Hydrogen Generation Using a CuO/ZnO-ZrO2 Nanocatalyst for Autothermal Reforming of Methanol in a Microchannel Reactor
Abstract
:1. Introduction
| Reaction | Scheme | Δ
H  (kJ mol−1) (kJ mol−1) |
|---|---|---|
| Methanol decomposition (MD) | CH3OH → 2 H2 + CO | +92.0 |
| Water-gas shift reaction (WGS) | CO + H2O → H2 + CO2 | −42.6 |
| Steam reforming of methanol (SRM) | CH3OH + H2O → 3 H2 + CO2 | +49.4 |
| Partial oxidation of methanol (POM) | CH3OH + ½ O2 → 2 H2 + CO2 | −192.2 |
| Total oxidation of methanol (TOM) | CH3OH + 3/2 O2 → CO2 + 2 H2O | +673.2 |
2. Results and Discussion
2.1. Morphology and crystalline structure identification
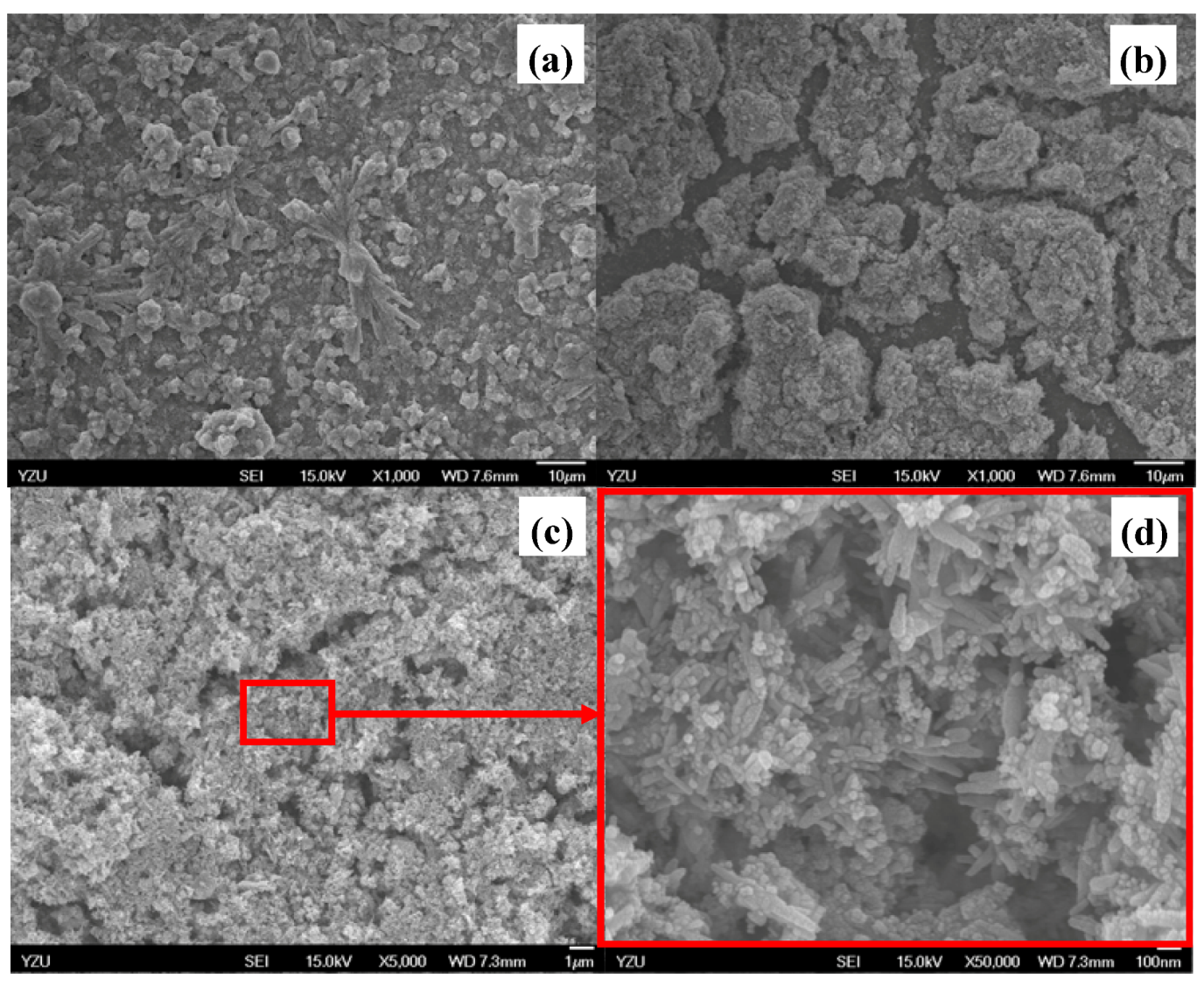
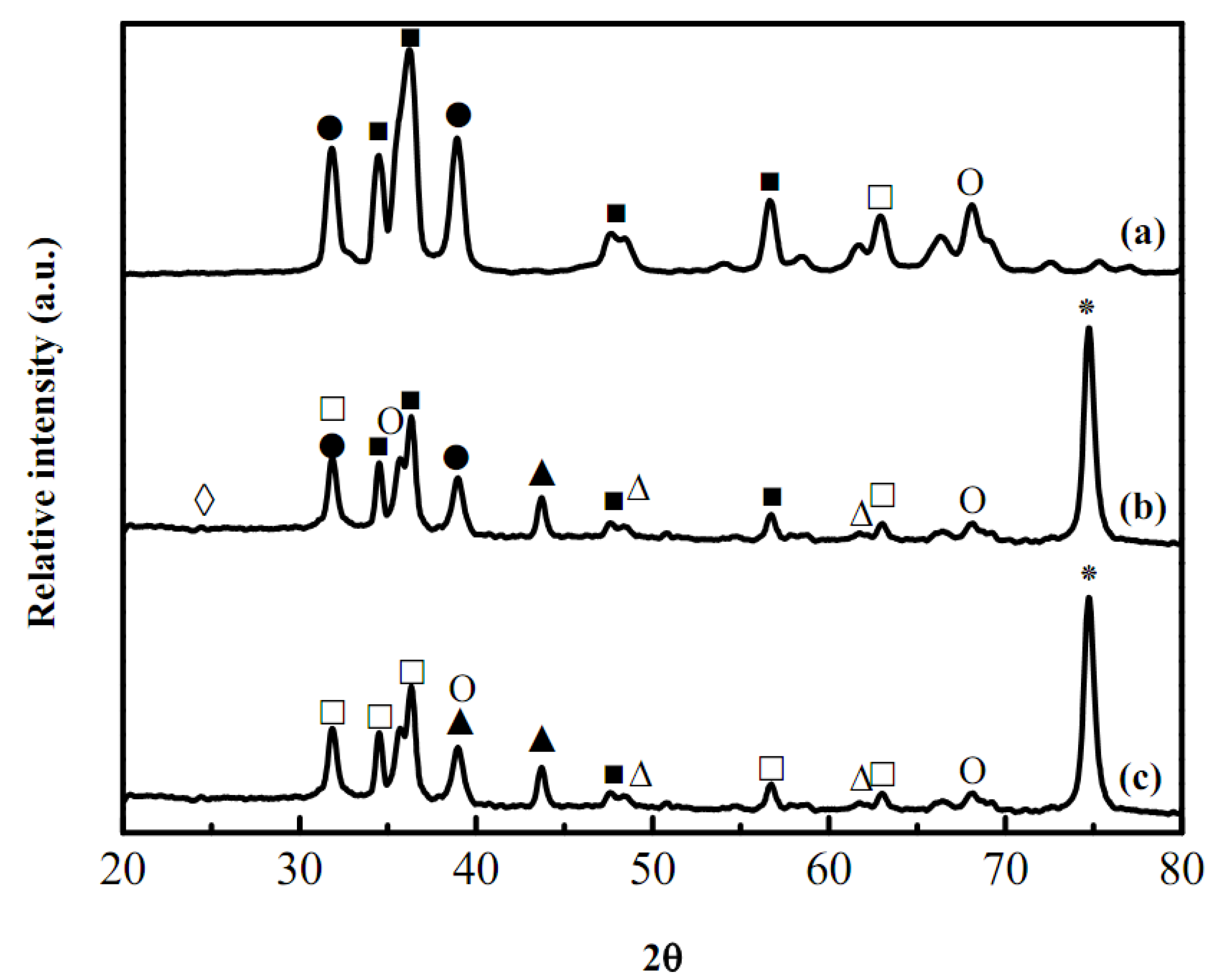
2.2. XRD pattern analyses
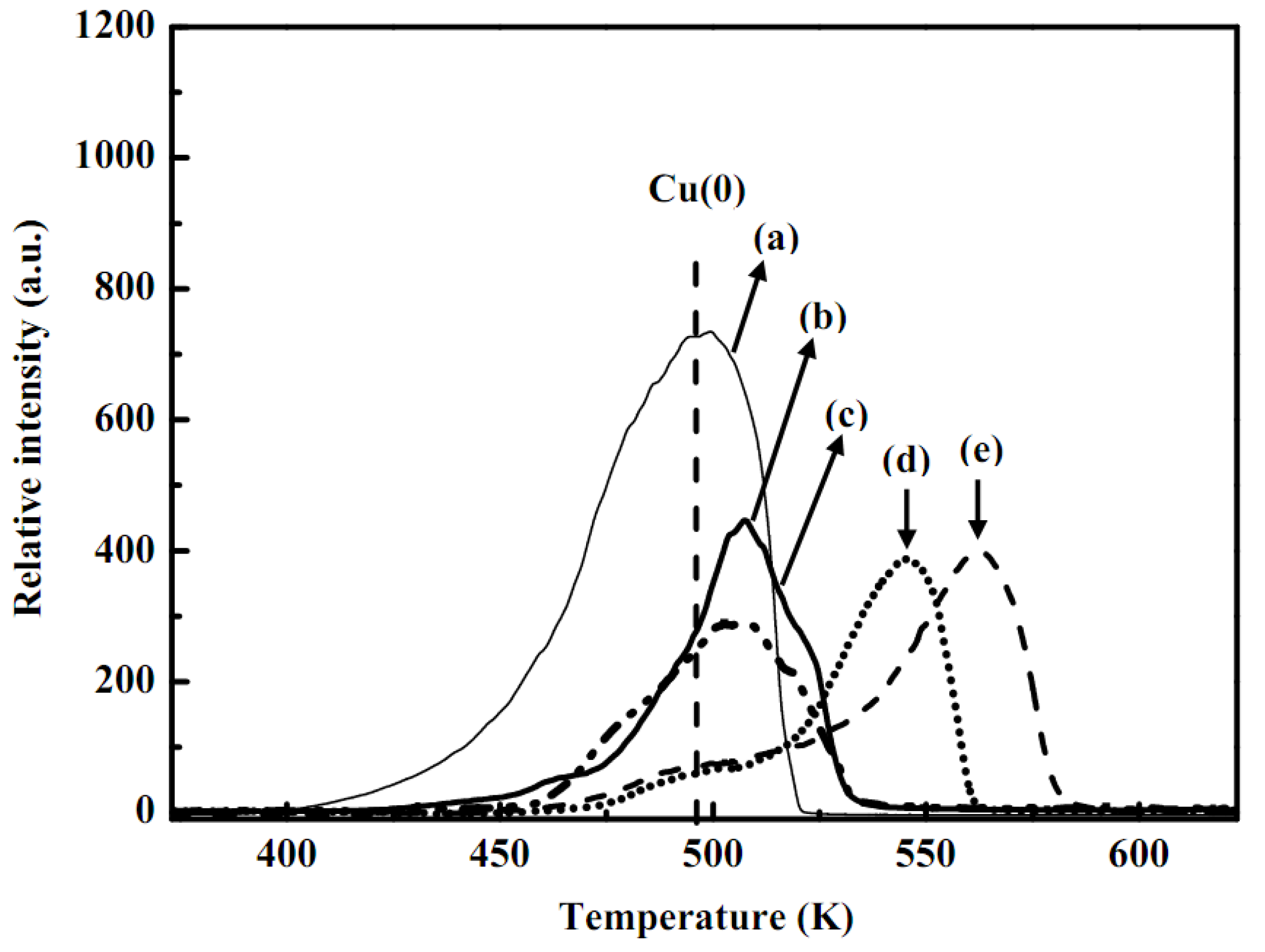
2.3. Temperature program reduction measurement
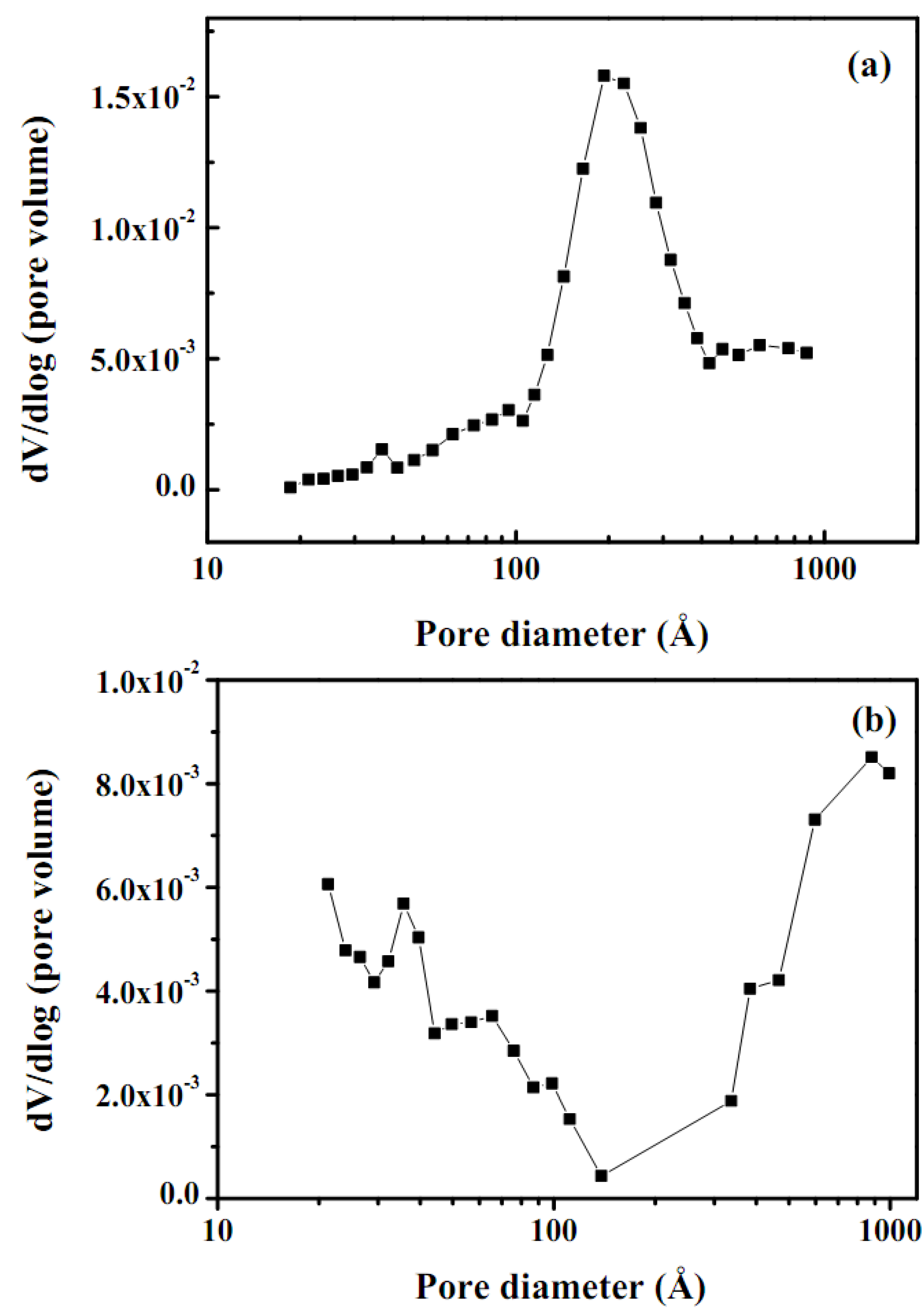
2.4. BET surface area and pore volume distribution
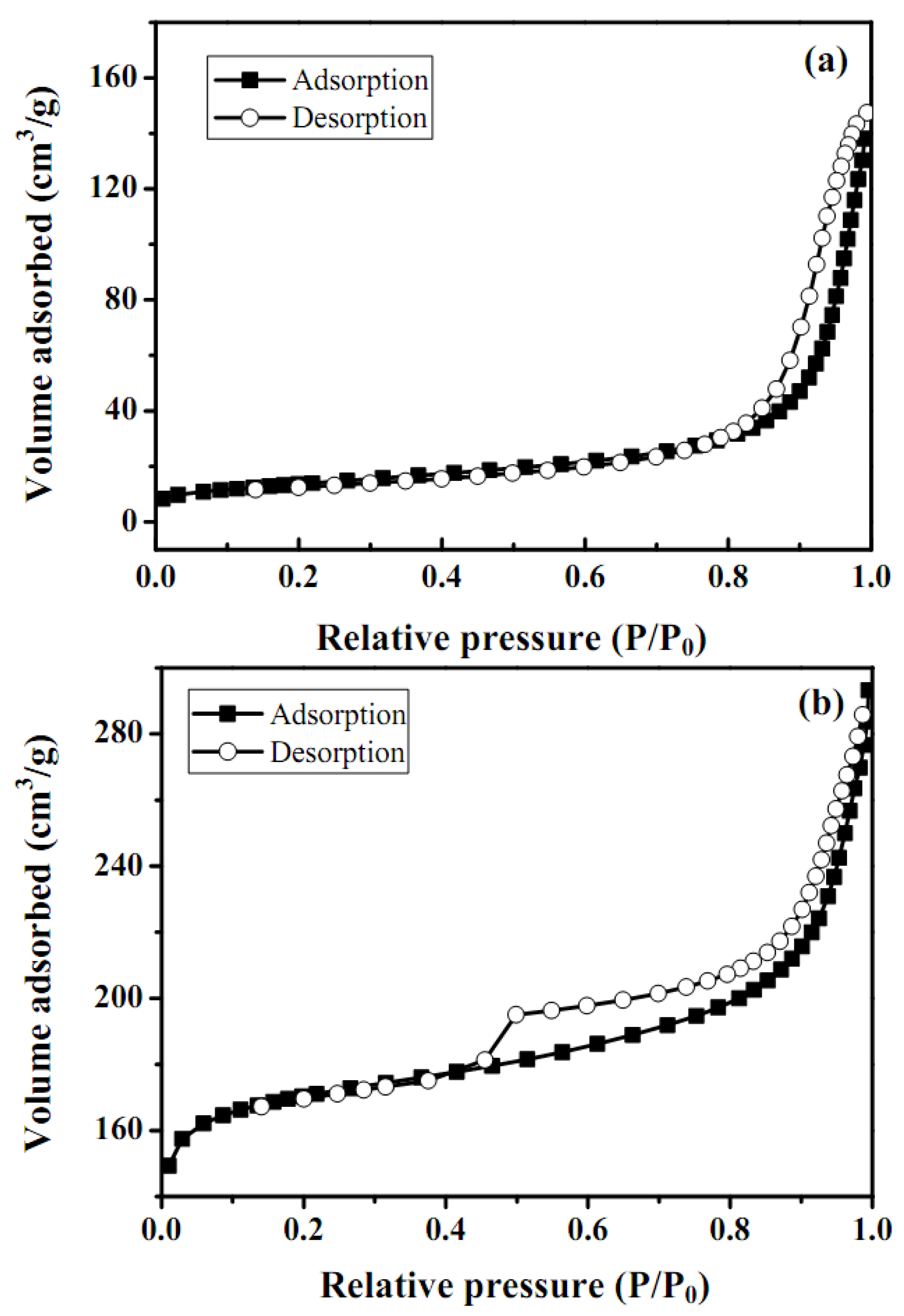
| Catalyst | SBETa± 2 | Vtb ± 0.005 | Pore size distribution | Crystalline size | |
|---|---|---|---|---|---|
| (m2 g−1) | (cm3 g−1) | Vmicroc (%) | Vmesod (%) | Cu(111) (nm) ± 0.2 | |
| Fresh catalyst | 62 | 0.080 | 0.025 (30) | 0.059 (70) | 8.7 |
| Catalyst/ZrO2 sol mixture | 108 | 0.160 | 0.017 (10) | 0.145 (90) | 8.1 |
2.5. XPS analyses and XANES/EXAFS measurement
| Species | Cu | Cu2O | CuO | ||
|---|---|---|---|---|---|
| Cu (2P3/2) | |||||
| Bonding energy (eV) | 932.7 | 932.5 | 933.7 | ||
| Concentration (%) (binding energy (eV)) | |||||
| Fresh CuO/ZnO/Al2O3 | 3.4 (932.7) | 10.0 (932.6) | 86.6 (934.5, 934.7, 935.4) | ||
| Cu/ZnO/Al2O3 + ZrO2 mixture | 42.5 (932.6) | 32.6 (932.5) | 24.9 (934.3, 934.6, 935,4) | ||
| Species | γ–Al2O3 | ZrO2 | ZnO | CuO | |
| O (1S) | |||||
| Concentration (%) (binding energy (eV)) | |||||
