New Steroidal Glycosides Isolated as CD40L Inhibitors of Activated Platelets
Abstract
:1. Introduction
2. Results and Discussion
| No. | 1 | 2 | 3 | No. | 1 | 2 | 3 |
|---|---|---|---|---|---|---|---|
| 1 | 0.78,1.51 (o) | 0.82, 1.51 (o) | 1.51,1.80 (o) | Inner Gal-1 | 4.85(d, J = 7.8 Hz) | 4.84(d, J = 7.8 Hz) | 4.84(d, J = 7.6 Hz) |
| 2 | 1.24,1.99 (o) | 1.25, 1.30 (o) | 1.07,1.64 (o) | 2 | 4.42(o) | 4.43(o) | 4.63(o) |
| 3 | 4.02(o) | 3.80 (o) | 4.28 (o) | 3 | 4.19(o) | 4.20(o) | 4.20 (o) |
| 4 | 1.31,1.77 (o) | 1.35, 1.75 (o) | 1.51,1.80, (o) | 4 | 4.58(m) | 4.56(m) | 4.54 (o) |
| 5 | 0.84(o) | 0.92 (o) | 2.19 (o) | 5 | 4.16(o) | 4.14(o) | 3.96 (o) |
| 6 | 1.08,1.11 (o) | 1.10, 1.60 (o) | 1.19,1.87 (o) | 6 | 4.21,4.68(o) | 4.67,4.21(o) | 4.39, 4.43(o) |
| 7 | 0.47,1.55 (o) | 1.57, 1.99 (o) | 1.31,1.89 (o) | Gal-Glc-1 | 5.56(d, J = 7.8 Hz) | 5.56(d, J = 7.8 Hz) | 5.25(d, J = 7.6 Hz) |
| 8 | 1.43(o) | 1.57 (o) | 1.66 (o) | 2 | 4.38(o) | 4.38(o) | 4.06 (o) |
