Microwave-Assisted One-Pot Synthesis of Pyrazolone Derivatives under Solvent-Free Conditions
Abstract
:1. Introduction
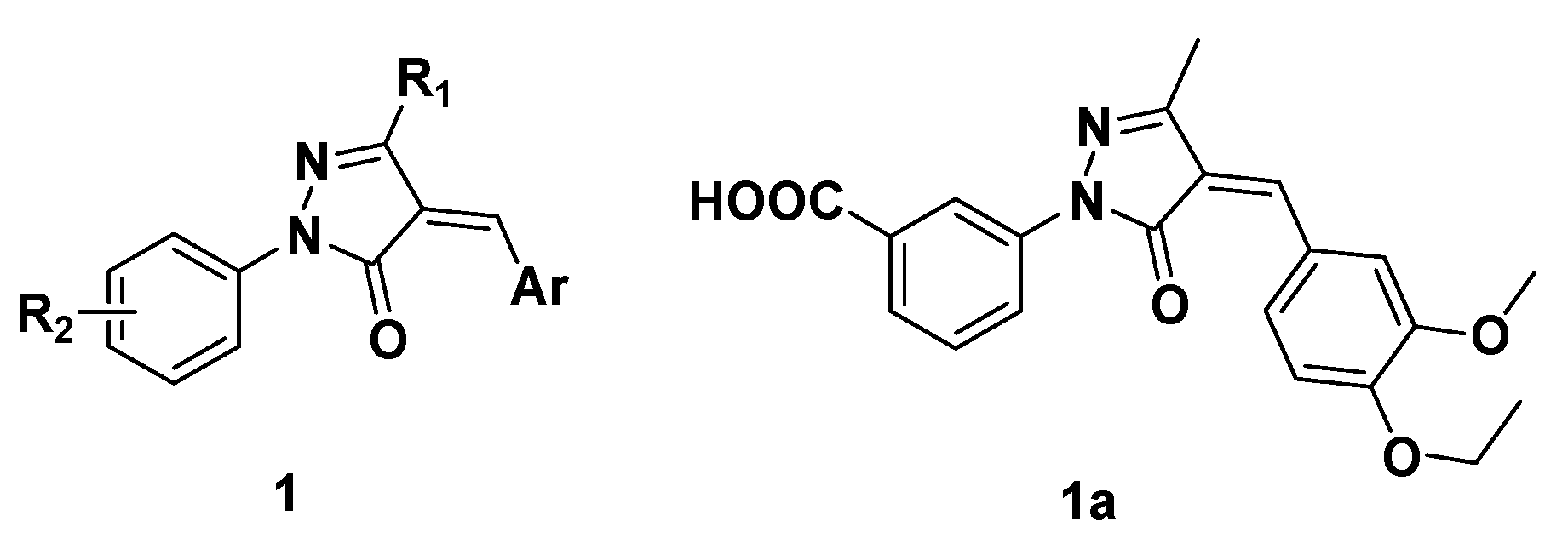
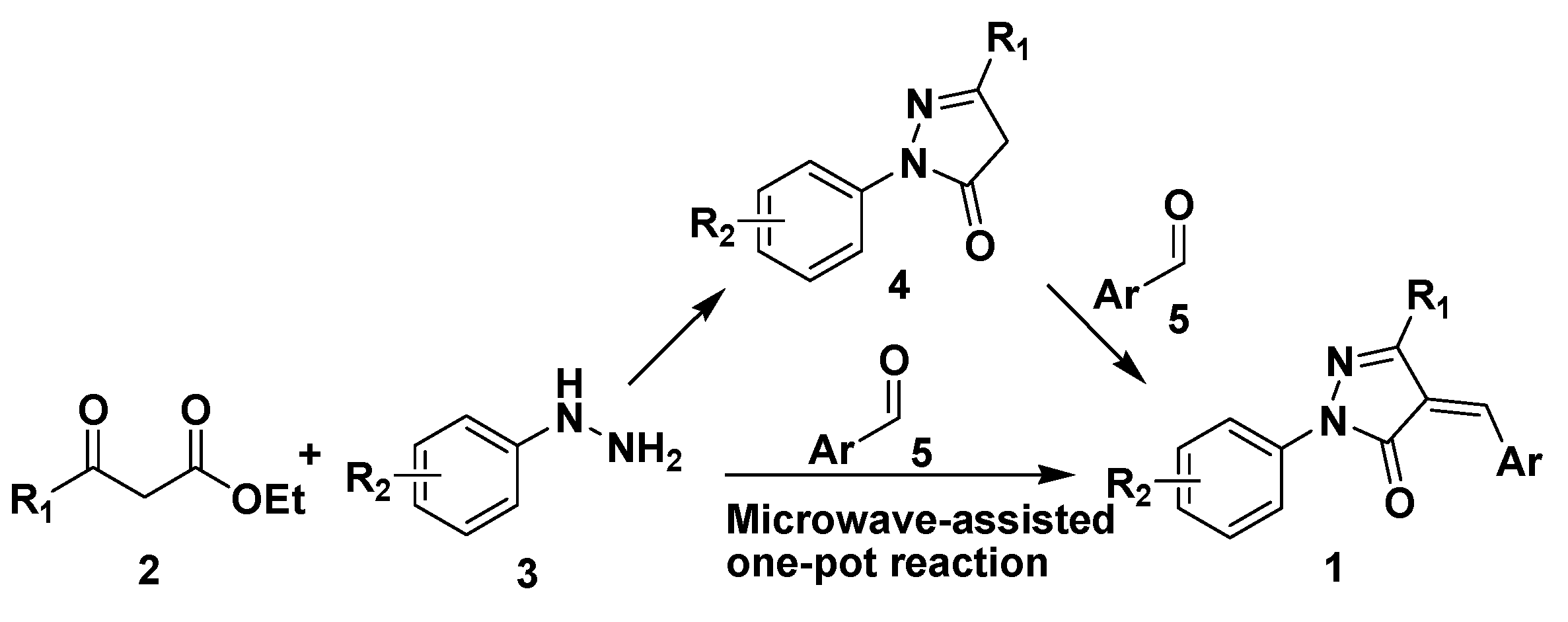
2. Results and Discussion
2.1. Optimization of the reaction conditions
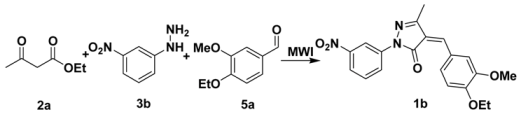 | |||
|---|---|---|---|
| Entry | Power (W) | Time (min) | Yield (%)b |
| 1 | 280 | 5 | 20 |
| 2 | 420 | 5 | 67 |
| 3 | 560 | 5 | 54 |
| 4 | 420 | 10 | 71 |
| 5 | 420 | 15 | 62 |
| Entry | Solid support | Reagent Ratio | Yield (%)b | ||
|---|---|---|---|---|---|
| 2a | 3b | 5a | |||
| 1 | - | 1 | 1.2 | 1 | 60 |
| 2 | - | 1.2 | 1 | 1 | 78 |
| 3 | - | 1 | 1 | 1.2 | 63 |
| 4 | - | 1.5 | 1 | 1 | 83 |
| 5 | - | 2 | 1 | 1 | 73 |
| 6 | - | 2.5 | 1 | 1 | 79 |
| 7 | - | 3 | 1 | 1 | 81 |
| 8 | SiO2 | 1.5 | 1 | 1 | 81 |
| 9 | Al2O3 | 1.5 | 1 | 1 | 80 |
2.2. Scope of microwave-assisted one-pot synthesis of 4-arylidenepyrazolone derivatives
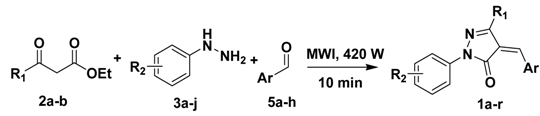 | ||||
|---|---|---|---|---|
| Compd. | R1 | R2 | Ar | Yield (%)b |
| 1a | Me, 2a | 3-CO2H, 3a | 3-MeO-4-EtO-Ph, 5a | 98 |
| 1b | Me, 2a | 3-NO2, 3b | 3-MeO-4-EtO-Ph, 5a | 83 |
| 1c | Me, 2a | 4-NO2, 3c | 3-MeO-4-EtO-Ph, 5a | 78 |
| 1d | Me, 2a | 4-CF3, 3d | 3-MeO-4-EtO-Ph, 5a | 53 |
| 1e | Me, 2a | 3-CF3, 3e | 3-MeO-4-EtO-Ph, 5a | 67c |
| 1f | Me, 2a | 3,5-di-CF3, 3f | 3-MeO-4-EtO-Ph, 5a | 54c |
| 1g | Me, 2a | 2-F, 3g | 3-MeO-4-EtO-Ph, 5a | 51 |
| 1h | Me, 2a | 3,4-di-Cl, 3h | 3-MeO-4-EtO-Ph, 5a | 86 |
| 1i | Me, 2a | 3,5-di-Cl, 3i | 3-MeO-4-EtO-Ph, 5a | 73c |
| 1j | Me, 2a | 3-CO2H, 3a | 3-MeO-4-OH-Ph, 5b | 68 |
| 1k | Me, 2a | 3-CO2H, 3a | 3,4-di-OH-Ph, 5c | 73 |
| 1l | Me, 2a | 3-NO2, 3b | 5-Me-thiophen-2-yl, 5d | 53 |
| 1m | Me, 2a | H, 3j | 3-MeO-4-EtO-Ph, 5a | 63 |
