Sequestration and Transport of Lignin Monomeric Precursors
Abstract
1. Introduction
2. Results and Discussion
2.1. Monolignol biosynthesis and subcellular localization of the related enzymes
2.2. Monolignol glucosylation and its potential roles in sequestration and transport of monolignols
2.3. Mechanisms for the transport of monolignols across membranes
2.3.1. Exocytosic transport via the Endoplasmic Reticulum- Golgi derived vesicles
2.3.2. Passive diffusion
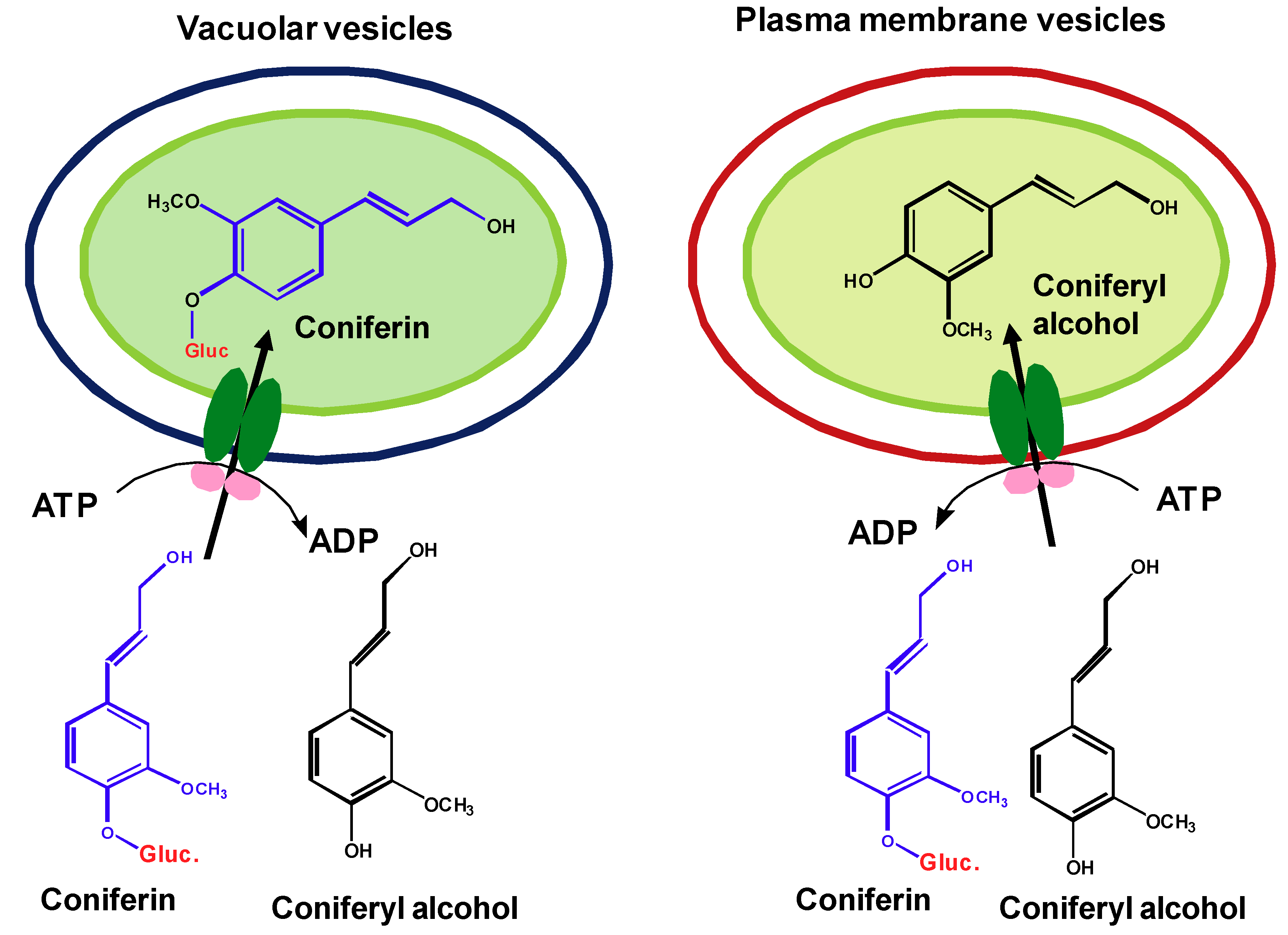
2.3.3. Transporter-mediated monolignol sequestration and export
2.4. Membrane transporters and their implication in phenolic transport
2.5. ABC-like transporters are involved in the transport of monolignols and their glucosides across membranes
3. Conclusions and Perspectives
Acknowledgements
References and Notes
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Ralph, J.; Lundquist, K.; Brunow, G.; Lu, F.; Kim, H.; Schatz, P.F.; Marita, J.M.; Hatfield, R.D.; Christensen, J.H.; Boerjan, W. Lignins: natural polymers from oxidative coupling of 4-hydroxyphenylpropanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar] [CrossRef]
- Vanholme, R.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin engineering. Curr. Opin. Plant Biol. 2008, 11, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Weng, J.K.; Chapple, C. Improvement of biomass through lignin modification. Plant J. 2008, 54, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Dixon, R.A. Lignin modification improves fermentable sugar yields for biofuel production. Nat. Biotechnol. 2007, 25, 759–761. [Google Scholar] [CrossRef] [PubMed]
- Takabe, K.; Fujita, M.; Harada, H.; Saiki, H. Autoradiographic investigations of lignification in the cell walls of cryptomeria (Cryptomeria japonica d. Don). Mokuzai Gakkaishi 1985, 31, 613–619. [Google Scholar]
- Nakashima, J.; Mizuno, T.; Takabe, K.; Fujita, M.; Saiki, H. Direct visualization of lignifying secondary wall thickenings in Zinna elegans cells in culture. Plant Cell Physiol. 1997, 38, 818–827. [Google Scholar] [CrossRef]
- Achnine, L.; Blancaflor, E.B.; Rasmussen, S.; Dixon, R.A. Colocalization of L-phenylalanine ammonia-lyase and cinnamate 4-hydroxylase for metabolic channeling in phenylpropanoid biosynthesis. Plant Cell 2004, 16, 3098–3109. [Google Scholar] [CrossRef] [PubMed]
- Whetten, R.; Sederoff, R. Lignin biosynthesis. Plant Cell 1995, 7, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Terazawa, M.; Miyake, M. Phenolic compounds in living tissue of woods. II. Seasonal variations of phenolic glycosides in the cambial sap of woods. Mokuzai Gakkaishi 1984, 30, 329–334. [Google Scholar]
- Leinhos, V.; Savidge, R.A. lsolation of protoplasts from developing xylem of Pinus banksiana and Pinus strobos. Can. J. For. Res. 1993, 23, 343–348. [Google Scholar] [CrossRef]
- Savidge, R.A. Coniferin, a biochemical indicator of commitment to tracheid differentiation in conifers. Can. J. Bot. 1989, 67, 2663–2668. [Google Scholar] [CrossRef]
- Savidge, R.A. A biochemical indicator of commitment to tracheid differentiation in Pinus contorta. Can. J. Bot. 1988, 66, 2009–2012. [Google Scholar] [CrossRef]