| 9 | 0.65(o) | 1.33 (o) | 2.11 (o) | 3 | 4.19(o) | 4.17(o) | 4.16 (o) |
| 10 | ---- | ------ | ---- | 4 | 3.84(o) | 3.86(o) | 4.29 (o) |
| 11 | 1.49,1.82 (o) | 1.63, 1.78 (o) | 1.63,1.72 (o) | 5 | 4.13(o) | 4.13(o) | 3.93 (o) |
| 12 | 3.52(m) | 3.98 (o) | 4.21 (o) | 6 | 4.51(o) | 4.50(o) | 4.38, 4.43 (o) |
| 13 | ---- | ------ | ---- | 3-Glc-1 | 5.28(d, J = 7.8 Hz) | 5.29(d, J = 7.8 Hz) | |
| 14 | 1.13(o) | 2.06 (o) | 2.12 (o) | 2 | 4.02(o) | 4.00(o) | |
| 15 | 1.52,2.08 (o) | 0.89 (o) | 1.53,2.13 (o) | 3 | 3.84(o) | 3.85(o) | |
| 16 | 5.02(o) | 5.02 (o) | 5.04 (m) | 4 | 4.23(o) | 4.23(o) | |
| 17 | 2.31(t, J = 8.2 Hz) | 3.21 (dd, J = 7.2, 8.3 Hz) | 3.22 (dd, J = 6.8, 8.7 Hz) | 5 | 3.85(o) | 3.83(o) | |
| 18 | 1.34 (s) | 0.99 (s) | 0.99 (s) | 6 | 4.18,4.27(o) | 4.18,4.27(o) | |
| 19 | 0.66(s) | 0.68 (s) | 1.02 (s) | 2-Glc-1 | 4.77(d, J = 7.8 Hz) | 4.76(d, J = 7.8 Hz) | |
| 20 | 2.48(m) | 2.28 (o) | 2.29 (m) | 2 | 4.08(o) | 4.09(o) | |
| 21 | 1.60(d, J = 6.8 Hz) | 1.38(d, J = 6.8 Hz) | 1.36(d, J = 6.8 Hz) | 3 | 4.21(o) | 4.21(o) | |
