1.374(5) | 1.379(2) |
| N(1)―C(9B) | 1.346(2) | 1.353(5) | 1.345(2) |
| N(1)―C(10) | 1.433(2) | 1.428(6) | 1.433(2) |
| N(2)―C(3) | 1.321(2) | 1.315(6) | 1.315(2) |
| C(3)―C(3A) | 1.408(3) | 1.400(7) | 1.408(3) |
| C(3A)―C(4) | 1.435(3) | 1.441(6) | 1.441(3) |
| C(3A)―C(9B) | 1.384(2) | 1.378(5) | 1.380(2) |
| C(5A)―C(9A) | 1.402(3) | 1.389(6) | 1.403(3) |
| C(9A)―C(9B) | 1.437(3) | 1.438(5) | 1.442(2) |
| O(8B)―N(8) | 1.195(3) | ||
| O(8A)―N(8) | 1.204(2) | ||
| Bond angles (º) | |||
| C(4)―O(5)―C(5A) | 123.60(15) | 123.8(4) | 124.12(15) |
| N(2)―N(1)―C(9B) | 111.82(14) | 111.3(3) | 111.53(12) |
| N(2)―N(1)―C(10) | 118.91(15) | 119.8(4) | 121.02(14) |
| C(9B)―N(1)―C(10) | 129.18(15) | 128.9(3) | 127.44(14) |
| N(1)―N(2)―C(3) | 105.86(15) | 105.9(4) | 105.81(15) |
| C(3)―C(3A)―C(4) | 131.53(15) | 131.9(4) | 132.13(14) |
| C(3)―C(3A)―C(9B) | 106.46(16) | 106.5(4) | 106.24(15) |
| O(4)―C(4)―O(5) | 116.68(17) | 117.0(4) | 115.98(18) |
| O(5)―C(4)―C(3A) | 114.98(14) | 114.5(4) | 114.44(14) |
| X(8)―C(8)―C(7) | 119.13(15) | 119.0(4) | 119.30(18) |
| N(1)―C(9B)―C(3A) | 105.87(15) | 105.9(3) | 106.10(15) |
| Comp. | D―H···Aa (symmetry code) | H···A/Å | D···A/Å | D―H···A/º |
|---|---|---|---|---|
| 2ab | C6―H6···Cg(4) (x, y, 1 + z) | 2.89 | 3.820(3) | 178 |
| C9―H9···Cg(4) (x, y, z) | 2.99 | 3.825(3) | 150(2) | |
| C16―H16A···Cg(3) (−x, −½ + y, −z) | 2.75(3) | 3.6659(18) | 157 | |
| 2b | C13―H13···O4 (x, y, z − 1) | 2.400 | 3.265(7) | 155 |
| C15―H15···O5 (2 − x, 1 − y,1 − z) | 2.570 | 3.443(6) | 157 | |
| C7―H7···Cg(4) (x, y − 1, z) | 2.57 | 3.460(2) | 161 | |
| C16―H16C···Cg(3) (1 − x, 1 − y, −z) | 2.78 | 3.535(2) | 136 | |
| 2c | C13―H13···O4 (x, y, z + 1) | 2.450 | 3.340(7) | 161 |
| C15―H15···O5 (−x, 1 − y, −z) | 2.580 | 3.450(6) | 156 | |
| C7―H7···Cg(4) (x, 1 + y, z) | 2.72 | 3.631(5) | 167 | |
| C16―H16B···Cg(3) (1 − x, 1 − y, −z) | 2.87 | 3.633(5) | 137 | |
| 2d | C13―H13···O4 (x, y, z + 1) | 2.53 | 3.464(3) | 179 |
