Effect of Five Different Stages of Ripening on Chemical Compounds in Medlar (Mespilus germanica L.)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Macroelements
2.2. Microelements
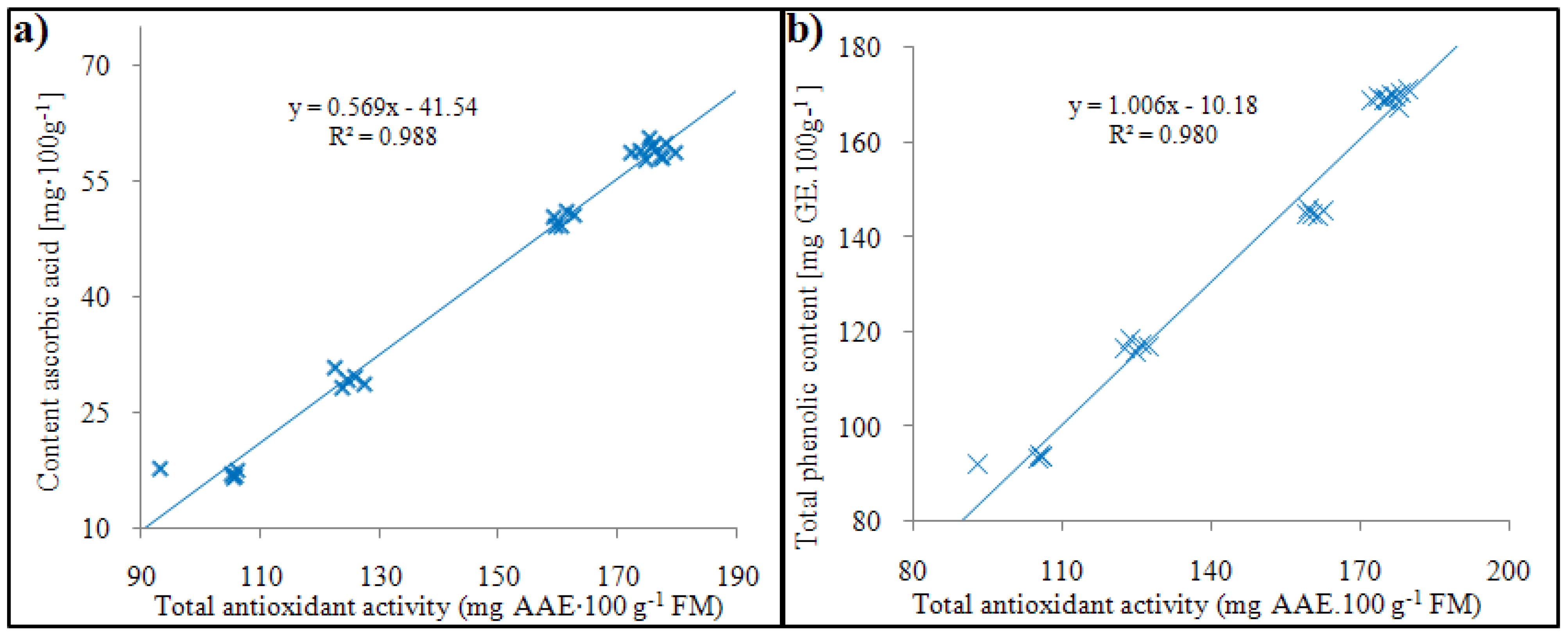
2.3. Total phenolic content, total antioxidant activity and ascorbic acid content
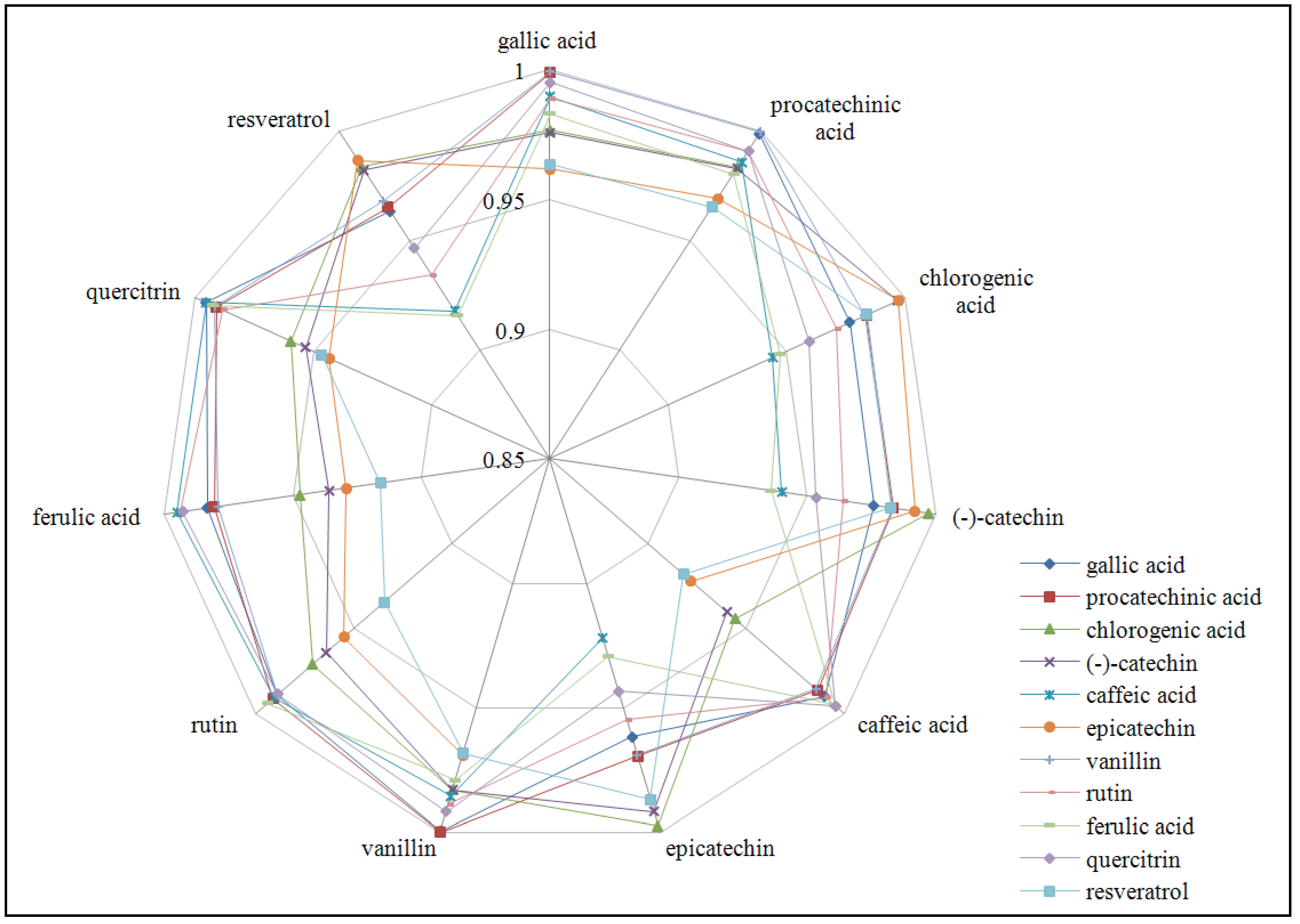
2.4. Determination of single phenolic compounds
3. Experimental
3.1. Plant material
3.2. Locality description and collection of samples
3.3. Chemicals
3.4. Extraction procedures
3.5. Determination of total phenolic content
3.6. Antioxidant activity assay
3.7. Mineral content assay
3.8. Determination of ascorbic acid
3.9. Determination of phenolic compounds
3.10. Statistical analysis
4. Conclusion
Acknowledgements
References and Notes
- Shulaev, V.; Korban, S.S.; Sosinski, B.; Abbott, A.G.; Aldwinckle, H.S.; Folta, K.M.; Iezzoni, A.; Main, D.; Arus, P.; Dandekar, A.M.; Lewers, K.; Brown, S.K.; Davis, T.M.; Gardiner, S.E.; Potter, D.; Veilleux, R.E. Multiple models for Rosaceae genomics. Plant Physiol. 2008, 147, 985–1003. [Google Scholar] [CrossRef] [PubMed]
- Dirlewanger, E.; Cosson, P.; Tavaud, M.; Aranzana, M.J.; Poizat, C.; Zanetto, A.; Arus, P.; Laigret, F. Development of microsatellite markers in peach [Prunus persica (L.) Batsch] and their use in genetic diversity analysis in peach and sweet cherry (Prunus avium L.). Theor. Appl. Genet. 2002, 105, 127–138. [Google Scholar] [PubMed]
- Kishore, G.M.; Shewmaker, C. Biotechnology: Enhancing human nutrition in developing and developed worlds. Proc. Natl. Acad. Sci. USA 1999, 96, 5968–5972. [Google Scholar] [CrossRef] [PubMed]
- Klejdus, B.; Vacek, J.; Adam, V.; Zehnalek, J.; Kizek, R.; Trnkova, L.; Kuban, V. Determination of isoflavones in soybean food and human urine using liquid chromatography with electrochemical detection. J. Chromatogr. B. 2004, 806, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Klejdus, B.; Mikelova, R.; Petrlova, J.; Potesil, D.; Adam, V.; Stiborova, M.; Hodek, P.; Vacek, J.; Kizek, R.; Kuban, V. Determination of isoflavones in soy bits by fast column high-performance liquid chromatography coupled with diode-array detector. J. Chromatogr. A. 2005, 