- Dharmawardhana, D.P.; Ellis, B.E.; Carlson, J.E. A β-glucosidase from lodgepole pine xylem specific for the lignin precursor coniferin. Plant Physiol. 1995, 107, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Dharmawardhana, D.P.; Ellis, B.E.; Carlson, J.E. cDNA cloning and heterologous expression of coniferin β-glucosidase. Plant Mol. Biol. 1999, 40, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Samuels, A.L.; Rensing, K.H.; Douglas, C.J.; Mansfield, S.D.; Dharmawardhana, D.P.; Ellis, B.E. Cellular machinery of wood production: Differentiation of secondary xylem in Pinus contorta var. Iatifolia. Planta 2002, 216, 72–82. [Google Scholar]
- Escamilla-Trevin˜o, L.L.; Chen, W.; Card, M.L.; Shih, M.-C.; Cheng, C.-L.; Poulton, J.E. Arabidopsis thaliana β-glucosidases BGLU45 and BGLU46 hydrolyse monolignol glucosides. Phytochemistry 2006, 67, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Steeves, C.; F¨orster, H.; Pommer, U.; Savidge, R. Coniferyl alcohol metabolism in conifers. I. Glucosidic turnover of cinnamyl aldehydes by UDPG: Coniferyl alcohol glucosyltransferase from pine cambium. Phytochemistry 2001, 57, 1085–1093. [Google Scholar] [CrossRef]
- Ibrahim, R.K. Glucosylation of lignin precursors by uridine diphosphate glucose: Coniferyl alcohol glucosyltransferase in higher plants. Z. Pflanzenphysiol. 1977, 85, 253–262. [Google Scholar] [CrossRef]
- Marcinowski, S.; Grisebach, H. Enzymology of lignification. Cell wall-bound β-glucosidase from coniferin from spruce (Picea abies) seedlings. Eur. J. Biochem. 1978, 87, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Hösel, W.; Surholt, E.; Borgmann, E. Characterization of beta-glucosidase isoenzymes possibly involved in lignification from chick pea (Cicer arietinum L.) cell suspension cultures. Eur. J. Biochem. 1978, 84, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Hösel, W.; Todenhagen, R. Characterization of a beta-glucosidase from Glycine max which hydrolyses coniferin and syringin. Phytochemistry 1980, 19, 1349–1353. [Google Scholar] [CrossRef]
- Leinhos, V.; Udagama-Randeniya, P.V.; Savage, R.A. Purification of an acidic coniferin-hydrolysing glucosidase from developing xylem of Pinus banksiana. Phytochem. 1994, 37, 311–315. [Google Scholar] [CrossRef]
- Marcinowski, S.; Falk, H.; Hammer, D.K.; Hoyer, B.; Grisebach, H. Appearance and localization of a beta-glucosidase hydrolyzing coniferin in spruce (Picea abies) seedlings. Planta 1979, 144, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, G.; Hosel, W. Immunohistochemical localization of beta-glucosidase in lignin and isoflavone metabolism in Cicer arietinum L. seedlings. Planta 1981, 152, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.K.; Li, Y.; Parr, A.; Jackson, R.; Ashford, D.A.; Bowles, D.J. Identification of glucosyltransferase genes involved in sinapate metabolism and lignin synthesis in Arabidopsis. J. Biol. Chem. 2001, 276, 4344–4349. [Google Scholar] [CrossRef] [PubMed]
- Lanot, A.; Hodge, D.; Jackson, R.G.; George, G.L.; Elias, L.; Lim, E.-K.; Vaistij, F.E.; Bowles, D.J. The glucosyltransferase UGT72E2 is responsible for monolignol 4-O-glucoside production in Arabidopsis thaliana. Plant J. 2006, 48, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, M.; Rensing, K.H.; Wong, J.C.; Banno, B.; Mansfield, S.D.; Samuels, A.L. Tracking monolignols during wood development in lodgepole pine. Plant Physiol. 2008, 147, 1750–1760. [Google Scholar] [CrossRef] [PubMed]