| 1n | Me, 2a | H, 3j | 4-Br-Ph, 5e | 53c |
| 1o | Ph, 2b | 3-NO2, 3b | 3-MeO-4-OH-Ph, 5c | 61 |
| 1p | Me, 2a | 3-CO2H, 3a | 3-MeO-4-PhCH2O-Ph, 5f | 84 |
| 1q | Me, 2a | 3-CO2H, 3a | 3-MeO-4-Me(CH2)4O-Ph, 5g | 83 |
| 1r | Me, 2a | 3-CO2H, 3a | 3-MeO-4-Me2CHO-Ph, 5h | 76 |
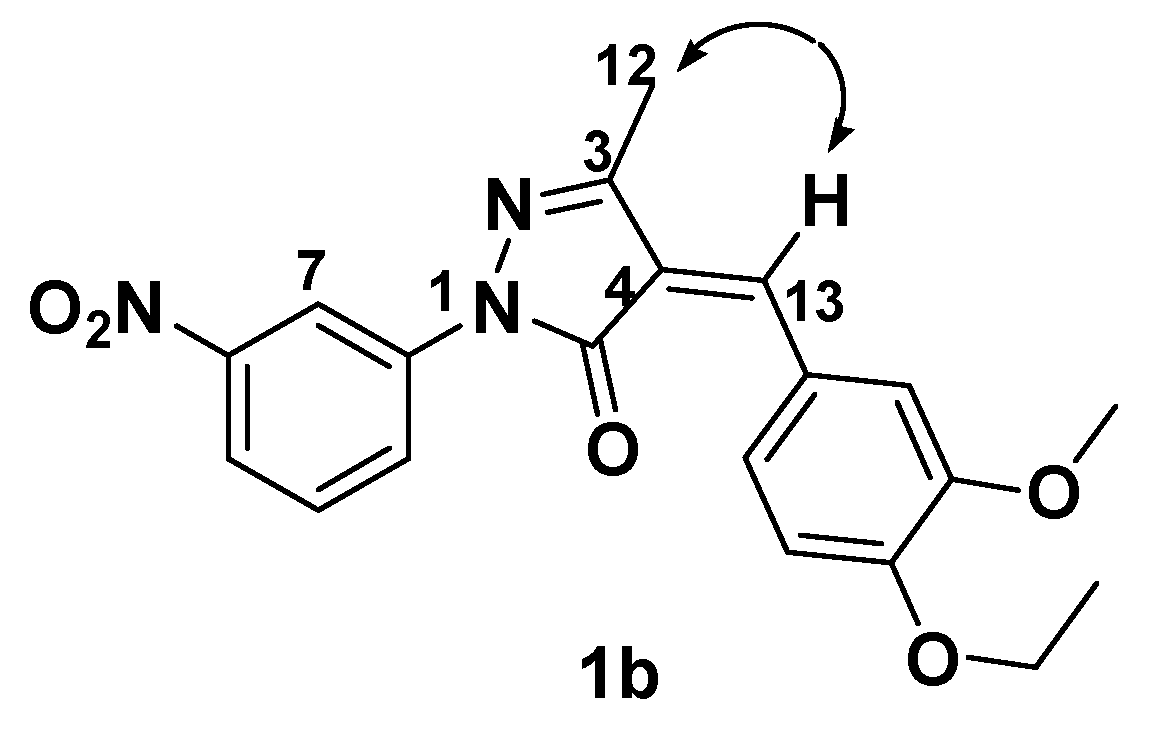
3. Experimental
3.1. General
3.2. General Procedure for the Preparation of 1a-r: Preparation of 1b
4. Conclusions
Acknowledgements
References
- Brogden, R.N. Pyrazolone derivatives. Drugs 1986, 32, 60–70. [Google Scholar]
- Castagnolo, D.; Manetti, F.; Radi, M.; Bechi, B.; Pagano, M.; De Logu, A.; Meleddu, R.; Saddi, M.; Botta, M. Synthesis, biological evaluation, and SAR study of novel pyrazole analogues as inhibitors of Mycobacterium tuberculosis: part 2. Synthesis of rigid pyrazolones. Bioorg. Med. Chem. 2009, 17, 5716–5721. [Google Scholar]
- Radi, M.; Bernardo, V.; Bechi, B.; Castagnolo, D. Microwave-assisted organocatalytic multicomponent Knoevenagel/hetero Diels-Alder reaction for the synthesis of 2,3-dihydropyran[2,3-c]pyrazoles. Tetrahedron Lett. 2009, 50, 6572–6575. [Google Scholar] [CrossRef]
- Mitra, P.; Mittra, A. Synthesis and fungitoxicity of different 2-pyrazolin-5-one Mannich bases. J. Indian Chem. Soc. 1981, 58, 695–696. [Google Scholar]
- Singh, Da.; Singh, De. Synthesis and antifubgal activity of some 4-arylmethylene derivatives of substituted pyrazolones. J. Indian Chem. Soc. 1991, 68, 165–167. [Google Scholar]
- Moreau, F.; Desroy, N.; Genevard, J.M.; Vongsouthi, V.; Gerusz, V.; Le Fralliec, G.; Oliveira, C.; Floquet, S.; Denis, A.; Escaich, S.; Wolf, K.; Busemann, M.; Aschenbrenner, A. Discovery of new Gram-negative antivirulence drugs: Structure and properties of novel E. coli WaaC inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 4022–4026. [Google Scholar]