| 22 | ---- | ------ | ---- | 4 | 4.17(o) | 4.18(o) | |
| 23 | 2.08(o) | 2.10 (o) | 2.04, 2.06 (o) | 5 | 3.92(o) | 3.92(o) | |
| 24 | 1.69,2.08 (o) | 1.70, 2.08 (o) | 1.71, 2.06 (o) | 6 | 4.20,4.00(o) | 4.21,4.00(o) | |
| 25 | 1.91(o) | 1.93 (o) | 1.94 (o) | C26 Glc-1 | 5.13(d, J = 7.8 Hz) | 5.12(d, J = 7.8 Hz) | 4.80(d, J = 7.6 Hz) |
| 26 | 3.61(m) 3.90(o) | 3.62 (o) | 3.63 (o) | 2 | 4.00(o) | 4.01(o) | 4.22 (o) |
| 3.99 (m) | 4.00 (m) | ||||||
| 27 | 0.97(d, J = 6.8 Hz) | 0.99(d, J = 6.8 Hz) | 0.99(d, J = 6.8 Hz) | 3 | 4.03(o) | 4.04(o) | 3.83 (o) |
| 4 | 4.23(o) | 4.23(o) | 3.99 (o) | ||||
| 5 | 4.17(o) | 4.16(o) | 4.22 (o) | ||||
| 6 | 4.20,4.49(o) | 4.20,4.47 (o) | 4.37,4.52 (o) |
| No. | 1 | 2 | 3 | No. | 1 | 2 | 3 |
|---|---|---|---|---|---|---|---|
| 1 | 37.1 | 37.0 | 30.8 | Inner Gal-1 | 102.4 | 102.3 | 102.3 |
| 2 | 29.8 | 29.9 | 26.8 | 2 | 73.1 | 73.1 | 81.7 |
| 3 | 75.1 | 77.5 | 75.1 | 3 | 75.5 | 75.5 | 75.1 |
| 4 | 34.7 | 34.8 | 30.9 | 4 | 80.1 | 80.2 | 69.8 |