| C7―H7···Cg(4) (1 + x, y, z) | 2.78 | 3.6999(3) | 171 | |
| C9―H9···Cg(4) | 2.79 | 3.632(3) | 152 |
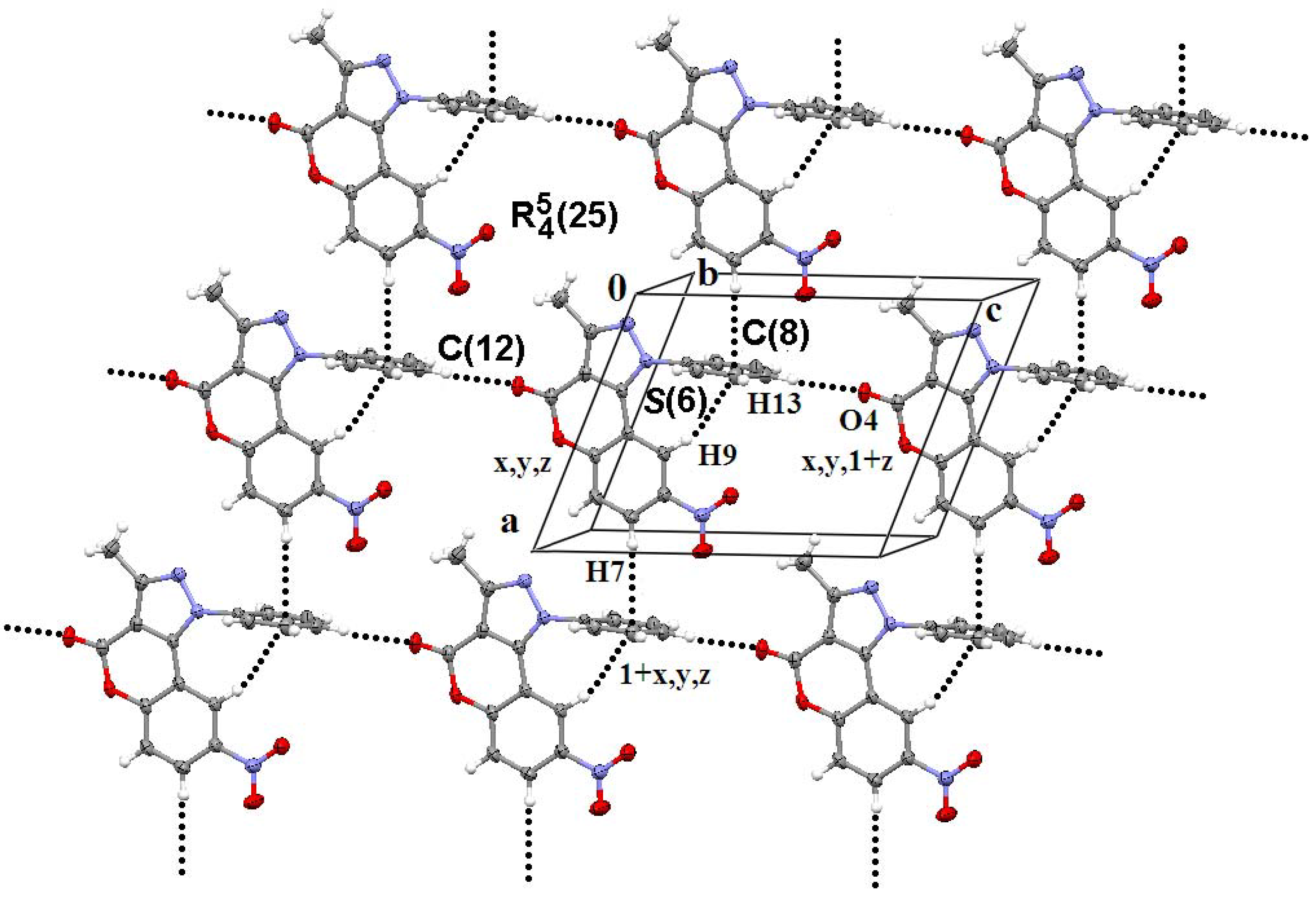
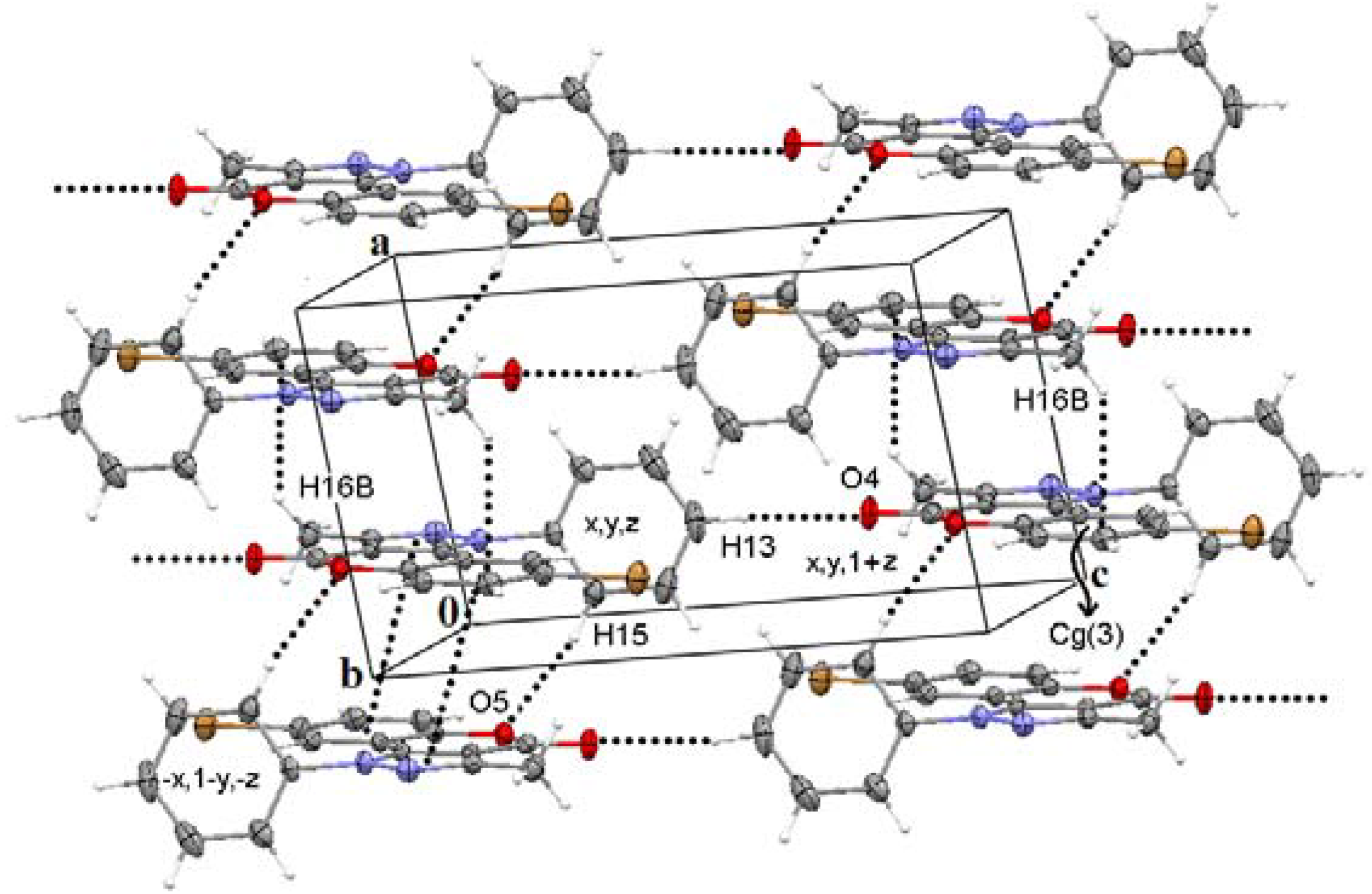
| Comp. | Centroidsa (symmetry code) | Intercentroid distance/A° | Dihedral angle/º | Interplanar distance/A° |
|---|---|---|---|---|
| 2ab | Cg(1)···Cg(2) (−x, −½ + y, −z) | 3.8508(9) | 0.000 | 3.4916(1) |
| 2b | Cg(1) · Cg(2) (1 − x, 1 − y, 1 − z) | 3.6117(10) | 0.30(8) | 3.3563(7) |
| Cg(1)···Cg(3) (2 − x, 1 − y, 1 − z) | 3.6664(11) | 1.33(9) | 3.3697(7) | |
| Cg(2)···Cg(3) (2 − x, 1 − y, 1 − z) | 3.6345(11) | 1.23(8) | 3.4103(6) | |
| 2c | Cg(1)···Cg(2) (1 − x, 1 − y, 1 − z) | 3.708(2) | 0.3(2) | 3.4328(17) |
| Cg(1)···Cg(3) (−x, 1 − y, −z) | 3.727(2) | 1.0(2) | 3.4367(17) | |
| Cg(2)···Cg(3) (−x, 1 − y, −z) | 3.6345(11) | 1.23(8) | 3.4103(6) | |
| 2d | Cg(1)···Cg(3) (1 − x, −½ + y, −z) | 3.8523(8) | 0.02(8) | 3.5032(7) |
3. Experimental
3.1. Materials and Methods
3.2. X-ray Data Collection and Structure Determination
| 2b | 2c | 2d | |
|---|---|---|---|
| Chemical formula | C17H11N2O2Cl1 | C17H11N2O2Br1 | C17H11N3O4 |
| Mw | 310.7 | 355.19 | 321.2 |
| Cell setting, Space group | Triclinic, P-1 | Triclinic, P-1 | Monoclinic, P 21/m |
| a (A°) | 7.1177 (8) | 7.1681(8) | 9.4294(11) |
| b (A°) | 9.2540 (10) | 9.3210(11) | 7.0064(8) |
| c (A°) | 11.7266(13) | 11.8449(14) | 12.0294(14) |
| α (º) | 110.450(2) | 109.820(2) | 90 |
| β (º) | 98.468(2) | 97.016(2) | 112.826(2) |
| γ (º) | 97.748(2) | 96.891(2) | 90 |
| V (Å 3) | 701.14(8) | 727.83(15) | 732.50(7) |
| Z | 2 | 2 | 2 |
| Density (mg cm−3) | 1.471 | 1.621 | 1.46 |
| μ (mm−1) | 0.281 | 2.831 | 0.11 |
| Crystal form, color | Block, pale yellow | Block, colorless | Block, pale yellow |
| Crystal size (mm3) | 0.48 × 0.22 × 0.19 | 0.40 × 0.20 × 0.20 | 0.45 × 0.33 × 0.30 |
| No. of measured, | 6092 | 7652 | 4922 |
| independent and | 3160 | 2853 | 2514 |
| observed reflections | 2840 | 2013 | 2261 |