- Bowles, D.; Lim, E.-K.; Poppenberger, B.; Vaistij, F.V. Glycosyltransferases of lipophilic small molecules. Annu. Rev. Plant Biol. 2006, 57, 567–597. [Google Scholar] [CrossRef] [PubMed]
- Lerouxel, O.; Cavalier, D.M.; Liepman, A.H.; Keegstra, K. Biosynthesis of plant cell wall polysaccharides- a complex process. Curr. Opin. Plant Biol. 2006, 9, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Pickett-Heaps, J.D. Xylem wall deposition: Radioautographic investigations using lignin precursors. Protoplasma 1968, 65, 181–205. [Google Scholar] [CrossRef]
- Fujita, M.; Harada, H. Autoradiographic investigations of cell wall development. II. Tritiated phenylalanine and ferulic acid assimilation in relation to lignification. Mokuzai Gakkaishi 1979, 25, 89–94. [Google Scholar]
- Morreel, K.; Ralph, J.; Kim, H.; Lu, F.; Goeminne, G.; Ralph, S.; Messens, E.; Boerjan, W. Profiling of oligolignols reveals monolignol coupling conditions in lignifying poplar Xylem. Plant Physiol. 2004, 136, 3537–3549. [Google Scholar] [CrossRef] [PubMed]
- Leple, J.C.; Dauwe, R.; Morreel, K.; Storme, V.; Lapierre, C.; Pollet, B.; Naumann, A.; Kang, K.Y.; Kim, H.; Ruel, K.; Lefebvre, A.; Joseleau, J.P.; Grima-Pettenati, J.; De Rycke, R.; Andersson-Gunneras, S.; Erban, A.; Fehrle, I.; Petit-Conil, M.; Kopka, J.; Polle, A.; Messens, E.; Sundberg, B.; Mansfield, S.D.; Ralph, J.; Pilate, G.; Boerjan, W. Downregulation of cinnamoyl-coenzyme A reductase in poplar: multiple-level phenotyping reveals effects on cell wall polymer metabolism and structure. Plant Cell 2007, 19, 3669–3691. [Google Scholar] [CrossRef] [PubMed]
- Ralph, J.; Kim, H.; Lu, F.; Grabber, J.H.; Leple, J.C.; Berrio-Sierra, J.; Derikvand, M.M.; Jouanin, L.; Boerjan, W.; Lapierre, C. Identification of the structure and origin of a thioacidolysis marker compound for ferulic acid incorporation into angiosperm lignins (and an indicator for cinnamoyl CoA reductase deficiency). Plant J. 2008, 53, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Ralph, J.; Morreel, K.; Messens, E.; Boerjan, W. Preparation and relevance of a cross-coupling product between sinapyl alcohol and sinapyl p-hydroxybenzoate. Org. Biomol. Chem. 2004, 2, 2888–2890. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, J.C.; Marques, G.; Rencoret, J.; Martinez, A.T.; Gutierrez, A. Occurrence of naturally acetylated lignin units. J. Agric. Food. Chem. 2007, 55, 5461–5468. [Google Scholar] [CrossRef] [PubMed]
- del Rio, J.C.; Rencoret, J.; Marques, G.; Gutierrez, A.; Ibarra, D.; Santos, J.I.; Jimenez-Barbero, J.; Zhang, L.; Martinez, A.T. Highly acylated (acetylated and/or p-coumaroylated) native lignins from diverse herbaceous plants. J. Agric. Food Chem. 2008, 56, 9525–9534. [Google Scholar] [CrossRef] [PubMed]
- Boija, E.; Lundquist, A.; Edwards, K.; Johansson, G. Evaluation of bilayer disks as plant cell membrane models in partition studies. Anal. Biochem. 2007, 364, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Boija, E.; Lundquist, A.; Nilsson, M.; Edwards, K.; Isaksson, R.; Johansson, G. Bilayer disk capillary electrophoresis: A novel method to study drug partitioning into membranes. Electrophoresis 2008, 29, 3377–3383. [Google Scholar] [CrossRef] [PubMed]
- Fergus, B.J.; Goring, D.A.I. The distribution of lignin in birch wood as determined by ultraviolet microscopy. Holzforschung 1970, 24, 118–124. [Google Scholar] [CrossRef]