| 5 | 44.7 | 44.8 | 37.0 | 5 | 76.1 | 76.1 | 76.5 |
| 6 | 28.9 | 29.0 | 27.1 | 6 | 60.5 | 60.5 | 62.1 |
| 7 | 32.2 | 29.8 | 26.7 | Gal-Glc-1 | 104.8 | 104.9 | 106.0 |
| 8 | 34.3 | 35.6 | 35.9 | 2 | 81.4 | 81.4 | 76.8 |
| 9 | 53.5 | 47.8 | 33.7 | 3 | 88.4 | 88.4 | 78.0 |
| 10 | 35.8 | 35.5 | 34.9 | 4 | 70.8 | 70.8 | 71.7 |
| 11 | 31.6 | 29.5 | 29.6 | 5 | 77.8 | 77.8 | 78.4 |
| 12 | 79.3 | 71.5 | 71.7 | 6 | 62.3 | 62.3 | 62.8 |
| 13 | 46.8 | 45.6 | 45.8 | 3-Glc-1 | 104.5 | 104.5 | |
| 14 | 55.0 | 48.3 | 48.6 | 2 | 75.1 | 75.1 | |
| 15 | 32.1 | 32.5 | 32.5 | 3 | 78.6 | 78.6 | |
| 16 | 81.1 | 80.9 | 81.0 | 4 | 70.9 | 70.9 | |
| 17 | 63.7 | 54.5 | 54.6 | 5 | 77.5 | 77.4 | |
| 18 | 11.3 | 17.4 | 17.5 | 6 | 62.7 | 62.3 | |
| 19 | 12.2 | 12.2 | 23.9 | 2-Glc-1 | 104.8 | 104.8 | |
| 20 | 41.6 | 40.9 | 40.9 | 2 | 75.1 | 75.2 | |
| 21 | 15.6 | 16.2 | 16.2 | 3 | 78.6 | 78.6 | |
| 22 | 110.8 | 110.6 | 110.7 | 4 | 71.7 | 71.7 | |
| 23 | 37.3 | 37.2 | 37.2 | 5 | 78.4 | 78.6 | |