1084, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Klejdus, B.; Mikelova, R.; Petrlova, J.; Potesil, D.; Adam, V.; Stiborova, M.; Hodek, P.; Vacek, J.; Kizek, R.; Kuban, V. Evaluation of isoflavones distribution in soy plants and soybeans by fast column high-performance liquid chromatography coupled with diode-array detector. J. Agr. Food Chem. 2005, 53, 5848–5852. [Google Scholar] [CrossRef] [PubMed]
- Haffner, K.; Vestrheim, S. Fruit quality of strawberry cultivars. In Third International Strawberry Symposium; vanderScheer, H.A.T., Lieten, F., Dijkstr, J., Eds.; International Society Horticultural Science: Veldhoven, The Netherlands, 1997; Volume 1-2, pp. 325–332. [Google Scholar]
- Mazur, W.M.; Uehara, M.; Wahala, K.; Adlercreutz, H. Phyto-oestrogen content of berries, and plasma concentrationsand urinary excretion of enterolactone after asingle strawberry-meal in human subjects. Br. J. Nutr. 2000, 83, 381–387. [Google Scholar] [PubMed]
- Oszmianski, J.; Wojdylo, A.; Lamer-Zarawska, E.; Swiader, K. Antioxidant tannins from Rosaceae plant roots. Food Chem. 2007, 100, 579–583. [Google Scholar] [CrossRef]
- Halvorsen, B.L.; Holte, K.; Myhrstad, M.C.W.; Barikmo, I.; Hvattum, E.; Remberg, S.F.; Wold, A.B.; Haffner, K.; Baugerod, H.; Andersen, L.F.; Moskaug, J.O.; Jacobs, D.R.; Blomhoff, R. A systematic screening of total antioxidants in dietary plants. J. Nutr. 2002, 132, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Kraft, T.F.B.; Dey, M.; Rogers, R.B.; Ribnicky, D.M.; Gipp, D.M.; Cefalu, W.T.; Raskin, I.; Lila, M.A. Phytochemical composition and metabolic performance-enhancing activity of dietary berries traditionally used by native North Americans. J. Agr. Food Chem. 2008, 56, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Kwok, B.H.L.; Kitts, D.D. Saskatoon berries (Amelanchier alnifolia Nutt.) scavenge free radicals and inhibit intracellular oxidation. Food Res. Int. 2005, 38, 1079–1085. [Google Scholar] [CrossRef]
- Campbell, C.S.; Donoghue, M.J.; Baldwin, B.G.; Wojciechowski, M.F. Phylogenetic-relationships in maloideae (rosaceae)—Evidence from sequences of the internal transcribed spacers of nuclear ribosomal dna and its congruence with morphology. Amer. J. Bot. 1995, 82, 903–918. [Google Scholar] [CrossRef]