| Rint | 0.024 | 0.054 | 0.024 |
| θmax(°) | 28.3 | 26 | 28.3 |
| Refinement on | F2 | F2 | F2 |
| R[F2 > 2σ(F2)], wR(F2),S | 0.048, 0.116, 1.089 | 0.057, 0.116, 1.029 | 0.043, 0.122, 1.056 |
| No. of reflections | 3160 | 2853 | 2514 |
| No. of parameters | 200 | 200 | 218 |
| Weighting scheme | 1/[σ2(Fo2) + (0.0542P)2 + 0.2899P] | 1/[σ2(Fo2) + (0.0542P)2 + 0.1266P] | 1/[σ2(Fo2) + (0.0576P)2 + 0.321P] |
| P = (Fo2 + 2Fc2)/3 | P = (Fo2 + 2Fc2)/3 | P = (Fo2 + 2Fc2)/3 | |
| Δρmax, Δρmin (eÅ−3) | 0.411, −0.281 | 0.670, −0.322 | 0.194, −0.199 |
3.3. General Methods of Synthesis
4. Conclusions
Acknowledgements
References and Notes
- Behr, L.C.; Fusco, R.; Jarboe, C.H. Pyrazoles, pyrazolines, pyrazolidines, indazoles and condensed rings; Wiley-Interscience Publishers: New York, NY, USA, 1967; pp. 10–16. [Google Scholar]
- Elguero, J. Comprehensive Heterocyclic Chemistry; Katritsky, A.R., Rees, C.W., Eds.; Pergamon: Oxford, UK, 1984; Volume 5, pp. 167–303. [Google Scholar]
- Tanitame, A.; Oyamada, Y.; Ofuji, K.; Fujimoto, M.; Iwai, N.; Hiyama, Y.; Susuki, K.; Ito, H.; Terauchi, H.; Kawasaki, M.; Nagai, K.; Wachi, M.; Yamagishi, J. Synthesis and antibacterial activity of a novel series of potent DNA gyrase inhibitors. Pyrazole derivatives. J. Med. Chem. 2004, 47, 3693–3696. [Google Scholar]
- Bekhit, A.A.; Ashour, H.M.A.; Guemei, A.A. Novel pyrazole derivatives as potential promising anti-inflammatory antimicrobial agents. Arch. Pharm. 2005, 338, 167–174. [Google Scholar] [CrossRef]
- Goodell, J.R.; Puig-Basagoiti, F.; Forshey, B.; Shi, P.; Ferguson, D. Identification of compounds with anti-west nile virus activity. J. Med. Chem. 2006, 49, 2127–2137. [Google Scholar] [CrossRef]
- Lougiakis, N.; Marakos, P.; Poul, N.; Balzarani, J. Synthesis and antiviral activity evaluation of some novel acyclic C-nucleosides. Chem. Pharm. Bull. 2008, 56, 775–780. [Google Scholar] [CrossRef]