| 24 | 28.4 | 28.4 | 28.4 | 6 | 62.8 | 62.8 | |
| 25 | 34.2 | 34.2 | 34.3 | C26 Glc-1 | 105.0 | 105.0 | 104.9 |
| 26 | 75.2 | 75.3 | 75.3 | 2 | 75.2 | 75.3 | 75.1 |
| 27 | 17.4 | 17.4 | 17.4 | 3 | 78.6 | 78.6 | 78.3 |
| 4 | 71.5 | 71.5 | 71.6 | ||||
| 5 | 78.5 | 78.4 | 78.5 | ||||
| 6 | 63.0 | 63.0 | 62.8 |
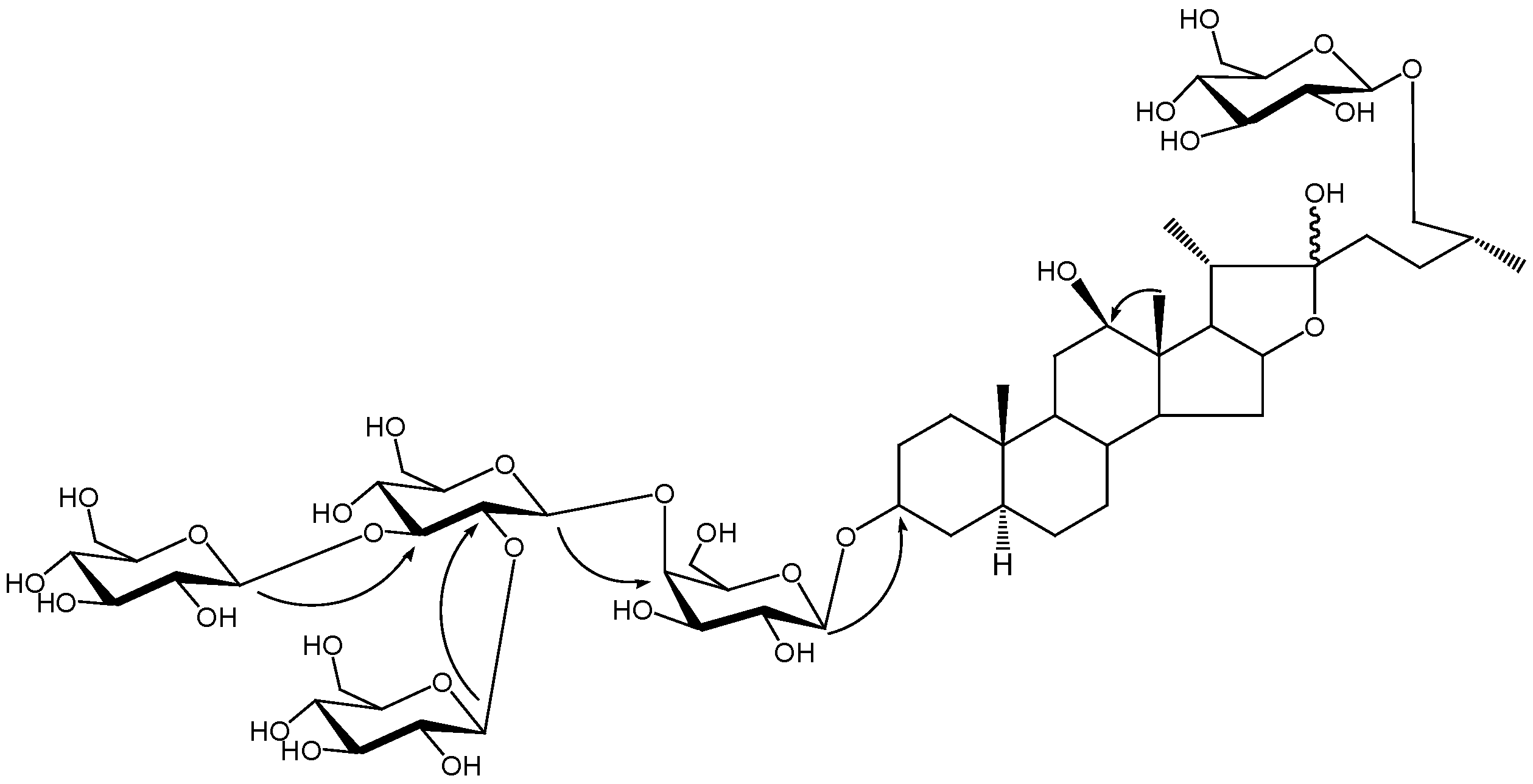
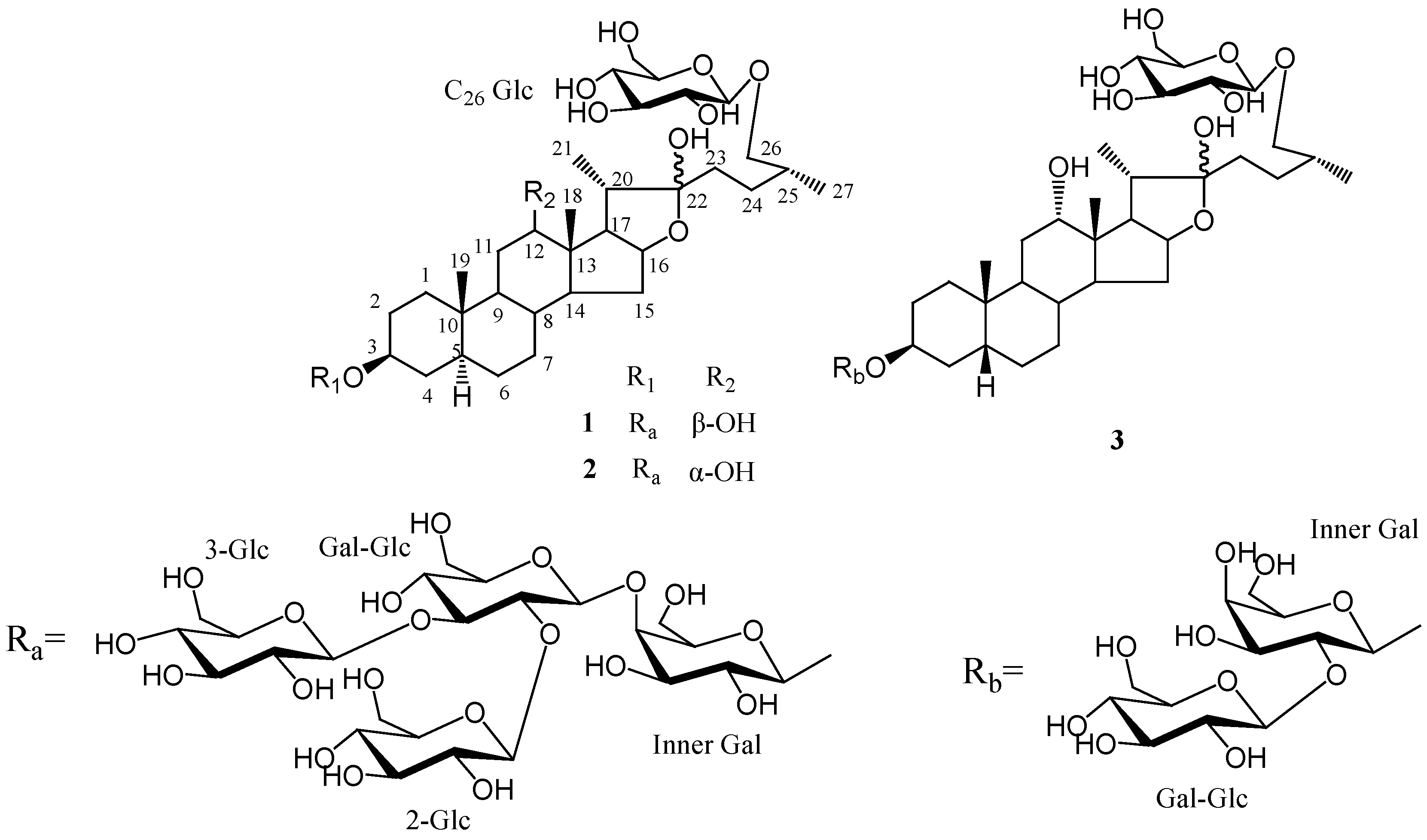
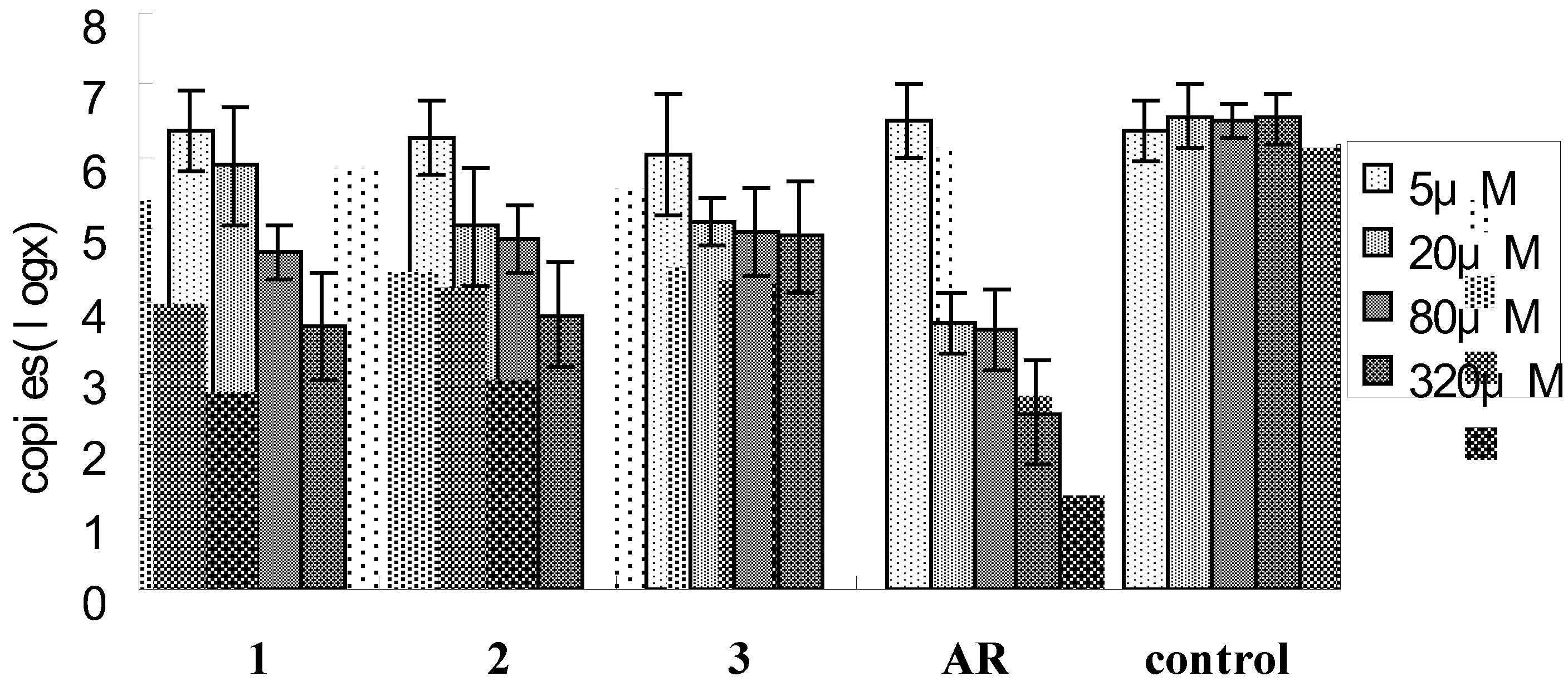
3. Experimental
3.1. General
3.2. Plant material
3.3. Extraction and isolation
 -26.4° (H2O,c 0.11). HR-ESIMS (positive mode) at m/z 1283.5862 [M+ Na]+ (calcd. 1283.5884). ESI-MS (positive mode) at m/z 1283 [M+ Na]+, 1265 [M+ Na- H2O]+, 1121 [M+ Na- 162]+, 1103 [M+ Na- H2O- 162]+, 941 [M+ Na- H2O- 162× 2]+, 779 [M+ Na- H2O- 162× 3]+; ESI-MS (negative mode) at m/z 1259 [M- H]-, 1097 [M- H- 162]-, 935 [M- H- 162× 2]-, 773 [M- H- 162× 3]-, 611 [M- H- 162× 4]-; IR νmax (KBr) cm-1: 3419 (OH), 2937 (CH), 1000-1100; 1H-NMR (C5D5N) data see Table 1; 13C-NMR, see Table 2.