- Evans, R.C.; Alice, L.A.; Campbell, C.S.; Kellogg, E.A.; Dickinson, T.A. The granule-bound starch synthase (GBSSI) gene in the rosaceae: Multiple loci and phylogenetic utility. Mol. Phylogenet. Evol. 2000, 17, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.C.; Campbell, C.S. The origin of the apple subfamily (Maloideae; Rosaceae) is clarified by DNA sequence data from duplicated GBSSI genes. Amer. J. Bot. 2002, 89, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, F.A.; Glew, R.H.; Huang, H.S.; Chuang, L.T.; VanderJagt, D.J.; Strnad, M. Evolution of fatty acids in mediar (Mespilus germanica L.) mesocarp at different stages of ripening. Grasas Aceites 2002, 53, 352–356. [Google Scholar]
- Glew, R.H.; Ayaz, F.A.; Sanz, C.; Vanderjagt, D.J.; Huang, H.S.; Chuang, L.T.; Strnad, M. Changes in sugars, organic acids and amino acids in medlar (Mespilus germanica L.) during fruit development and maturation. Food Chem. 2003, 83, 363–369. [Google Scholar] [CrossRef]
- Dincer, B.; Colak, A.; Aydin, N.; Kadioglu, A.; Guner, S. Characterization of polyphenoloxidase from medlar fruits (Mespilus germanica L., Rosaceae). Food Chem. 2002, 77, 1–7. [Google Scholar] [CrossRef]
- Haciseferogullari, H.; Ozcan, M.; Sonmete, M.H.; Ozbek, O. Some physical and chemical parameters of wild medlar (Mespilus germanica L.) fruit grown in Turkey. J. Food Eng. 2005, 69, 1–7. [Google Scholar] [CrossRef]
- Murcia, M.A.; Martinez-Tome, M.; Jimenez-Monreal, A.M. Evaluation of antioxidant activity of tomato treated with an ethylene inhibitor. FEBS J. 2005, 272, 417–417. [Google Scholar]
- de Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Quantitative analysis of flavan-3-ols in Spanish foodstuffs and beverages. J. Agr. Food Chem. 2000, 48, 5331–5337. [Google Scholar] [CrossRef]
- Glew, R.H.; Ayaz, F.A.; Vanderjagt, D.J.; Millson, M.; Dris, R.; Niskanen, R. A research note mineral composition of medlar (Mespilus germanica) fruit at different stages of maturity. J. Food Qual. 2003, 26, 441–447. [Google Scholar] [CrossRef]