- Chapple, C.; Vogt, T.; Ellis, B.E.; Somerville, C. Arabidopsis mutant defective in phenylpropanoid pathway. Plant Cell 1994, 4, 1413–1424. [Google Scholar] [CrossRef]
- Whiting, P.; Goring, D.A.I. Chemical characterization of tissue fractions from the middle lamella and secondary wall of black spruce tracheids. Wood Sci. Technol. 1982, 16, 261–267. [Google Scholar] [CrossRef]
- Terashima, N.; Fukushima, K.; He, L.-F.; Takabe, K. Comprehensive model of the lignified plant cell wall. In Forage Cell Wall Structure and Digestibility; Jung, H.G., Buxton, D.R., Hatfield, R.D., Ralph, J., Eds.; ASA-CSSA-SSSA: Madison, WI, USA, 1993; pp. 247–270. [Google Scholar]
- Terashima, N.; Fukushima, K.; Tsuchiya, S. Heterogeneity in formation of lignin VII. An autoradiographic study on the formation of guaiacyl and syringyl lignin in poplar. J. Wood Chem. Technol. 1986, 6, 495–504. [Google Scholar] [CrossRef]
- Terashima, N.; Fukushima, K.; Takabe, K. Heterogeneity in formation of lignin VIII. An autoradiographic study on the formation of guaiacyl and syringyl lignin in Magnolia kobus dc. Holzforschung 1986, 40, 101–105. [Google Scholar]
- Allona, I.; Quinn, M.; Shoop, E.; Swope, K.; Cyr, S.S.; Carlis, J.; Riedl, J.; Retzel, E.; Campbell, M.M.; Sederoff, R.E.A. Analysis of xylem formation in pine by cDNA sequencing. Proc. Natl. Acad. Sci. USA 1998, 95, 9693–9698. [Google Scholar] [CrossRef] [PubMed]
- Hertzberg, M.; Aspeborg, H.; Schrader, J.; Andersson, A.; Erlandsson, R.; Blomqvist, K.; Bhalerao, R.; Uhlen, M.; Teeri, T.T.; Lundeberg, J.; Sundberg, B.; Nilsson, P.; Sandberg, G. A transcriptional roadmap to wood formation. Proc. Natl. Acad. Sci. USA 2001, 98, 14732–14737. [Google Scholar] [CrossRef] [PubMed]
- Kirst, M.; Johnson, A.F.; Baucom, C.; Ulrich, E.; Hubbard, K.; Staggs, R.; Paule, C.; Retzel, E.; Whetten, R.; Sederoff, R. Apparent homology of expressed genes from wood-forming tissues of loblolly pine (Pinus taeda) with Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2003, 100, 7383–7388. [Google Scholar] [CrossRef] [PubMed]
- Egertsdotter, U.; van Zyl, L.M.; MacKay, J.; Peter, G.; Kirst, M.; Clark, C.; Whetten, R.; Sederoff, R. Gene expression during formation of earlywood and latewood in loblolly pine: expression profiles of 350 genes. Plant Biol. (Stuttg) 2004, 6, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Whetten, R.; Sun, Y.H.; Zhang, Y.; Sederoff, R. Functional genomics and cell wall biosynthesis in loblolly pine. Plant Mol. Biol. 2001, 47, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Ehlting, J.; Mattheus, N.; Aeschliman, D.S.; Li, E.; Hamberger, B.; Cullis, I.F.; Zhuang, J.; Kaneda, M.; Mansfield, S.D.; Samuels, L.; Ritland, K.; Ellis, B.E.; Bohlmann, J.; Douglas, C.J. Global transcript profiling of primary stems from Arabidopsis thaliana identifies candidate genes for missing links in lignin biosynthesis and transcriptional regulators of fiber differentiation. Plant J. 2005, 42, 618–640. [Google Scholar] [CrossRef] [PubMed]