| Fresh CuO/ZnO/Al2O3 | 38.1 (531.0) | Not available | 33.7 (530.4) | 28.2 (529.9) | |
| Cu/ZnO/Al2O3 + ZrO2 mixture | 10.1 (530.9) | 2.2 (530.2) | 30.0 (530.4) | 47.7 (529.8) | |
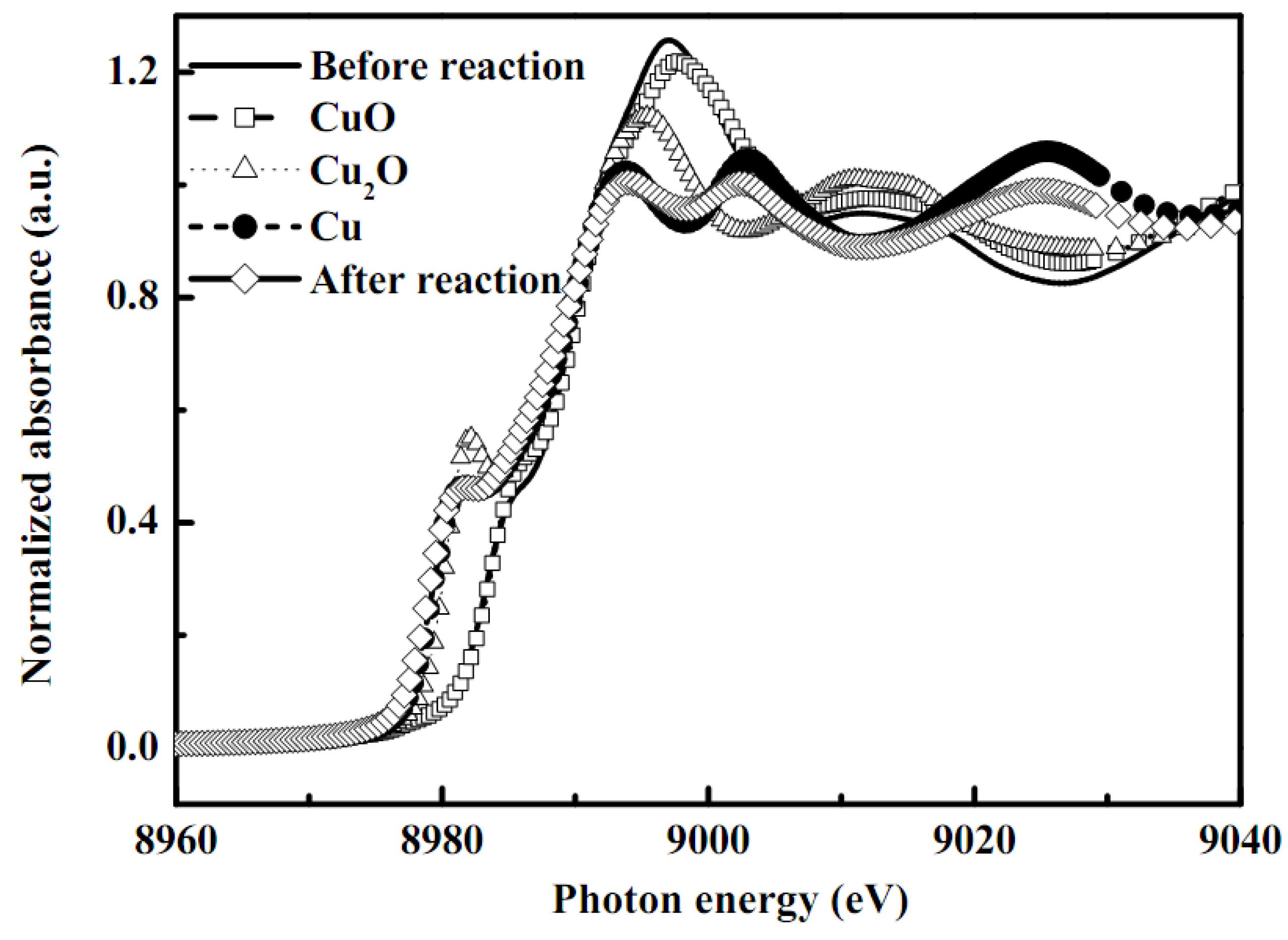
| Sample | Shell | CNa(±0.05) | Rb (±0.02 Å) | Δσ2(Å2 )c |
|---|---|---|---|---|
| Fresh Cu/ZnO/Al2O3 | Cu-O | 3.46 | 1.88 | 0.0043 |
| Cu-Cu | 3.95 | 2.69 | 0.0078 | |
| Used Cu/ZnO/Al2O3 | Cu-O | 2.86 | 1.93 | 0.0026 |
| Cu-Cu | 4.09 | 2.13 | 0.0039 | |
| Elemental Copper (Cu) | Cu-Cu | 11.05 | 2.54 | 0.0086 |
| Curous oxide (Cu2O) | Cu-O | 1.55 | 1.85 | 0.0029 |
| Cupric oxide (CuO) | Cu-O | 2.81 | 1.95 | 0.0043 |
2.6. Performance of the microreactor in a SRM reaction

| Catalyst | S/Ca | WHSVb | SCO (%) | TCc (°C) | Binder | References |
|---|---|---|---|---|---|---|
| Cu40Zn50Al10 | 1.3 | 16.2 | 0.1–1.2 | 543 (98) | ZrO2 | This work |
| Cu50Zn33Al8 | 1.1 | 54.0 | 2.90 | 533 (99) | Al2O3 | Park et al. [15] |
| Cu50Zn33Al8 | 1.5 | 14.8 | 1.10 | 543 (80) | ZrO2 | Lim et al. [21] |
| Cu65Zn28Ce7 | 1.3 | 8.3 | 2.05 | 565 (97) | Al2O3 | Yu et al. [38] |
| Cu48Zn48Ce4 | 1.3 | 8.3 | 1.30 | 565 (98) | Al2O3 | Yu et al. [38] |
| Cu38Zn58Ce4 | 1.3 | 8.3 | 1.60 | 565 (91) | Al2O3 | Yu et al. [38] |
| Cu48Zn48Ce4 | 1.3 | 8.3 | 2.10 | 565 (88) | Al2O3 | Yu et al. [38] |
| Cu30Zn60Al10 | 1.1 | 14.1 | 0.47 | 523 (74) | N.A. | Huang et al. [39] |
| Cu40Zn50Al10 | 1.1 | 14.1 | 0.49 | 523 (85) | N.A. | Huang et al. [39] |
| Cu50Zn40Al10 | 1.1 | 14.1 | 0.50 | 573 (89) | N.A. | Huang et al. [39] |
| Cu60Zn30Al10 | 1.1 | 14.1 | 0.49 | 573 (75) | N.A. | Huang et al. [39] |
3. Experimental
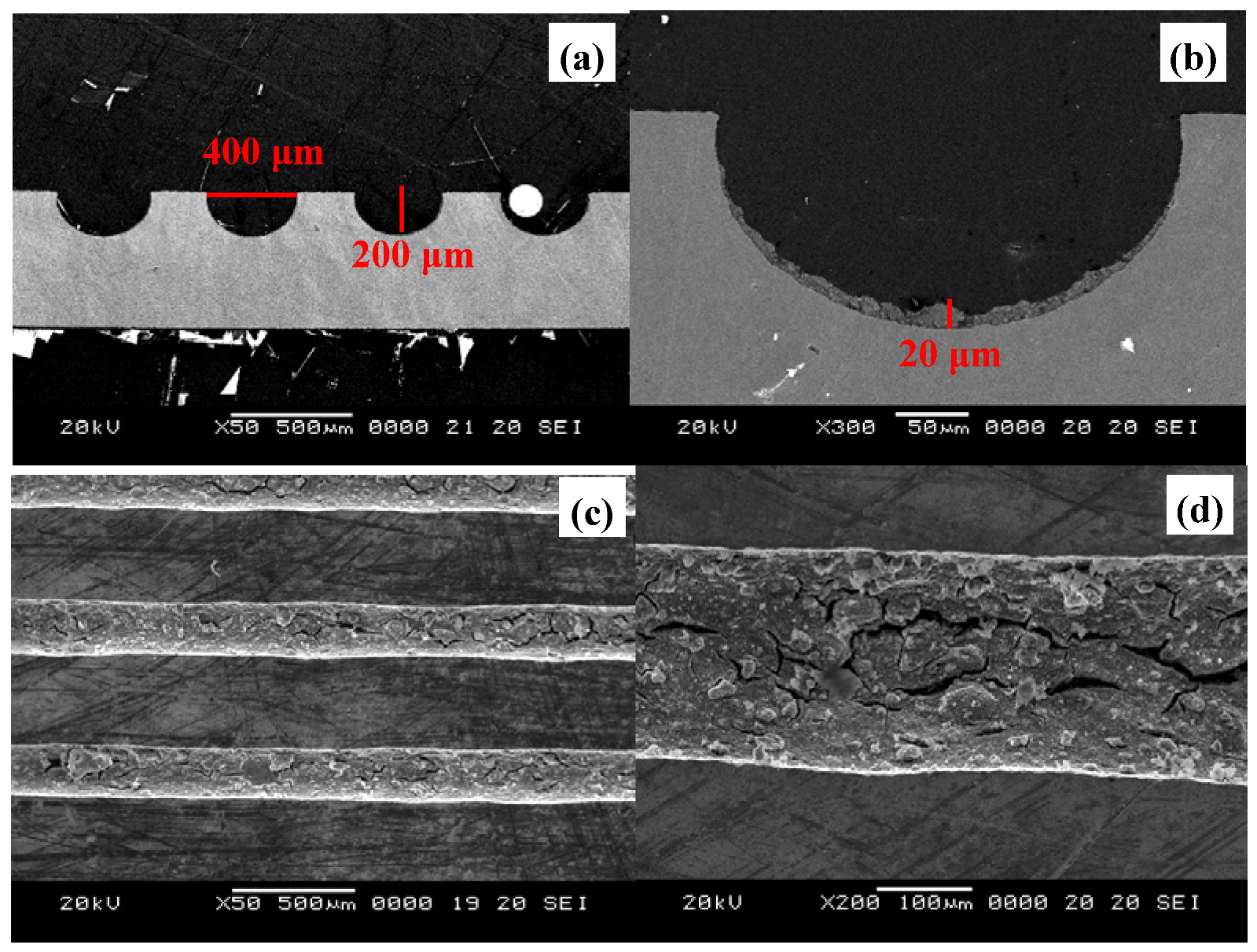
3.1. Microchannel reactor setup
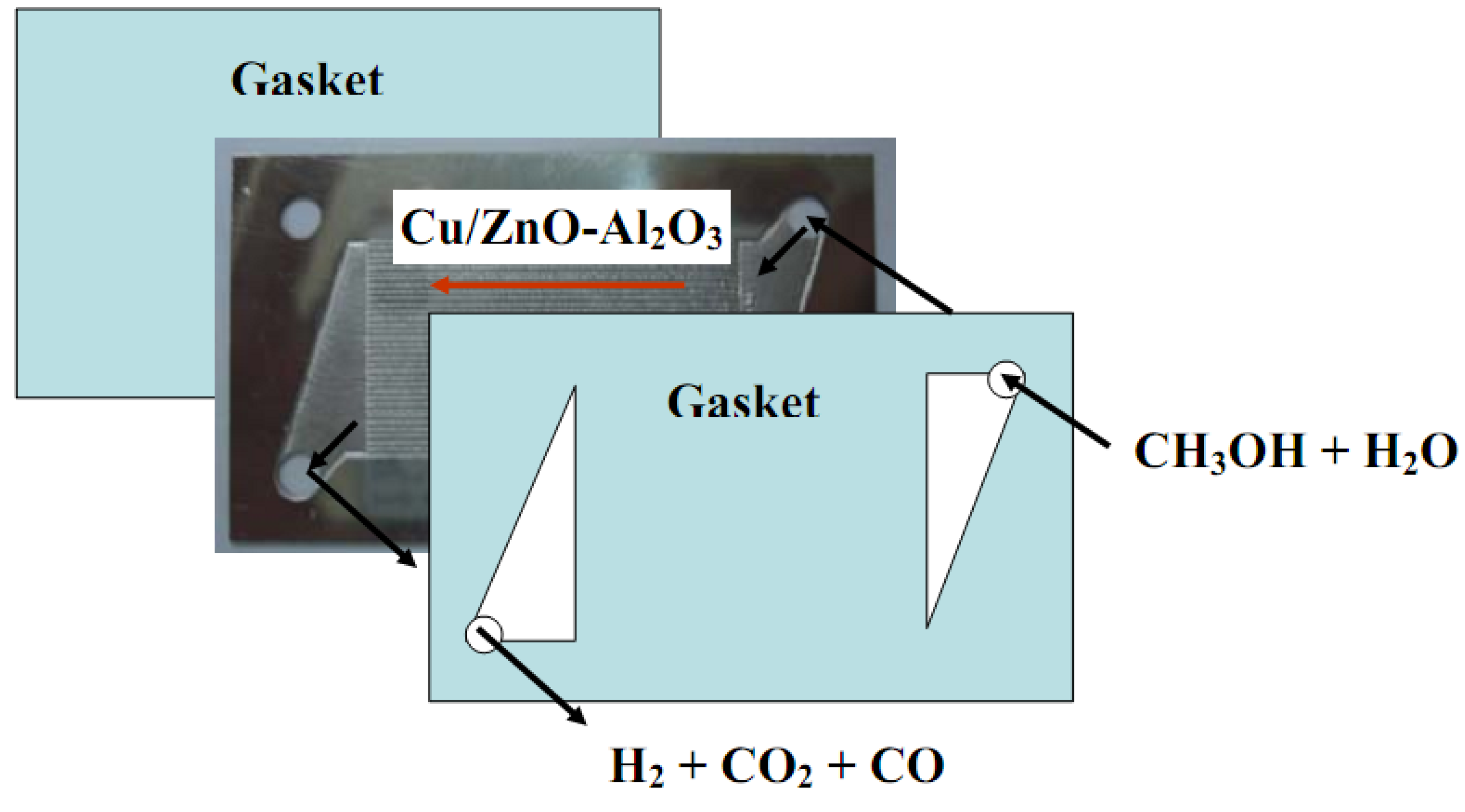
3.2. Test of microreactor performance for methanol conversion