- Roppe, J.; Smith, N.D.; Huang, D.; Tehrani, L.; Wang, B.; Anderson, J.; Brodkin, J.; Chung, J.; Jiang, X.; King, C.; Munoz, B.; Varney, M.; Prasit, P.; Cosford, N. Discovery of novel heteroarylazoles that are metabotropic glutamate subtype 5 receptor antagonists with anxiolytic activity. J. Med. Chem. 2004, 47, 4645–4648. [Google Scholar]
- Melani, F.; Cecchi, L.; Palazzino, G.; Filiacchioni, G.; Pennacchi, E; Lucacchini, A. Pyrazolo[4,5-c]quinolines. 2. Synthesis and specific inhibition of bezodiazepine receptor binding. J. Med. Chem. 1986, 29, 291–295. [Google Scholar] [CrossRef]
- Khode, S.; Maddi, V.; Aragede, P.; Palkar, M.; Kumar, R.P.; Mamledesai, S.; Thippeswamy, A.H.M.; Satyanarayana, D. Synthesis and pharmacological evaluation of a novel series of 5-(substituted) aryl-3-(3-coumarinyl)-1-phenyl-2-pyrazolines as novel anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2009, 44, 1682–1688. [Google Scholar]
- Ren, X.L.; Li, H.B.; Wu, C.; Yang, H.Z. Synthesis of a small library containing substituted pyrazoles. Arkivoc 2005, 15, 59–67. [Google Scholar]
- Li, H.B.; Zhu, Y.Q.; Song, X.W.; Hu, F.Z.; Liu, B.; Li, Y.H.; Niu, Z.X.; Liu, P.; Wang, Z.H.; Song, H.B.; Zou, X.M.; Yang, H.Z. Novel protoporphyrinogen oxidase inhibitors: 3H-pyrazolo[3,4-d][1,2,3]triazin-4-one derivatives. J. Agric. Food Chem. 2008, 56, 9535–9542. [Google Scholar]
- Meegalla, S.K.; Doller, D.; Sha, D.Y.; Soll, R.; Wisnewski, N.M.; Silver, G.M.; Dhanoa, D. Synthesis and GABA receptor potency of 3-thiomethyl-4-(hetero)aryl-5-amino-1-phenylpyrazoles. Bioorg. Med. Chem. 2004, 14, 4949–4953. [Google Scholar] [CrossRef]
- Colotta, V.; Cecchi, L.; Filacchioni, G.; Melani, F.; Palazzino, G.; Martini, C.; Giannaccini, G.; Lucaccini, A. Synthesis, binding studies and structure-activity relationships of 1-aryl- and 2-aryl[1]benzopyranopyrazol-4-ones, central benzodiazepine receptor ligands. J. Med. Chem. 1988, 31, 1–3. [Google Scholar] [CrossRef]