- Douglas, C.J.; Ehlting, J. Arabidopsis thaliana full genome longmer microarrays: a powerful gene discovery tool for agriculture and forestry. Transgenic Res. 2005, 14, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.; Bernfur, K.; Gustavsson, N.; Bygdell, J.; Wingsle, G.; Larsson, C. Proteomics of plasma membranes from poplar trees reveals tissue distribution of transporters, receptors, and proteins in cell wall formation. Mol. Cell Proteomics 2010, 9, 368–387. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.H.; Paulsen, I.T.; Skurray, R.A. The multidrug efflux protein NorM is a prototype of a new family of transporters. Mol. Microbiol. 1999, 31, 393–395. [Google Scholar] [CrossRef]
- Higgins, C.F. ABC transporters: From microorganisms to man. Annu. Rev. Cell Biol. 1992, 8, 67–113. [Google Scholar] [CrossRef] [PubMed]
- Sa´nchez-Ferna´ndez, R.; Davies, E.T.G.; Coleman, J.O.D.; Rea, P.A. The Arabidopsis thaliana ABC protein superfamily: a complete inventory. J. Biol. Chem. 2001, 276, 30231–30244. [Google Scholar] [CrossRef] [PubMed]
- Verrier, P.J.; Bird, D.; Burla, B.; Dassa, E.; Forestier, C.; Geisler, M.; Klein, M.; Kolukisaoglu, U.; Lee, Y.; Martinoia, E.; Murphy, A.; Rea, P.A.; Samuels, L.; Schulz, B.; Spalding, E.J.; Yazaki, K.; Theodoulou, F.L. Plant ABC proteins – a unified nomenclature and updated inventory. Trends Plant Sci. 2008, 13, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Rea, P.A. Plant ATP-Binding Cassette transporters. Annu. Rev. Plant Biol. 2007, 58, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.F.; Linton, K.J. The ATP switch model for ABC transporters. Nat. Struct. Mol. Biol. 2004, 11, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Schulza, B.; Kolukisaoglu, H.U. Genomics of plant ABC transporters: The alphabet of photosynthetic life forms or just holes in membranes? FEBS Letters 2006, 580, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Jasinski, M.; Ducos, E.; Martinoia, E.; Boutry, M. The ATP-binding cassette transporters: Structure, function, and gene family comparison between rice and arabidopsis. Plant Physiol. 2003, 131, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.P.; Li, Z.S.; Drozdowicz, Y.M.; Hortensteiner, S.; Martinoia, E.; Rea, P.A. AtMRP2, an Arabidopsis ATP binding cassette transporter able to transport glutathione S-conjugates and chlorophyll catabolites: functional comparisons with Atmrp1. Plant Cell 1998, 10, 267–282. [Google Scholar] [PubMed]
- Tommasini, R.; Vogt, E.; Fromenteau, M.; Hortensteiner, S.; Matile, P.; Amrhein, N.; Martinoia, E. An ABC-transporter of Arabidopsis thaliana has both glutathione-conjugate and chlorophyll catabolite transport activity. Plant J. 1998, 13, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Kispal, G.; Csere, P.; Prohl, C.; Lill, R. The mitochondrial proteins Atm1p and Nfs1p are essential for biogenesis of cytosolic Fe/S proteins. Embo. J. 1999, 18, 3981–3989. [Google Scholar] [CrossRef] [PubMed]
- Zolman, B.K.; Silva, I.D.; Bartel, B. The Arabidopsis pxa1 mutant is defective in an ATP-binding cassette transporter-like protein required for peroxisomal fatty acid beta-oxidation. Plant Physiol. 2001, 127, 1266–1278. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, K.; Strader, L.C.; Bailly, A.; Yang, H.; Blakeslee, J.; Langowski, L.; Nejedla, E.; Fujita, H.; Itoh, H.; Syono, K.; Hejatko, J.; Gray, W.M.; Martinoia, E.; Geisler, M.; Bartel, B.; Murphy, A.S.; Friml, J. Arabidopsis PIS1 encodes the ABCG37 transporter of auxinic compounds including the auxin precursor indole-3-butyric acid. Proc. Natl. Acad. Sci. USA 2010, 107, 10749–10753. [Google Scholar] [CrossRef] [PubMed]