3.3. Catalyst washcoat
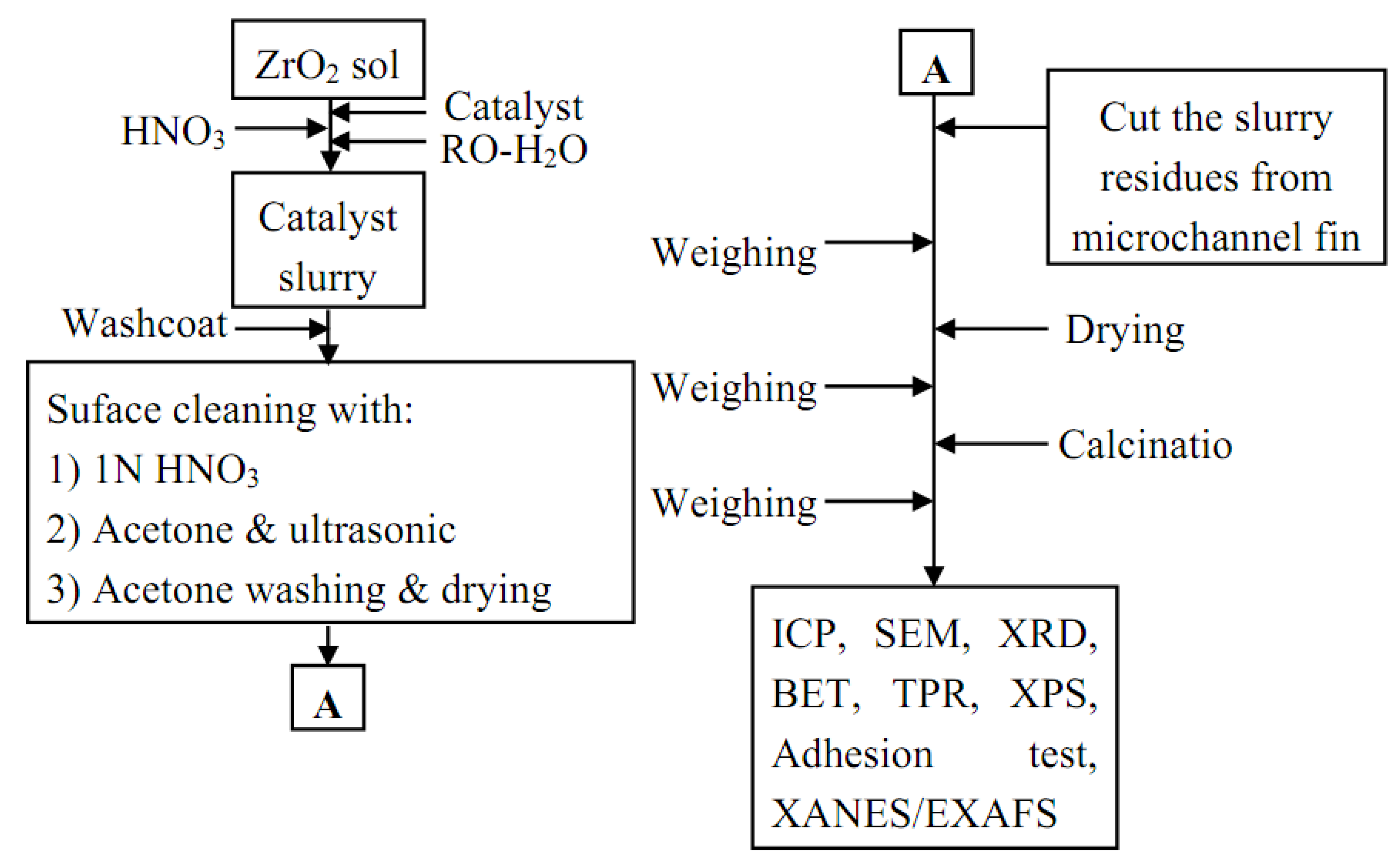
3.4. Catalyst characterization
4. Conclusions
Acknowledgements
References
- Holladay, J.D.; Jones, E.O.; Phelps, M.; Hu, J. Microfuel processor for use in a miniature power supply. J. Power Sourc. 2002, 108, 21–27. [Google Scholar] [CrossRef]
- Horny, C.; Renken, A. Compact string reactor for autothermal hydrogen production. Catal. Today 2007, 120, 45–53. [Google Scholar]
- De Wild, P.J.; Verhaak, M.J.F.M. Catalytic production of hydrogen from methanol. Catal. Today 2000, 60, 3–10. [Google Scholar]
- Kundu, A.; Park, J.M.; Ahn, J.E.; Park, S.S.; Shul, Y.G.; Han, H.S. Micro-channel reactor for steam reforming of methanol. Fuel 2007, 86, 1331–1336. [Google Scholar] [CrossRef]
- Kundu, A.; Park, J.M.; Ahn, J.E.; Park, S.S.; Shul, Y.G.; Han, H.S. Process intensification by micro-channel reactor for steam reforming of methanol. Chem. Eng. J. 2008, 135, 113–119. [Google Scholar] [CrossRef]
- Asprey, S.P.; Wojciechowski, B.W.; Peppley, B.A. Kinetic studies using temperature-scanning: the steam-reforming of methanol. Appl. Catal. A Gen. 1999, 179, 51–70. [Google Scholar] [CrossRef]
- Hong, X.; Ren, S. Selective hydrogen production from methanol oxidative steam reforming over Zn–Cr catalysts with or without Cu loading. Int. J. Hydrogen Energy 2008, 33, 700–708. [Google Scholar] [CrossRef]
- Shah, K.; Besser, R.S. Key issues in the microchemical systems-based methanol fuel processor: Energy density, thermal integration, and heat loss mechanisms. J. Power Sourc. 2007, 166, 177–193. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Tsetsekou, A.; Ekonomakou, A. The effect of particle size on the adhesion properties of oxide washcoats on cordierite honeycombs. J. Mater. Sci. Lett. 1999, 18, 1421–1424. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Tsetsekou, A. The effect of processing parameters on the properties of γ-alumina washcoats deposited on ceramic honeycombs. J. Mater. Sci. Lett. 2000, 35, 951–960. [Google Scholar]