- Kidwai, M.; Singhal, P.K.; Rastogi, S. A convenient K2CO3 catalysed regioselective synthesis for benzopyrano[4,3-c]pyrazoles in aqueous medium. Heterocycles 2007, 71, 569–576. [Google Scholar] [CrossRef]
- Chantegrel, B.; Nadi, A.-I.; Gelin, S. 4-Oxo-1H- and 2H-[1]benzopyrano[4,3-c]pyrazoles. Preparation from 4-hydroxycoumarin or 3-chromonecarboxylic acid derivatives. Tetrahedron Lett. 1983, 24, 381–384. [Google Scholar] [CrossRef]
- Colotta, V.; Cecchi, L.; Melani, F.; Palazzino, G.; Filacchioni, G. The correct synthesis of 2,3-dihydro-2-aryl-4-R-[1]benzopyrano[4,3-c]pyrazole-3-ones. Tetrahedron Lett. 1987, 28, 5165–5168. [Google Scholar] [CrossRef]
- Stadlbauer, W.; Hojas, G. Ring closure reactions of 3-arylhydrazonoalkyl-quinolin-2-ones to 1-aryl-pyrazolo[4,3-c]quinolin-2-ones. J. Heterocycl. Chem. 2004, 41, 681–690. [Google Scholar] [CrossRef]
- Hassan, N.A.; Mohamed, T.K.; Abdel-Hafez, O.M.; Lutz, M.; Karl, C.C.; Wirschun, W.; Al-Soud, Y.A.; Jochims, J.C. Cycloadditions of 1-aza-2-azoniaallene salts derived from coumarin and camphor. J. Prakt. Chem. 1998, 340, 151–159. [Google Scholar] [CrossRef]
- Hassaneen, H.M.; Mousa, H.A.H.; Shawali, A.S. Chemistry of C-heteroarylnitrilimines. Synthesis and cycloaddition reactions of N-phenyl-C-(2-thienyl)nitrilimine. J. Heterocycl. Chem. 1987, 24, 1665–1668. [Google Scholar] [CrossRef]
- Allen, F.H. The Cambridge Structural Database: a quarter of million crystal structures and rising. Acta Cryst. 2002, B58, 380–388. [Google Scholar]
- Hamada, T.; Ye, X.; Stahl, S.S. Copper-catalyzed aerobic oxidative amidation of terminal alkynes: efficient synthesis of ynamides. J. Am. Chem. Soc. 2008, 130, 833–835. [Google Scholar] [CrossRef]
- Monguchi, D.; Fujiwara, T.; Furukawa, H.; Mori, A. Direct amination of azoles via catalytic C-H, N-H Coupling. Org. Lett. 2009, 11, 1607–1610. [Google Scholar] [CrossRef]