- Geisler, M.; Blakeslee, J.J.; Bouchard, R.; Lee, O.R.; Vincenzetti, V.; Bandyopadhyay, A.; Titapiwatanakun, B.; Peer, W.A.; Bailly, A.; Richards, E.L.; Ejendal, K.F.; Smith, A.P.; Baroux, C.; Grossniklaus, U.; Muller, A.; Hrycyna, C.A.; Dudler, R.; Murphy, A.S.; Martinoia, E. Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J. 2005, 44, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Strader, L.C.; Bartel, B. The Arabidopsis PLEIOTROPIC DRUG RESISTANCE8/ABCG36 ATP binding cassette transporter modulates sensitivity to the auxin precursor indole-3-butyric acid. Plant Cell 2009, 21, 1992–2007. [Google Scholar] [CrossRef] [PubMed]
- Kuromori, T.; Miyaji, T.; Yabuuchi, H.; Shimizu, H.; Sugimoto, E.; Kamiya, A.; Moriyama, Y.; Shinozaki, K. ABC transporter AtABCG25 is involved in abscisic acid transport and responses. Proc. Natl. Acad. Sci. USA 2010, 107, 2361–2366. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Hwang, J.U.; Lee, M.; Kim, Y.Y.; Assmann, S.M.; Martinoia, E.; Lee, Y. PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proc. Natl. Acad. Sci. USA 2010, 107, 2355–2360. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.J.; Wang, Y.F.; Frelet, A.; Leonhardt, N.; Klein, M.; Forestier, C.; Mueller-Roeber, B.; Cho, M.H.; Martinoia, E.; Schroeder, J.I. The ATP binding cassette transporter AtMRP5 modulates anion and calcium channel activities in Arabidopsis guard cells. J. Biol. Chem. 2007, 282, 1916–1924. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Choi, Y.; Burla, B.; Kim, Y.Y.; Jeon, B.; Maeshima, M.; Yoo, J.Y.; Martinoia, E.; Lee, Y. The ABC transporter AtABCB14 is a malate importer and modulates stomatal response to CO2. Nat. Cell Biol. 2008, 10, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Pighin, J.A.; Zheng, H.; Balakshin, L.J.; Goodman, I.P.; Western, T.L.; Jetter, R.; Kunst, L.; Samuels, A.L. Plant cuticular lipid export requires an ABC transporter. Science 2004, 306, 702–704. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, H.E.; Shin, J.J.; Bird, D.A.; Samuels, A.L. Arabidopsis ABCG transporters, which are required for export of diverse cuticular lipids, dimerize in different combinations. Plant Cell 2010, 22, 3066–3075. [Google Scholar] [CrossRef] [PubMed]
- Jasinski, M.; Stukkens, Y.; Degand, H.; Purnelle, B.; Marchand-Brynaert, J.; Boutry, M. A plant plasma membrane ATP binding cassette-type transporter is involved in antifungal terpenoid secretion. Plant Cell 2001, 13, 1095–1107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goossens, A.; Hakkinen, S.T.; Laakso, I.; Oksman-Caldentey, K.M.; Inze, D. Secretion of secondary metabolites by ATP-binding cassette transporters in plant cell suspension cultures. Plant Physiol. 2003, 131, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Shitan, N.; Bazin, I.; Dan, K.; Obata, K.; Kigawa, K.; Ueda, K.; Sato, F.; Forestier, C.; Yazaki, K. Involvement of CjMDR1, a plant multidrugresistance-type ATP-binding cassette protein, in alkaloid transport in Coptis japonica. Proc. Natl. Acad. Sci. USA 2003, 100, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Shitan, N.; Yazaki, K. Accumulation and membrane transport of plant alkaloids. Curr. Pharm. Biotechnol. 