- Agrafiotis, C.; Tsetsekou, A.; Leon, I. Effect of slurry rheological properties on the coating of ceramic honeycombs with yttria-stabilized-zirconia washcoats. J. Am. Ceram. Soc. 2000, 83, 1033–1038. [Google Scholar]
- Choi, Y.; Stenger, H.G. Fuel cell grade hydrogen from methanol on a commercial Cu/ZnO/Al2O3 catalyst. Appl. Catal. B Environ. 2002, 38, 259–269. [Google Scholar] [CrossRef]
- Park, D.E.; Kim, T.; Kwon, S.; Kim, C.K.; Yoon, E. Micromachined methanol steam reforming system as a hydrogen supplier for portable proton exchange membrane fuel cells. Sens. Actuat. A 2007, 135, 58–66. [Google Scholar] [CrossRef]
- Park, G.G.; Yim, S.D.; Yoon, Y.G.; Kim, W.Y.; Seo, D.J.; Eguchi, K. Hydrogen production with integrated microchannel fuel processor using methanol for portable fuel cell systems. Catal. Today 2005, 110, 108–113. [Google Scholar]
- Park, G.G.; Yim, S.D.; Yoon, Y.G.; Lee, W.Y.; Kim, C.S.; Seo, D.J.; Eguchi, K. Hydrogen production with integrated microchannel fuel processor for portable fuel cell systems. J. Power Sourc. 2005, 145, 702–706. [Google Scholar] [CrossRef]
- Kawamura, Y.; Ogura, N.; Yamamoto, T.; Igarashi, A. A miniaturized methanol reformer with Si-based microreactor for a small PEMFC. Chem. Eng. Sci. 2006, 61, 1092–1101. [Google Scholar] [CrossRef]
- Germani, G.; Stefanescu, A.; Schuurman, Y.; Veen, A.C.V. Preparation and characterization of porous alumina-based catalyst coatings in microchannels. Chem. Eng. Sci. 2007, 62, 5084–5091. [Google Scholar] [CrossRef]