- Tantawy, A.; Eisa, H.; Ismail, A. Synthesis of 1-(substituted) 4-arylhydrazono-3-methyl-2-pyrazolin-5-ones as potential antiinflammatory agents. Alexandria, M. E. J. Pharm. Sci. 1988, 2, 113–116. [Google Scholar]
- Pasha, F.A.; Muddassar, M.; Neaz, M.M.; Cho, S.J. Pharmacophore and docking-based combined in-silico study of KDR inhibitors. J. Mol. Graph. Model. 2009, 28, 54–61. [Google Scholar] [CrossRef]
- Deng, G.; Li, W.; Shen, J.; Jiang, H.; Chen, K.; Liu, H. Pyrazolidine-3,5-dione derivatives as potent non-steroidal agonists of farnesoid X receptor: Virtual screening, synthesis, and biological evaluation. Bioorg. Med. Chem. 2008, 18, 5497–5502. [Google Scholar] [CrossRef]
- Abdelrazek, F.M.; Metz, P.; Kataeva, O.; Jager, A.; EI-Mahrouky, S.F. Synthesis and molluscicidal activity of new chromene and pyrano[2,3-c]pyrazole derivatives. Arch. Pharm. Chem. Life Sci. 2007, 340, 543–548. [Google Scholar] [CrossRef]
- Azev, Y.A.; Gryazeva, O.V.; Golomolzin, B.V. Azines and their acyclic derivatives as transferers of one-carbon fragment in reactions with pyrazolones. Chem. Heterocycl. Comp. 2003, 39, 1478–1486. [Google Scholar]
- Ceulemans, E.; Voets, M.; Emmers, S.; Dehaen, W. A highly diastereoselective intramolecular hetero Diels-Alder approach towards tetracyclic pyrazoles. Synlett. 1997, 10, 1155–1156. [Google Scholar]
- Bevk, D.; Jakse, R.; Svete, J.; Golobic, A.; Golic, L.; Stanovnik, B. Transformations of alkyl (5-oxo-1-phenyl-4,5-dihydro-1H-pyrazol-3-yl)acetates into 5-heteroaryl-3-OXO-2-phenyl-3,5-dihydro-2H-pyrazolo[4,3-c]pyridine-7- carboxylates. Heterocycles 2003, 61, 197–224. [Google Scholar] [CrossRef]