- Glew, R.H.; Ayaz, F.A.; Sanz, C.; VanderJagt, D.J.; Huang, H.S.; Chuang, L.T.; Strnad, M. Effect of postharvest period on sugars, organic acids and fatty acids composition in commercially sold medlar (Mespilus germanica 'Dutch') fruit. Eur. Food Res. Technol. 2003, 216, 390–394. [Google Scholar] [CrossRef]
- Romero-Rodriguez, A.; Simal-Lozano, J.; Vazquez-Oderiz, L.; Lopez-Hernandez, J.; Gonzalez-Castro, M.J. Physical, physicochemical and chemical changes during maturation of medlards and persimmons. Dtsch. Lebensm.-Rundsch. 2000, 96, 142–145. [Google Scholar]
- Altunkaya, A.; Becker, E.M.; Gokmen, V.; Skibsted, L.H. Antioxidant activity of lettuce extract (Lactuca sativa) and synergism with added phenolic antioxidants. Food Chem. 2009, 115, 163–168. [Google Scholar] [CrossRef]
- Gorinstein, S.; Zachwieja, Z.; Folta, M.; Barton, H.; Piotrowicz, J.; Zemser, M.; Weisz, M.; Trakhtenberg, S.; Martin-Belloso, O. Comparative contents of dietary fiber, total phenolics, and minerals in persimmons and apples. J. Agr. Food Chem. 2001, 49, 952–957. [Google Scholar] [CrossRef]
- Brown, G.S.; Kitchener, A.E.; McGlasson, W.B.; Barnes, S. The effects of copper and calcium foliar sprays on cherry and apple fruit quality. Sci. Hort. 1996, 67, 219–227. [Google Scholar] [CrossRef]
- Sabrine, H.; Afif, H.; Mohamed, B.; Hamadi, B.; Maria, H. Effects of cadmium and copper on pollen germination and fruit set in pea (Pisum sativum L.). Sci. Hort. 2010, 125, 551–555. [Google Scholar] [CrossRef]
- Sharma, Y.M.; Rathore, G.S.; Jesani, J.C. Effect of soil and foliar application of zinc and copper on yield and fruit quality of seedless lemon (Citrus limon). Indian J. Agr. Sci. 1999, 69, 236–238. [Google Scholar]
- Parr, A.J.; Bolwell, G.P. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J. Sci. Food Agr. 2000, 80, 985–1012. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Gazdik, Z.; Krska, B.; Adam, V.; Saloun, J.; Pokorna, T.; Reznicek, V.; Horna, A.; Kizek, R. Electrochemical Determination of the Antioxidant Potential of Some Less Common Fruit Species. Sensors 2008, 8, 7564–7570. [Google Scholar] [CrossRef] [PubMed]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, Rubus, and Ribes. J. Agr. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef]