-26.4° (H2O,c 0.11). HR-ESIMS (positive mode) at m/z 1283.5862 [M+ Na]+ (calcd. 1283.5884). ESI-MS (positive mode) at m/z 1283 [M+ Na]+, 1265 [M+ Na- H2O]+, 1121 [M+ Na- 162]+, 1103 [M+ Na- H2O- 162]+, 941 [M+ Na- H2O- 162× 2]+, 779 [M+ Na- H2O- 162× 3]+; ESI-MS (negative mode) at m/z 1259 [M- H]-, 1097 [M- H- 162]-, 935 [M- H- 162× 2]-, 773 [M- H- 162× 3]-, 611 [M- H- 162× 4]-; IR νmax (KBr) cm-1: 3419 (OH), 2937 (CH), 1000-1100; 1H-NMR (C5D5N) data see Table 1; 13C-NMR, see Table 2. -3.5° (H2O, c 0.16). HR-ESIMS (positive mode) at m/z 937.5075 [M+ H]+ (calcd. 937.5008). ESI-MS (positive mode) at m/z 959 [M+ Na]+, 941 [M+ Na- H2O]+, 919 [M+ H- H2O]+, 797 [M+ Na- 162]+, 617 [M+ Na- H2O- 162× 2]+, 595 [M+ H- H2O- 162× 2]+, 433 [M+ H- H2O- 162×2- 162]+; ESI-MS (negative mode) at m/z 935 [M- H]-, 773 [M- H- 162]-, 611 [M- H- 162× 2]-; IR νmax (KBr) cm -1: 3415 (OH), 2925 (CH), 1045; 1H-NMR (C5D5N) data see Table 1; 13C-NMR, see Table 2.
-3.5° (H2O, c 0.16). HR-ESIMS (positive mode) at m/z 937.5075 [M+ H]+ (calcd. 937.5008). ESI-MS (positive mode) at m/z 959 [M+ Na]+, 941 [M+ Na- H2O]+, 919 [M+ H- H2O]+, 797 [M+ Na- 162]+, 617 [M+ Na- H2O- 162× 2]+, 595 [M+ H- H2O- 162× 2]+, 433 [M+ H- H2O- 162×2- 162]+; ESI-MS (negative mode) at m/z 935 [M- H]-, 773 [M- H- 162]-, 611 [M- H- 162× 2]-; IR νmax (KBr) cm -1: 3415 (OH), 2925 (CH), 1045; 1H-NMR (C5D5N) data see Table 1; 13C-NMR, see Table 2. -28.7° (H2O, c 0.12). HR-ESIMS (positive mode) at m/z 1283.5891 [M+Na]+ (calcd 1283.5884). ESI-MS (positive mode) at m/z 1283 [M+ Na]+, 1121 [M+ Na- 162]+, 959 [M+ Na- 162× 2]+, 797 [M+ Na- 162× 3]+, 779 [M+ Na- 162× 3- H2O]+; ESIMS (negative mode) at m/z 1259 [M- H]-, 1097 [M- H- 162]-, 935 [M- H- 162× 2]-, 773 [M- H- 162× 3]-, 611 [M- H- 162× 4]-; IR νmax (KBr) cm -1: 3429 (OH), 2933 (CH), 1039; 1H-NMR (C5D5N) data see Table 1; 13C-NMR, see Table 2.
-28.7° (H2O, c 0.12). HR-ESIMS (positive mode) at m/z 1283.5891 [M+Na]+ (calcd 1283.5884). ESI-MS (positive mode) at m/z 1283 [M+ Na]+, 1121 [M+ Na- 162]+, 959 [M+ Na- 162× 2]+, 797 [M+ Na- 162× 3]+, 779 [M+ Na- 162× 3- H2O]+; ESIMS (negative mode) at m/z 1259 [M- H]-, 1097 [M- H- 162]-, 935 [M- H- 162× 2]-, 773 [M- H- 162× 3]-, 611 [M- H- 162× 4]-; IR νmax (KBr) cm -1: 3429 (OH), 2933 (CH), 1039; 1H-NMR (C5D5N) data see Table 1; 13C-NMR, see Table 2.3.4. Acid hydrolysis of saponins
3.5. Platelets preparation
3.6. Real-time reverse-transcriptase-polymerase chain reaction
3.7. Statistical analysis
Acknowledgements
References
- Pitchford, S.C. Novel uses for anti-platelet agents as anti-inflammatory drugs. Brit. J. Pharmacol. 2007, 152, 987–1002. [Google Scholar] [CrossRef]
- Henn, V.; Slupsky, J.R.; Grafe, M.; Anagnostopoulos, I.; Forster, R.; Muller, B.G.; Kroczek, R.A. CD40L on activated platelets platelets triggers an inflammatory reaction on endothelial cells. Nature 1998, 391, 591–594. [Google Scholar] [CrossRef]
- Prasad, K.S.; Andre, P.; He, M.; Bao, M.; Manganello, J.; Phillips, D.R. Soluble CD40 ligand induces beta3 integrin tyrosine phosphorylation and triggers platelet activation by outside-in signaling. Proc. Natl. Acad. Sci.USA 2003, 100, 12367–12371. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Kereiakes, D.J.; Mascelli, M.A.; Deckelbaum, L.I.; Barnathan, E.S.; Patel, K.K.; Frederick, B.; Nakada, M.T.; Topol, E.J. Abciximab suppresses the rise in levels of circulating inflammatory markers after percutaneous coronary revascularization. Circulation 2001, 104, 163–167. [Google Scholar]