2007, 8, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Baerson, S.R.; Sanchez-Moreiras, A.; Pedrol-Bonjoch, N.; Schulz, M.; Kagan, I.A.; Agarwal, A.K.; Reigosa, M.J.; Duke, S.O. Detoxification and transcriptome response in Arabidopsis seedlings exposed to the allelochemical benzoxazolin-2(3H)-one. J. Biol. Chem. 2005, 280, 21867–21881. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Dixon, R.A. The ‘ins’ and ‘outs’ of flavonoid transport. Trends Plant Sci. 2010, 15, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Martinoia, E.; Hoffmann-Thoma, G.; Weissenbock, G. A membrane-potential dependent ABC-like transporter mediates the vacuolar uptake of rye flavone glucuronides: regulation of glucuronide uptake by glutathione and its conjugates. Plant J. 2000, 21, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Frangne, N.; Eggmann, T.; Koblischke, C.; Weissenbo¨ck, G.; Martinoia, E.; Klein, M. Flavone glucoside uptake into barley mesophyll and Arabidopsis cell culture vacuoles. Energization occurs by H+-antiport and ATP-binding cassettetype mechanisms. Plant Physiol. 2002, 128, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.D.; Casati, P.; Walbot, V. A multidrug resistance-associated protein involved in anthocyanin transport in Zea mays. Plant Cell 2004, 16, 1812–1826. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Shitan, N.; Yazaki, K. Involvement of a soybean ATP-binding cassette-type transporter in the secretion of genistein, a signal flavonoid in legume-Rhizobium symbiosis. Plant Physiol. 2007, 144, 2000–2008. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Compartmentation of secondary metabolites and xenobiotics in plant vacuoles. In The Plant Vacuole; Leigh, R.A., Sanders, D., Eds.; Academic Press: San Diego, CA, USA, 1997; pp. 141–169. [Google Scholar]
- Yazaki, K. Transporters of secondary metabolites. Curr. Opin. Plant Biol. 2005, 8, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Diener, A.C.; Gaxiola, R.A.; Fink, G.R. Arabidopsis ALF5, a Multidrug Efflux Transporter Gene Family Member, Confers Resistance to Toxins. Plant Cell 2001, 13, 1625–1637. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, Z.; Pandey, G.K.; Tsuchiya, T.; Luan, S. Functional Cloning and Characterization of a Plant Efflux Carrier for Multidrug and Heavy Metal Detoxification. J. Biol. Chem. 2002, 277, 5360–5368. [Google Scholar] [CrossRef] [PubMed]
- Debeaujon, I.; Peeters, A.J.M.; Leon-Kloosterziel, K.M.; Korneef, M. The TRANSPARENT TESTA 12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell 2001, 13, 853–871. [Google Scholar] [CrossRef] [PubMed]
- Marinova, K.; Pourcel, L.; Weder, B.; Schwarz, M.; Barron, D.; Routaboul, J.M.; Debeaujon, I.; Klein, M. The Arabidopsis MATE transporter TT12 acts as a vacuolar flavonoid/H+ -antiporter active in proanthocyanidin-accumulating cells of the seed coat. Plant Cell 2007, 19, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Dixon, R.A. MATE Transporters Facilitate Vacuolar Uptake of Epicatechin 3′-O-Glucoside for Proanthocyanidin Biosynthesis in Medicago truncatula and Arabidopsis. Plant Cell 2009, 21, 2323–2340. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.; Terrier, N.; Torregrosa, L.; Vialet, S.; Fournier-Level, A.; Verries, C.; Souquet, J.M.; Mazauric, J.P.; Klein, M.; Cheynier, V.; Ageorges, A. Grapevine MATE-type proteins act as vacuolar H+-dependent acylated anthocyanin transporters. Plant Physiol. 