- Colotta, V.; Cecchi, L.; Melani, F.; Filacchioni, G.; Martini, C.; Gelli, S.; Lucacchini, A. Triciclyc heteroaromatic systems: [1]bezopyrano-pyrazol-4-ones as benzodiazepine receptor ligans. J. Pharm. Sci. 1991, 80, 276–279. [Google Scholar] [CrossRef]
- Braun, N.A.; Ousmer, M.; Bray, J.D.; Bouchu, D.; Peters, K.; Peters, E.-M.; Ciufolini, M.A. New oxidative transformations of phenolic and indolic oxazolines: an avenue to useful azaspirocyclic building blocks. J. Org. Chem. 2000, 65, 4397–4408. [Google Scholar]
- Dang, T.-T.; Dang, T.-T.; Langer, P. One-pot synthesis of pyrazole-5-carboxylates by cyclization of hydrazone 1,4-dianions with diethyl oxalate. Tetrahedron Lett. 2007, 48, 3591–3593. [Google Scholar] [CrossRef]
- Santos-Contreras, R.; Martínez-Martínez, F.J.; Padilla-Martínez, I.I.; Peraza, A.L.; Höpfl, H. Carbonyl-carbonyl, carbonyl-pi and carbonyl-halogen dipolar interactions as the directing motifs of the supramolecular structure of ethyl 6-chloro-2-oxo-2H-chromene-3-carboxylate and ethyl 6-bromo-2-oxo-2H-chromene-3-carboxylate. Acta Cryst. 2007, C63, o239–o242. [Google Scholar]
- Strakova, I.; Petrova, M.; Belyakov, S.; Strakovs, A. Reactions of 4-chloro-3-formylcoumarin with arylhydrazines. Chem. Heterocycl. Comp. 2003, 39, 1608–1616. [Google Scholar] [CrossRef]
- Chopra, D.; Venugopala, K.N.; Rao, G.K.; Row, T.N.G. 3-Dibromoacetyl-2H-chromen-2-one. Acta Cryst. 2007, E63, o2826–o1971. [Google Scholar]
- Umezawa, Y.; Tsuboyama, S.; Honda, K.; Uzawa, J.; Nishio, M. CH/π Interaction in the crystal structure of organic compounds. A database study. Bull. Chem. Soc. Jpn. 1998, 71, 1207–1213. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.L. Patterns in hydrogen bonding: functionality and graph set analysis in crystals. Angew. Chem. Int. Ed. Engl. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Williams, J.H. The molecular electric quadrupole moment and solid-state architecture. Acc. Chem. Res. 1993, 26, 593–598. [Google Scholar] [CrossRef]
- Kovalevsky, A.Y. CCDC private communication, 2000.