- Meille, V. Review on methods to deposit catalysts on structured surfaces. Appl. Catal. A Gen. 2006, 315, 1–17. [Google Scholar] [CrossRef]
- Lee, M.T.; Greif, R.; Grigoropoulos, C.P.; Park, H.G.; Hsu, F.K. Transport in packed-bed and wall-coated steam-methanol reformers. J. Power Sourc. 2007, 166, 194–201. [Google Scholar] [CrossRef]
- Qi, A.; Peppley, B.; Karan, K. Integrated fuel processors for fuel cell application: A review. Fuel Process. Technol. 2007, 88, 3–22. [Google Scholar] [CrossRef]
- Lim, M.S.; Kim, M.R.; Noh, J.; Woo, S.I. A plate-type reactor coated with zirconia-sol and catalyst mixture for methanol steam-reforming. J. Power Sourc. 2005, 140, 66–71. [Google Scholar] [CrossRef]
- Pfeifer, P.; Schubert, K.; Emig, G. Preparation of copper catalyst washcoats for methanol steam reforming in microchannels based on nanoparticles. Appl. Catal. A Gen. 2005, 286, 175–185. [Google Scholar] [CrossRef]
- Stutz, M.J.; Poulikakos, D. Optimum washcoat thickness of a monolith reactor for syngas production by partial oxidation of methane. Chem. Eng. Sci. 2008, 63, 1761–1770. [Google Scholar] [CrossRef]
- Yu, X.; Tu, S.T.; Wang, Z.; Qi, Y. On-board production of hydrogen for fuel cells over Cu/ZnO/Al2O3 catalyst coating in a micro-channel reactor. J. Power Sourc. 2005, 150, 57–66. [Google Scholar] [CrossRef]
- Agrell, J.; Boutonnet, M.; Cabrera, I.M.; Fierro, J.L.G. Production of hydrogen from methanol over binary Cu/ZnO catalysts: Part I. Catalyst preparation and characterization. Appl. Catal. A Gen. 2003, 253, 201–211. [Google Scholar] [CrossRef]
- Bravo, J.; Karim, A.; Conant, T.; Lopez, G.P.; Datye, A. Wall coating of a CuO/ZnO/Al2O3 methanol steam reforming catalyst for micro-channel reformers. Chem. Eng. J. 2004, 101, 113–121. [Google Scholar] [CrossRef]