2009, 150, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Inai, K.; Yazaki, Y.; Sato, Y.; Takase, H.; Shitan, N.; Yazaki, K.; Goto, Y.; Toyooka, K.; Matsuoka, K.; Hashimoto, T. Multidrug and toxic compound extrusion-type transporters implicated in vacuolar sequestration of nicotine in tobacco roots. Plant Physiol. 2009, 149, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Shitan, N.; Sawada, K.; Van Montagu, M.C.; Inze, D.; Rischer, H.; Goossens, A.; Oksman-Caldentey, K.M.; Moriyama, Y.; Yazaki, K. Vacuolar transport of nicotine is mediated by a multidrug and toxic compound extrusion (MATE) transporter in Nicotiana tabacum. Proc. Natl. Acad. Sci. USA 2009, 106, 2447–2452. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, K. ABC transporters involved in the transport of plant secondary metabolites. FEBS Lett. 2006, 580, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Weissenbock, G.; Dufaud, A.; Gaillard, C.; Kreuz, K.; Martinoia, E. Different energization mechanisms drive the vacuolar uptake of a flavonoid glucoside and a herbicide glucoside. J. Biol. Chem. 1996, 271, 29666–29671. [Google Scholar] [CrossRef] [PubMed]
- Bartholomew, D.M.; Van Dyk, D.E.; Lau, S.C.; O’Keefe, D.P.; Rea, P.A.; Viitanen, P.V. Alternate energy-dependent pathways for the vacuolar uptake of glucose and glutathione conjugates. Plant Physiol. 2002, 130, 1562–1572. [Google Scholar] [CrossRef] [PubMed]
- Walczak, H.A.; Dean, J.V. Vacuolar transport of the glutathione conjugate of trans-cinnamic acid. Phytochem. 2000, 53, 441–446. [Google Scholar] [CrossRef]
- Rea, P.A.; Li, Z.S.; Lu, Y.P.; Drozdowicz, Y.M.; Martinoia, E. From vacuolar GS-X pumps to multispecific ABC transporters. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 727–760. [Google Scholar] [CrossRef] [PubMed]
- Kolukisaoglu, H.U.; Bovet, L.; Klein, M.; Eggmann, T.; Geisler, M.; Wanke, D.; Martinoia, E.; Schulz, B. Family business: the multidrug resistance related protein (MRP) ABC transporter genes in Arabidopsis thaliana. Planta 2002, 216, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Badri, D.V.; Loyola-Vargas, V.M.; Broeckling, C.D.; DelaPena, C.; Jasinski, M.; Santelia, D.; Martinoia, E.; Sumner, L.W.; Banta, L.M.; Stermitz, F.; Vivanco, J.M. Altered profile of secondary metabolites in the root exudates of Arabidopsis ATP-binding cassette transporter mutants. Plant Physiol. 2008, 146, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.C.; Liu, C.J. ATP-binding cassette-like transporters are involved in the transport of lignin precursors across plasma and vacuolar membranes. Proc. Natl. Acad. Sci. USA 2011, in press. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, C.-J.; Miao, Y.-C.; Zhang, K.-W. Sequestration and Transport of Lignin Monomeric Precursors. Molecules 2011, 16, 710-727. https://doi.org/10.3390/molecules16010710
Liu C-J, Miao Y-C, Zhang K-W. Sequestration and Transport of Lignin Monomeric Precursors. Molecules. 2011; 16(1):710-727. https://doi.org/10.3390/molecules16010710
Chicago/Turabian StyleLiu, Chang-Jun, Yu-Chen Miao, and Ke-Wei Zhang. 2011. "Sequestration and Transport of Lignin Monomeric Precursors" Molecules 16, no. 1: 710-727. https://doi.org/10.3390/molecules16010710
APA StyleLiu, C.-J., Miao, Y.-C., & Zhang, K.-W. (2011). Sequestration and Transport of Lignin Monomeric Precursors. Molecules, 16(1), 710-727. https://doi.org/10.3390/molecules16010710