- Weaver, C.; Charley, H. Enzymatic browning of ripening bananas. J. Food Sci. 1974, 39, 1200–1202. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef] [PubMed]
- Andersen, F.; Lygren, B.; Maage, A.; Waagbo, R. Interaction between two dietary levels of iron and two forms of ascorbic acid and the effect on growth, antioxidant status and some non-specific immune parameters in Atlantic salmon (Salmo salar) smolts. Aquaculture 1998, 161, 437–451. [Google Scholar] [CrossRef]
- Benvenuti, S.; Pellati, F.; Melegari, M.; Bertelli, D. Polyphenols, anthocyanins, ascorbic acid, and radical scavenging activity of Rubus, Ribes, and Aronia. J. Food Sci. 2004, 69, C164–C169. [Google Scholar] [CrossRef]
- Hasler, A.; Sticher, O.; Meier, B. High-performance liquid-chromatographic determination of 5 widespread flavonoid aglycones. J. Chromatogr. 1990, 508, 236–240. [Google Scholar] [CrossRef]
- Marin, F.R.; Del Rio, J.A. Selection of hybrids and edible Citrus species with a high content in the diosmin functional compound. Modulating effect of plant growth regulators on contents. J. Agr. Food Chem. 2001, 49, 3356–3362. [Google Scholar] [CrossRef]
- Versari, A.; Parpinello, G.P.; Tornielli, G.B.; Ferrarini, R.; Giulivo, C. Stilbene compounds and stilbene synthase expression during ripening, wilting, and UV treatment in grape cv. Corvina. J. Agr. Food Chem. 2001, 49, 5531–5536. [Google Scholar] [CrossRef]
- Guyot, S.; Marnet, N.; Laraba, D.; Sanoner, P.; Drilleau, J.F. Reversed-phase HPLC following thiolysis for quantitative estimation and characterization of the four main classes of phenolic compounds in different tissue zones of a French cider apple variety (Malus domestica var. Kermerrien). J. Agr. Food Chem. 1998, 46, 1698–1705. [Google Scholar] [CrossRef]
- Kobayashi, H.; Wang, C.Z.; Pomper, K.W. Phenolic content and antioxidant capacity of pawpaw fruit (Asimina triloba L.) at different ripening stages. Hortscience 2008, 43, 268–270. [Google Scholar]
- Kuca, P.; Majsky, J.; Kopecek, F.; Jongepierova, I. Biele Karpaty, 1st ed.; Ekologia: Bratislava, Slovak Republic, 1992; pp. 258–262. [Google Scholar]
- Rupasinghe, H.P.V.; Jayasankar, S.; Lay, W. Variation in total phenolics and antioxidant capacity among European plum genotypes. Sci. Hort. 2006, 108, 243–246. [Google Scholar] [CrossRef]
