Supramolecular Coordination Assemblies Constructed From Multifunctional Azole-Containing Carboxylic Acids
Abstract
:1. Introduction

2. Methodology
3. One-Dimensional Coordination Chains
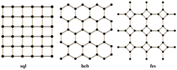
3.1. Chain
| CICZUJ[64] | OGALEO[71] | FEGGAA[78] | MEFZUS[87] | VIKCOI[97] | CEYLOI[104] |
| PIFBAI[65] | OGALIS[71] | FOHFUE[79] | MENGAO[88] | VIQYEA[98] | ECULUK[105] |
| PODZOY[66] | OKEHIV[72] | GOYSES[80] | NENDAL[89] | VIQYIE[98] | OKEHIV01[67] |
| AVUPUC[67] | OKEHOB[72] | GOYSIW[80] | NIQGUQ[90] | WUPHEU[99] | RAJNOG[106] |
| AVUQOX[67] | VOBKUT[73] | HIHJIS[81] | PAJJOA[91] | XIBPAA[100] | KOBGUE[40] |
| DOGMAO[68] | BIPJEQ[74] | IDIXOJ[82] | PAJJOA01[92] | XIKWAQ[101] | |
| DOGMES[68] | DATMUH[75] | IYASEG[83] | PEXSIV[93] | YIFQUZ[102] | |
| HUXTUP[69] | DATNAO[75] | LAJZIG[84] | PEXSIV01[94] | TIWRUN[103] | |
| NIQWUG[70] | DOGZAB[76] | LAQPAV[85] | QANDUF[95] | KEXWIU[37] | |
| OGAKUD[71] | EDURUR[77] | LASSEE[86] | SENJEB[96] | PEXVEU[37] |
3.2. Chain and monomer
| CEYLEY[104] |
3.3. Chains of cubes and rings
3.4. Ladder
| ABAYEI[108] | XENCEZ[109] | DILGIP[111] | KEPYIO[113] | ROMRUH[115] | LIWLUZ[36] |
| AVUQAJ[67] | XOKRIZ[110] | HOSTEO[112] | POHSUB[114] | PEFVIF[116] | VODCEX[43] |
3.5. Pipe
| YIFSIP[102] |
4. Two-Dimensional Coordination Layers
4.1. sql
| TIGCAO[117] | JEXSIP[120] | LIQVEN[122] | SONJUA[126] | POLDIE[129] | MISHAY[50] |
| EVONOS[118] | KEPYOU[121] | LIWKIM[123] | YASSEQ[127] | KOBGOY[40] | PEZROC[38] |
| HOGDEN[114] | KEPYOU01[68] | OFITAZ[124] | ODIVIH[128] | KOBGOY01[42] | XOHPAM[48] |
| JEDYEX[119] | LAQNUN[85] | OFITAZ01[125] | ODIVON[128] | LIWLOT[36] | XOHPEQ[48] |
4.2. hcb
| BOKXUV[130] | FIBJEG[133] | TIVZOO[132] | WOFVET[94] | NOFGAR[136] |
| FENSUN[131] | KEKWIH[134] | TIVZUU[132] | VIQZAX[98] | NOFGEV[136] |
| FENSUN03[132] | TIVZII[132] | TIWBAD[132] | NETXIU[135] | EHAGAW[137] |
4.3. fes
| FENSUN01[138] | OFIVIJ[125] | TIWBEH[132] | YELYIY01[140] |
| FENSUN02[92] | QEXPAL[139] | YELYIY[92] | LIMNOL[35] |
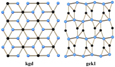
4.4. kgd
| JEXSAH[120] | JEXSEL[120] | SEYVEY[141] |
4.5. gek1
| TIKWUG[142] |
4.6. New two-dimensional topologies
5. Three-Dimensional Coordination Frameworks
5.1. dia
| AGOMOZ[149] | SEYVIC[150] | LUMDEC[150] | LUMDIG[150] | METYIU[151] | NEHZIK[152] |
5.2. sra
| RAPBEP[153] | GAMFEG[154] | INOXUE[30] | INOYAL[30] | KOCWAB[41] | QEYXAU[155] |
5.3. etb
5.4. etc
| WOMFIO[157] |
5.5. pcu
| ODIVUT[128] |
5.6. ths
5.7. rtl
5.8. pts
| LARBOW[32] | REHRAY[33] | LARBOW01[35] |
5.9. ant
5.10. bbf
5.11. dmc
| XOHPOA[161] |
5.12. pyr
5.13. sqc5577
5.14. stp
5.15. tfz
| REJLOI[164] |
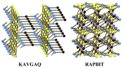
5.16. KAVGAQ
| KAVGAQ[159] | REJLEY[164] | REJLIC[164] | XECBUD[165] |
5.17. RAPBIT
5.18. New three-dimensional topologies
6. Summary and Conclusions
Acknowledgments
References
- Caulder, D.L.; Raymond, K.N. Supermolecules by design. Acc. Chem. Res. 1999, 32, 975–982. [Google Scholar] [CrossRef]
- Seidel, S.R.; Stang, P.J. High-symmetry coordination cages via self-assembly. Acc. Chem. Res. 2002, 35, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Kesanli, B.; Lin, W.B. Chiral porous coordination networks: Rational design and applications in enantioselective processes. Coord. Chem. Rev. 2003, 246, 305–326. [Google Scholar] [CrossRef]