- Danese, S.; De La, M.C.; Rivera Reyes, B.M.; Sans, M.; Levine, A.D.; Fiocchi, C. T cells trigger CD40-dependent platelet activation and granular RANTES release: a novel pathway for immune response amplification. J. Immunol. 2004, 172, 2011–2015. [Google Scholar]
- Buchner, K.; Henn, V.; Grafe, M.; De Boer, O.J.; Becker, A.E.; Kroczek, R.A. CD40 ligand is selectively expressed on CD4t T cells and platelets: implications for CD40–CD40L signalling in atherosclerosis. J. Pathol. 2003, 201, 288–295. [Google Scholar] [CrossRef]
- Peng, J.P.; Yao, X.S.; Kobayashi, H.; Ma, C.Y. Novel furostanol glycosides from Allium macrostemon. Planta Med. 1995, 61, 58–61. [Google Scholar] [CrossRef]
- Ikeda, T.; Tsumagari, H.; Okawa, M.; Nohara, T. Pregnane- and Furostane-Type Oligoglycosides from the Seeds of Allium tuberosum. Chem. Pharm. Bull. 2004, 52, 142–145. [Google Scholar] [CrossRef]
- Peng, J.P.; Yao, X.S.; Okada, Y.; Okuyama, T. Structures of macrostemonoside J, K and L from Allium macrostemon Bunge. Acta Pharm. Sinica 1994, 29, 526–531. [Google Scholar]
- Peng, J.P.; Wang, X.; Yao, X.S. Studies on two new furostanol glycosides from Allium Macrostemon Bunge. Acta Pharm. Sinica 1993, 28, 526–531. [Google Scholar]
- Chen, H.F.; Wang, N.L.; Sun, H.L.; Yang, B.F.; Yao, X.S. Novel furostanol saponins from the bulbs of Allium macrostemon B. and their bioactive on [Ca2+]i increase induced by KCl. J. Asian Nat. Prod. Res. 2006, 8, 21–28. [Google Scholar] [CrossRef]
- Kostova, I.; Dinchev, D.; Rentsch, G.H.; Dimitrov, V.; Ivanova, A. Zeitschrift fuer Naturforschung, C: J. Biosci. 2002, 57, 33–38.
- Agrawal, P.K. Assigning stereodiversity of the 27-Me group of furostane-type steroidal saponins via NMR chemical shifts. Steroids 2005, 70, 715–724. [Google Scholar] [CrossRef]
- Acharyaa, D.; Mitaine Offera, A.C.; Kaushikb, N.; Miyamotoc, T.; Paululatd, T.; Lacaille Dubois, M.A. Furostane-Type Steroidal Saponins from the Roots of Chlorophytum borivilianum. Helvet. Chim. Acta 2008, 91, 2262–2269. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compound 1, 2 and 3 are available from authors.
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, H.; Ou, W.; Wang, G.; Wang, N.; Zhang, L.; Yao, X. New Steroidal Glycosides Isolated as CD40L Inhibitors of Activated Platelets. Molecules 2010, 15, 4589-4598. https://doi.org/10.3390/molecules15074589
Chen H, Ou W, Wang G, Wang N, Zhang L, Yao X. New Steroidal Glycosides Isolated as CD40L Inhibitors of Activated Platelets. Molecules. 2010; 15(7):4589-4598. https://doi.org/10.3390/molecules15074589
Chicago/Turabian StyleChen, Haifeng, Wenchao Ou, Guanghui Wang, Naili Wang, Linnan Zhang, and Xinsheng Yao. 2010. "New Steroidal Glycosides Isolated as CD40L Inhibitors of Activated Platelets" Molecules 15, no. 7: 4589-4598. https://doi.org/10.3390/molecules15074589
APA StyleChen, H., Ou, W., Wang, G., Wang, N., Zhang, L., & Yao, X. (2010). New Steroidal Glycosides Isolated as CD40L Inhibitors of Activated Platelets. Molecules, 15(7), 4589-4598. https://doi.org/10.3390/molecules15074589