- Sulc, M.; Lachman, J.; Hamouz, K.; Orsak, M.; Dvorak, P.; Horackova, V. Selection and evaluation of methods for determination of antioxidant activity of purple- and red-fleshed potato varieties. Chem. Listy 2007, 101, 584–591. [Google Scholar]
- Gazdik, Z.; Zitka, O.; Petrlova, J.; Adam, V.; Zehnalek, J.; Horna, A.; Reznicek, V.; Beklova, M.; Kizek, R. Determination of Vitamin C (Ascorbic Acid) Using High Performance Liquid Chromatography Coupled with Electrochemical Detection. Sensors 2008, 8, 7097–7112. [Google Scholar] [CrossRef] [PubMed]
- Sochor, J.; Zitka, O.; Skutkova, H.; Pavlik, D.; Babula, P.; Krska, B.; Horna, A.; Adam, V.; Provaznik, I.; Kizek, R. Content of phenolic compounds and antioxidant capacity in fruits of selected genotypes of apricot with resistance against Plum pox virus. Molecules 2010, 15, 6285–6305. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds of interest are available from the authors. |




| Ripening stage (DAFB) | |||||
|---|---|---|---|---|---|
| Element | 134 | 144 | 154 | 164 | 174 |
| Phosphorus | 939 ± 46 | 945 ± 35 | 940 ± 43 | 961 ± 41 | 938 ± 32 |
| Potassium | 8766 ± 101 | 8751 ± 85 | 8737 ± 77 | 8725 ± 92 | 8320 ± 93 |
| Calcium | 3094 ± 82 | 3111 ± 79 | 3095 ± 88 | 2754 ± 86 | 2695 ± 115 |
| Magnesium | 1038 ± 40 | 1035 ± 44 | 1021 ± 31 | 913 ± 50 | 842 ± 41 |
| Sodium | 118 ± 19 | 115 ± 17 | 115 ± 15 | 124 ± 12 | 121 ± 16 |
| Ripening Stage (DAFB) | |||||
|---|---|---|---|---|---|
| Element | 134 | 144 | 154 | 164 | 174 |
| Iron | 27.05 ± 2.04 | 27.14 ± 1.25 | 27.35 ± 1.67 | 27.52 ± 2.20 | 27.60 ± 1.45 |
| Manganese | 14.99 ± 2.11 | 14.25 ± 2.57 | 14.52 ± 2.50 | 14.17 ± 2.96 | 14.95 ± 2.15 |
| Zinc | 5.82 ± 0.51 | 5.71 ± 0.44 | 5.80 ± 0.67 | 5.90 ± 0.39 | 6.10 ± 0.50 |
| Copper | 5.37 ± 0.92 | 5.43 ±1.10 | 5.66 ± 0.79 | 5.94 ± 0.86 | 5.10 ± 1.13 |
| Molybdenum | 0.50 ± 0.05 | 0.50 ± 0.05 | 0.53 ± 0.05 | 0.60 ± 0.05 | 0.60 ± 0.05 |
| Calcium | Magnesium | Molybdenum | Iron | |
|---|---|---|---|---|
| Calcium | X | 0.980 | X | X |
| Magnesium | 0.980 | X | -0.969 | X |
| Molybdenum | X | -0.969 | X | 0.960 |
| Iron | X | X | 0.960 | X |