- Patel, S.; Pant, K.K. Activity and stability enhancement of copper–alumina catalysts using cerium and zinc promoters for the selective production of hydrogen via steam reforming of methanol. J. Power Sourc. 2006, 159, 139–143. [Google Scholar] [CrossRef]
- Zhou, W.; Tang, Y.; Pan, M.; Wei, X.; Chen, H.; Xiang, J. A performance study of methanol steam reforming microreactor with porous copper sintered felt as catalyst support for fuel cell. Int. J. Hydrogen Energy 2009, 34, 9745–9753. [Google Scholar] [CrossRef]
- Lamberti, C.; Bordiga, S.; Bonino, F.; Prestipino, C.; Berlier, G.; Capello, L.; D'Acapito, F.; Xamena, F.X.L.I.; Zecchina, A. Determination of the oxidation and coordination state of copper on different Cu-based catalysts by XANES spectroscopy in situ or in operando conditions. Phys. Chem. Chem. Phys. 2003, 5, 4502–4509. [Google Scholar]
- Kim, T.; Lee, D.H.; Park, D.E.; Kwon, S. Micromachined Methanol Reformer for Portable PEM Fuel Cells. J. Fuel Cell Sci. Technol. 2008, 5. art. no. 011008. [Google Scholar]
- Haa, J.W.; Jang, J.H.; Gil, J.H.; Kim, S.H. The fabrication and performance of a poly(dimethylsiloxane) (PDMS)-based microreformer for application to electronics. Int. J. Hydrogen Energy 2008, 33, 2059–2063. [Google Scholar] [CrossRef]
- Men, Y.; Kolb, G.; Zapf, R.; Tiemann, D.; Wichert, M.; Hessel, V.; Löwe, H. A complete miniaturized microstructured methanol fuel processor/fuel cell system for low power applications. Int. J. Hydrogen Energy 2008, 33, 1374–1382. [Google Scholar] [CrossRef]
- Seo, D.J.; Yoon, W.L.; Yoon, Y.G.; Park, S.H.; Park, G.G.; Kim, C.S. Development of a micro fuel processor for PEMFCs. Electrochim. Acta 2004, 50, 719–723. [Google Scholar] [CrossRef]
- Stefanidis, G.D.; Vlachos, D.G. High vs. low temperature reforming for hydrogen production via microtechnology. Chem. Eng. Sci. 2010, 64, 4856–4865. [Google Scholar]