- Holzer, W.; Hallak, L. Synthesis and NMR spectroscopic investigations with 3-amino-, 3-hydroxy-, and 3-methoxy-4-acyl-1-phenyl-2-pyrazolin-5-ones. Heterocycles 2004, 63, 1311–1334. [Google Scholar] [CrossRef]
- Kreutzberger, A.; Kolter, K. Antiviral agents. XXVII: Aminomethynylation of 5-oxo-2-pyrazoline-3-carboxylic acid derivatives. Arch. Pharm. 1986, 319, 18–25. [Google Scholar] [CrossRef]
- Brooker, L.G.S.; Keyes, G.H.; Sprague, R.H.; VanDyke, R.H.; VanLare, E.; VanZandt, G.; White, F.L. The cyanine dye series. XI. The merocyanines. J. Am. Chem. Soc. 1951, 73, 5326–5330. [Google Scholar]
- Knorr, L. Einwirkung von Acetessigester auf Hydrazinchinizinderivate. Chem. Ber. 1884, 17, 546–552. [Google Scholar]
- Knorr, L. Synthetische Versuche mit dem Acetessigester. II. Mittheilung: Ueberführung des Diacetbernsteinsäureesters und des Acetessigesters in Pyrrolderivate. Ann. Chem. 1886, 236, 290–336. [Google Scholar] [CrossRef]
- Wu, T.Y.; Schultz, P.G.; Ding, S. One-Pot Two-Step Microwave-Assisted Reaction in Constructing 4,5-Disubstituted Pyrazolopyrimidines. Org. Lett. 2003, 5, 3587–3590. [Google Scholar] [CrossRef]
- Kappe, C.O. Synthetic methods. Controlled microwave heating in modern organic synthesis. Angew. Chem., Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef]
- Burczyk, A.; Loupy, A.; Bogdal, D.; Petit, A. Improvement in the synthesis of metallophthalocyanines using microwave irradiation. Tetrahedron 2005, 61, 179–188. [Google Scholar]
- Krishna Mohan, K.V.V.; Narender, N.; Kulkarni, S.J. Zeolite catalyzed acylation of alcohols and amines with acetic acid under microwave irradiation. Green Chem. 2006, 8, 368–372. [Google Scholar] [CrossRef]
- Appukkuttan, P.; Mehta, V.P.; Van der Eycken, E.V. Microwave-assisted cycloaddition reactions. Chem. Soc. Rev. 2010, 39, 1467–1477. [Google Scholar]
