2-(Penta-1,3-diynyl)-5-(3,4-dihydroxybut-1-ynyl)thiophene, a Novel NQO1 Inducing Agent from Echinops grijsii Hance
Abstract
:1. Introduction
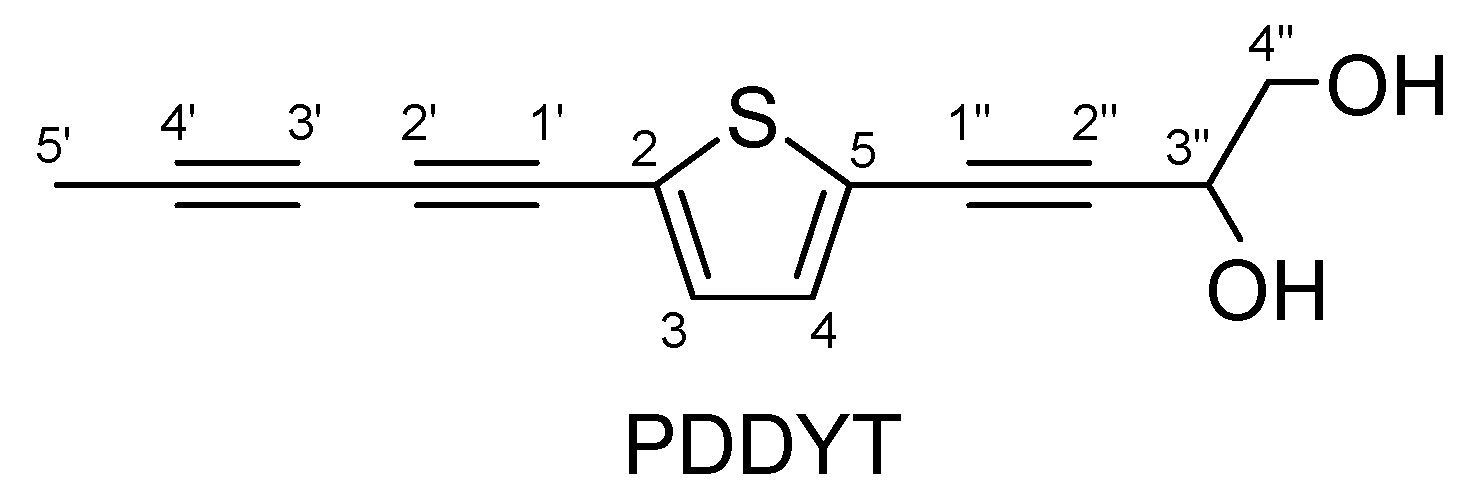
2. Results and Discussion
2.1. PDDYT is a Potent NQO1 Inducing Agent

2.2. The Activation of Keap1-Nrf2 Pathway of PDDYT is via Activating Akt and Depleting Cellular GSH



3. Conclusions
4. Experimental
4.1. Materials and Reagents
4.2. Isolation of PDDYT from E. grijisii
4.3. Cell Culture
4.4. Crystal Violet Assay for Determining Cell Viability
4.5. NQO1 Induction Assay
4.6. Western Blot Analysis
4.7. Glutathione (GSH) Assay
4.8. Detection of Intracellular ROS
4.9. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS) Analysis of GSH Conjugates
Supplementary Materials
Supplementary File 1Acknowledgements
References and Notes
- Tanigawa, S.; Fujii, M.; Hou, D. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic. Biol. Med. 2007, 42, 1690–1703. [Google Scholar] [CrossRef]
- Zhang, W.; Go, M. Functionalized 3-benzylidene-indolin-2-ones: Inducers of NAD(P)H-quinone oxidoreductase 1 (NQO1) with antiproliferative activity. Bioorg. Med. Chem. 2009, 17, 2077–2090. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.I.; Watai, Y.; Tong, K.I.; Shibata, T.; Uchida, K.; Yamamoto, M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol. Cell Biol. 2006, 26, 221–229. [Google Scholar]
- Eggler, A.L.; Liu, G.; Pezzuto, J.M.; van Breemen, R.B.; Mesecar, A.D. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc. Natl. Acad. Sci. USA 2005, 102, 10070–10075. [Google Scholar]
- Hur, W.; Sun, Z.; Jiang, T.; Mason, D.E.; Peters, E.C.; Zhang, D.D.; Luesch, H.; Schultz, P.G.; Gray, N.S. A small-molecule inducer of the antioxidant response element. Chem. Biol. 2010, 17, 537–547. [Google Scholar] [CrossRef]
- Kensler, T.W.; Wakabayashi, N. Nrf2: Friend or foe for chemoprevention? Carcinogenesis 2010, 31, 90–99. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, X.; Zhang, M.; Zhang, P.; Song, Z.; Ma, Z.; Cheng, Y.; Qu, H. Characterization of chemopreventive agents from the dichloromethane extract of Eurycorymbus cavaleriei by liquid chromatography–ion trap mass spectrometry. J. Chromatogr. A 2009, 1216, 4859–4867. [Google Scholar] [CrossRef]
- Holland, R.; Fishbein, J.C. Chemistry of the cysteine sensors in Keap1. Antioxid. Redox Signal. 2010. [Google Scholar] [CrossRef]