- Fazeli, A.; Behnam, M. Hydrogen production in a zigzag and straight catalytic wall coated micro channel reactor by CFD modeling. Int. J. Hydrogen Energy 2010, 35, 9496–9503. [Google Scholar] [CrossRef]
- Moreno, A.M.; Wilhite, B.A. Autothermal hydrogen generation from methanol in a ceramic microchannel network. J. Power Sourc. 2010, 195, 1964–1970. [Google Scholar] [CrossRef]
- Chen, F.; Chang, M.H.; Kuo, C.Y.; Hsueh, C.Y.; Yan, W.M. Analysis of a plate-type microreformer for methanol steam reforming reaction. Energy Fuels 2010, 23, 5092–5098. [Google Scholar]
- Yu, X.; Tu, S.T.; Wang, Z.; Qi, Y. Development of a microchannel reactor concerning steam reforming of methanol. Chem. Eng. J. 2006, 116, 123–132. [Google Scholar] [CrossRef]
- Huang, G.; Liaw, B.J.; Jhang, C.J.; Chen, Y.Z. Steam reforming of methanol over CuO/ZnO/CeO2/ZrO2/Al2O3 catalysts. Appl. Catal. A 2009, 358, 7–12. [Google Scholar] [CrossRef]
- Santo, K.H.; Fichtner, M.; Schubert, K. Preparation of microstructure compatible porous supports by sol–gel synthesis for catalyst coatings. Appl. Catal. A Gen. 2001, 220, 79–92. [Google Scholar] [CrossRef]
- Battistoni, C.; Cantelli, V.; Debenedetti, M.; Kačiulis, S.; Mattogno, G.; Napoli, A. XPS study of ceramic three-way catalysts. Appl. Surface Sci. 1999, 144/145, 390–394. [Google Scholar]
- Suh, J.S.; Lee, M.T.; Greif, R.; Grigoropoulos, C.P. Transport phenomena in a steam-methanol reforming microreactor with internal heating. Int. J. Hydrogen Energy 2009, 34, 314–322. [Google Scholar]
- Yasaki, Y.Y.S.; Ihara, K.; Ohkubo, K. Method of manufacturing an exhaust gas purifying catalyst. U.S. Patent 5,208,206, 4 May 1993. [Google Scholar]