- Allen, F.H.; Baalham, C.A.; Lommerse, J.P.M.; Raithby, P.R. Carbonyl-carbonyl interactions can be competitive with hydrogen bonds. Acta Cryst. 1998, B54, 320–329. [Google Scholar]
- García-Báez, E.V.; Martínez-Martínez, F.J.; Höpfl, H.; Padilla-Martínez, I.I. Pi-stacking interactions and C-H···X (X = O, aryl) hydrogen bonding as directing features of the supramolecular self-association in 3-carboxy and 3-amido coumarin derivatives. Cryst. Growth. Des. 2003, 3, 34–45. [Google Scholar]
- Santos-Contreras, R.J.; Martínez-Martínez, F.J.; Mancilla-Margalli, N.A.; Peraza-Campos, A.L.; Morín-Sánchez, L.M.; García-Báez, E.V.; Padilla-Martínez, I.I. Competition between OH···O and multiple halogen-dipole interactions on the formation of intramolecular three-centred hydrogen bond in 3-acyl coumarins. CrystEngCommunity 2009, 11, 1451–1461. [Google Scholar] [CrossRef]
- Flores-Larios, I.Y.; López-Garrido, L.; Martínez-Martínez, F.J.; González, J.; García-Báez, E.V.; Cruz, A.; Padilla-Martínez, I.I. Thermal [4+2] Cycloadditions of 3-acetyl-, 3-carbamoyl-, and 3-ethoxycarbonyl-coumarins with 2,3-dimethyl-1,3-butadiene under solventless conditions: a structural study. Molecules 2010, 15, 1513–1530. [Google Scholar] [CrossRef]
- Bruker. APEX II, SAINT, SADABS and SHELXTL, Bruker AXS Inc: Madison, WI, USA, 2004.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: visualization and analysis of crystal structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 2e-i are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Padilla-Martinez, I.I.; Flores-Larios, I.Y.; García-Baez, E.V.; Gonzalez, J.; Cruz, A.; Martínez-Martinez, F.J. X-Ray Supramolecular Structure, NMR Spectroscopy and Synthesis of 3-Methyl-1-phenyl-1H-chromeno[4,3-c]pyrazol-4-ones Formed by the Unexpected Cyclization of 3-[1-(Phenyl-hydrazono)ethyl]-chromen-2-ones. Molecules 2011, 16, 915-932. https://doi.org/10.3390/molecules16010915
Padilla-Martinez II, Flores-Larios IY, García-Baez EV, Gonzalez J, Cruz A, Martínez-Martinez FJ. X-Ray Supramolecular Structure, NMR Spectroscopy and Synthesis of 3-Methyl-1-phenyl-1H-chromeno[4,3-c]pyrazol-4-ones Formed by the Unexpected Cyclization of 3-[1-(Phenyl-hydrazono)ethyl]-chromen-2-ones. Molecules. 2011; 16(1):915-932. https://doi.org/10.3390/molecules16010915
Chicago/Turabian StylePadilla-Martinez, Itzia I., Irma Y. Flores-Larios, Efren V. García-Baez, Jorge Gonzalez, Alejandro Cruz, and Francisco J. Martínez-Martinez. 2011. "X-Ray Supramolecular Structure, NMR Spectroscopy and Synthesis of 3-Methyl-1-phenyl-1H-chromeno[4,3-c]pyrazol-4-ones Formed by the Unexpected Cyclization of 3-[1-(Phenyl-hydrazono)ethyl]-chromen-2-ones" Molecules 16, no. 1: 915-932. https://doi.org/10.3390/molecules16010915
APA StylePadilla-Martinez, I. I., Flores-Larios, I. Y., García-Baez, E. V., Gonzalez, J., Cruz, A., & Martínez-Martinez, F. J. (2011). X-Ray Supramolecular Structure, NMR Spectroscopy and Synthesis of 3-Methyl-1-phenyl-1H-chromeno[4,3-c]pyrazol-4-ones Formed by the Unexpected Cyclization of 3-[1-(Phenyl-hydrazono)ethyl]-chromen-2-ones. Molecules, 16(1), 915-932. https://doi.org/10.3390/molecules16010915