| Days after full bloom | Ascorbic acid content (mg·100 g-1 FM) | Total phenolic content (mg GAE·100 g-1 FM) | Total antioxidant activity (mg AAE·100 g-1 FM) |
|---|---|---|---|
| 134 | 59 ± 2 | 170 ± 1 | 180 ± 4 |
| 144 | 58 ± 2 | 169 ± 1 | 175 ± 5 |
| 154 | 50 ± 2 | 145 ± 1 | 160 ± 4 |
| 164 | 29 ± 2 | 117 ± 1 | 120 ± 5 |
| 174 | 17 ± 1 | 93 ± 1 | 100 ± 4 |
| pH | P | K | Ca | Mg | Mn | Cu | Zn | Mo |
|---|---|---|---|---|---|---|---|---|
| 6.8 | 33 | 122 | 2411 | 186 | 514 | 4.5 | 17.4 | 2.5 |
| Month | Temperature [°C] | Precipitation [mm] | Atmospheric moisture [%] | Global radiation [w · m-2] |
|---|---|---|---|---|
| March | 3.8 | 25 | 0.7 | 75 |
| April | 9 | 49 | 47.0 | 189 |
| May | 13.7 | 57 | 54.0 | 198 |
| June | 20.3 | 47 | 59.9 | 210 |
| July | 19.9 | 71 | 59.0 | 193 |
| August | 14.3 | 38 | 66.5 | 175 |
| September | 10.3 | 29 | 80.7 | 117 |
| October | 6.7 | 13 | 80.1 | 65 |
| November | 2.2 | 29 | 82.9 | 36 |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rop, O.; Sochor, J.; Jurikova, T.; Zitka, O.; Skutkova, H.; Mlcek, J.; Salas, P.; Krska, B.; Babula, P.; Adam, V.; et al. Effect of Five Different Stages of Ripening on Chemical Compounds in Medlar (Mespilus germanica L.). Molecules 2011, 16, 74-91. https://doi.org/10.3390/molecules16010074
Rop O, Sochor J, Jurikova T, Zitka O, Skutkova H, Mlcek J, Salas P, Krska B, Babula P, Adam V, et al. Effect of Five Different Stages of Ripening on Chemical Compounds in Medlar (Mespilus germanica L.). Molecules. 2011; 16(1):74-91. https://doi.org/10.3390/molecules16010074
Chicago/Turabian StyleRop, Otakar, Jiri Sochor, Tunde Jurikova, Ondrej Zitka, Helena Skutkova, Jiri Mlcek, Petr Salas, Boris Krska, Petr Babula, Vojtech Adam, and et al. 2011. "Effect of Five Different Stages of Ripening on Chemical Compounds in Medlar (Mespilus germanica L.)" Molecules 16, no. 1: 74-91. https://doi.org/10.3390/molecules16010074
APA StyleRop, O., Sochor, J., Jurikova, T., Zitka, O., Skutkova, H., Mlcek, J., Salas, P., Krska, B., Babula, P., Adam, V., Kramarova, D., Beklova, M., Provaznik, I., & Kizek, R. (2011). Effect of Five Different Stages of Ripening on Chemical Compounds in Medlar (Mespilus germanica L.). Molecules, 16(1), 74-91. https://doi.org/10.3390/molecules16010074