- Valentini, M.; Groppi, G.; Cristiani, C.; Levi, M.; Tronconi, E.; Forzatti, P. The deposition of γ-Al2O3 layers on ceramic and metallic supports for the preparation of structured catalysts. Catal. Today 2001, 69, 307–314. [Google Scholar]
- Lytle, F.W. The EXAFS family tree: A personal history of the development of extended X-ray absorption fine structure. J. Synchrotron Rad. 1999, 6, 123–134. [Google Scholar] [CrossRef]
- Nesvizhskii, A.I.; Rehr, J.J. L-edge XANES of 3d-transition metals. J. Synchrotron Rad. 1999, 6, 315–316. [Google Scholar] [CrossRef]
- Resslert, T. WinXAS: A program for X-ray absorption spectroscopy data analysis under MS-windows. J. Synchrotron Rad. 1998, 5, 118–122. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lin, K.-S.; Pan, C.-Y.; Chowdhury, S.; Tu, M.-T.; Hong, W.-T.; Yeh, C.-T. Hydrogen Generation Using a CuO/ZnO-ZrO2 Nanocatalyst for Autothermal Reforming of Methanol in a Microchannel Reactor. Molecules 2011, 16, 348-366. https://doi.org/10.3390/molecules16010348
Lin K-S, Pan C-Y, Chowdhury S, Tu M-T, Hong W-T, Yeh C-T. Hydrogen Generation Using a CuO/ZnO-ZrO2 Nanocatalyst for Autothermal Reforming of Methanol in a Microchannel Reactor. Molecules. 2011; 16(1):348-366. https://doi.org/10.3390/molecules16010348
Chicago/Turabian StyleLin, Kuen-Song, Cheng-Yu Pan, Sujan Chowdhury, Mu-Ting Tu, Wan-Ting Hong, and Chuin-Tih Yeh. 2011. "Hydrogen Generation Using a CuO/ZnO-ZrO2 Nanocatalyst for Autothermal Reforming of Methanol in a Microchannel Reactor" Molecules 16, no. 1: 348-366. https://doi.org/10.3390/molecules16010348
APA StyleLin, K.-S., Pan, C.-Y., Chowdhury, S., Tu, M.-T., Hong, W.-T., & Yeh, C.-T. (2011). Hydrogen Generation Using a CuO/ZnO-ZrO2 Nanocatalyst for Autothermal Reforming of Methanol in a Microchannel Reactor. Molecules, 16(1), 348-366. https://doi.org/10.3390/molecules16010348