- Jin, W.; Shi, Q.; Hong, C.; Cheng, Y.; Ma, Z.; Qu, H. Cytotoxic properties of thiophenes from Echinops grijissi Hance. Phytomedicine 2008, 15, 768–774. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Z. Characterization of bioactive thiophenes from the dichloromethane extract of Echinops grijisii as Michael addition acceptors. Anal. Bioanal. Chem. 2010, 397, 1975–1984. [Google Scholar] [CrossRef]
- Fokialakis, N.; Cantrell, C.L.; Duke, S.O.; Skaltsounis, A.L.; Wedge, D.E. Antifungal activity of thiophenes from Echinops ritro. J. Agric. Food Chem. 2006, 54, 1651–1655. [Google Scholar] [CrossRef]
- Sogawa, K.; Fujii-Kuriyama, Y.Y. Ah receptor, a novel ligand-activated transcription factor. J. Biochem. 1997, 122, 1075–1079. [Google Scholar] [CrossRef]
- Lima, J.H.; Kima, K.M.; Kimb, S.W.; Hwangb, Q.; Choi, H.J. Bromocriptine activates NQO1 via Nrf2-PI3K/Akt signaling: Novel cytoprotective mechanism against oxidative damage. Pharmacol. Res. 2008, 57, 325–331. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Kim, H.G.; Han, E.H.; Jeong, H.G. Metallothionein-III protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a PI3K and ERK/Nrf2-dependent manner. Toxicol. Appl. Pharm. 2008, 231, 318–327. [Google Scholar] [CrossRef]
- Jugea, N.; Mithena, R.F.; Traka, M. Molecular basis for chemoprevention by sulforaphane: A comprehensive review. Cell Mol. Life Sci. 2007, 64, 1105–1127. [Google Scholar] [CrossRef]
- Zhang, Y. Role of glutathione in the accumulation of anticarcinogenic isothiocyanates and their glutathione conjugates by murine hepatoma cells. Carcinogenesis 2000, 21, 1175–1182. [Google Scholar] [CrossRef]
- Sample Availability: Samples of PDDYT are available from the authors.
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shi, J.; Zhang, X.; Jiang, H. 2-(Penta-1,3-diynyl)-5-(3,4-dihydroxybut-1-ynyl)thiophene, a Novel NQO1 Inducing Agent from Echinops grijsii Hance. Molecules 2010, 15, 5273-5281. https://doi.org/10.3390/molecules15085273
Shi J, Zhang X, Jiang H. 2-(Penta-1,3-diynyl)-5-(3,4-dihydroxybut-1-ynyl)thiophene, a Novel NQO1 Inducing Agent from Echinops grijsii Hance. Molecules. 2010; 15(8):5273-5281. https://doi.org/10.3390/molecules15085273
Chicago/Turabian StyleShi, Jing, Xiaoyu Zhang, and Hai Jiang. 2010. "2-(Penta-1,3-diynyl)-5-(3,4-dihydroxybut-1-ynyl)thiophene, a Novel NQO1 Inducing Agent from Echinops grijsii Hance" Molecules 15, no. 8: 5273-5281. https://doi.org/10.3390/molecules15085273
APA StyleShi, J., Zhang, X., & Jiang, H. (2010). 2-(Penta-1,3-diynyl)-5-(3,4-dihydroxybut-1-ynyl)thiophene, a Novel NQO1 Inducing Agent from Echinops grijsii Hance. Molecules, 15(8), 5273-5281. https://doi.org/10.3390/molecules15085273