- Alcazar, J.; Oehlrich, D. Recent applications of microwave irradiation to medicinal chemistry. Future Med. Chem. 2010, 2, 169–176. [Google Scholar] [CrossRef]
- Jiang, B.; Sh, F.; Tu, S.J. Microwave-Assisted Multicomponent Reactions in the Heterocyclic Chemistry. Curr. Org. Chem. 2010, 14, 357–378. [Google Scholar] [CrossRef]
- Radi, M.; Bernardo, V.; Bechi, B.; Castagnolo, D.; Pagano, M.; Botta, M. Microwave-assisted organocatalytic multicomponent Knoevenagel/hetero Diels-Alder reaction for the synthesis of 2,3-dihydropyran[2,3-c]pyrazoles. Tetrahedron Lett. 2009, 50, 6572–6575. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds reported in this paper are available from the authors.
© 2010 by the authors;
Share and Cite
Ma, R.; Zhu, J.; Liu, J.; Chen, L.; Shen, X.; Jiang, H.; Li, J. Microwave-Assisted One-Pot Synthesis of Pyrazolone Derivatives under Solvent-Free Conditions. Molecules 2010, 15, 3593-3601. https://doi.org/10.3390/molecules15053593
Ma R, Zhu J, Liu J, Chen L, Shen X, Jiang H, Li J. Microwave-Assisted One-Pot Synthesis of Pyrazolone Derivatives under Solvent-Free Conditions. Molecules. 2010; 15(5):3593-3601. https://doi.org/10.3390/molecules15053593
Chicago/Turabian StyleMa, Ruoqun, Jin Zhu, Jie Liu, Lili Chen, Xu Shen, Hualiang Jiang, and Jian Li. 2010. "Microwave-Assisted One-Pot Synthesis of Pyrazolone Derivatives under Solvent-Free Conditions" Molecules 15, no. 5: 3593-3601. https://doi.org/10.3390/molecules15053593
APA StyleMa, R., Zhu, J., Liu, J., Chen, L., Shen, X., Jiang, H., & Li, J. (2010). Microwave-Assisted One-Pot Synthesis of Pyrazolone Derivatives under Solvent-Free Conditions. Molecules, 15(5), 3593-3601. https://doi.org/10.3390/molecules15053593