- Frey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F. Crystallized frameworks with giant pores: Are there limits to the possible? Acc. Chem. Res. 2005, 38, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, D.; Claridge, J.B.; Cussen, E.J.; Prior, T.J.; Rosseinsky, M.J. Design, chirality, and flexibility in nanoporous molecule-based materials. Acc. Chem. Res. 2005, 38, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Tominaga, M.; Hori, A.; Therrien, B. Coordination assemblies from a Pd(II)-cornered square complex. Acc. Chem. Res. 2005, 38, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Robin, A.Y.; Fromm, K.M. Coordination polymer networks with O- and N-donors: what they are, why and how they are made. Coord. Chem. Rev. 2006, 250, 2127–2157. [Google Scholar] [CrossRef]
- Wei, Y.Q.; Yu, Y.F.; Wu, K.C. Highly stable diamondoid network coordination polymer [Mn(NCP)2]n with notable NLO, magnetic, and luminescence properties. Cryst. Growth Des. 2007, 7, 2262–2264. [Google Scholar] [CrossRef]
- Su, C.Y.; Smith, M.D.; Goforth, A.M.; Zur Loye, H. A Three-dimensional, noninterpenetrating metal-organic framework with the moganite topology: A simple (42.62.82)(4.64.8)2 net containing two kinds of topologically nonequivalent points. Inorg. Chem. 2004, 43, 6881–6883. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Masaoka, S. Metal complexes of hexaazatriphenylene (hat) and its derivatives - from oligonuclear complexes to coordination polymers. Coord. Chem. Rev. 2003, 246, 73–88. [Google Scholar] [CrossRef]
- Bu, X.H.; Tong, M.L.; Chang, H.C.; Kitagawa, S.; Batten, S.R. A neutral 3D copper coordination polymer showing 1D open channels and the first interpenetrating NbO-type network. Angew. Chem. Int. Ed. 2004, 43, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Yong, G.P.; Wang, Z.Y.; Cui, Y. Synthesis, structural characterization and properties of copper(Ⅱ) and zinc(Ⅱ) coordination polymers with a new bridging chelating ligand. Eur. J. Inorg. Chem. 2004, 4317–4323. [Google Scholar] [CrossRef]
- Chang, F.; Wang, Z.M.; Sun, H.L.; Wen, G.H.; Zhang, X.X. [Cu2(bpdado)2(H2O)2]·H2O}n: A 1D nanotubular coordination polymer with wall made of edge-sharing hexagons, where bpdado=2,2′-bipyridine-3,3′-dicarboxylate-1,1′-dioxide. Dalton Trans. 2005, 2976–2978. [Google Scholar] [CrossRef] [PubMed]
- Zaworotko, M.J. From disymmetric molecules to chiral polymers: a new twist for supramolecular synthesis? Angew. Chem., Int. Ed. 1998, 37, 1211–1213. [Google Scholar] [CrossRef]
- Cao, R.; Sun, D.F.; Liang, Y.C.; Hong, M.C.; Tatsumi, K.; Shi, Q. Syntheses and characterizations of three-dimensional channel-like polymeric lanthanide complexes constructed by 1,2,4,5-benzenetetracarboxylic acid. Inorg. Chem. 2002, 41, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.C.; Cao, R.; Su, W.P.; Hong, M.C.; Zhang, W.J. Syntheses, structures, and magnetic properties of two gadolinium(III)-copper(II) coordination polymers by a hydrothermal reaction. Angew. Chem., Int. Ed. 2000, 39, 3304–3307. [Google Scholar] [CrossRef]
- Chapman, M.E.; Ayyappan, P.; Foxman, B.M.; Yee, G.T.; Lin, W.B. Synthesis, x-ray structures, and magnetic properties of copper(II) pyridinecarboxylate coordination networks. Cryst. Growth Des. 2001, 1, 159–163. [Google Scholar] [CrossRef]
- Lu, J.Y.; Schauss, V. Crystal engineering of a three-dimensional coordination polymer based on both covalent and O–H···O hydrogen bonding interactions of bifunctional ligands. CrystEngComm. 2001, 26, 111–113. [Google Scholar] [CrossRef]
- Noro, S.; Kitagawa, S.; Yamashita, M.; Wada, T. New microporous coordination polymer affording guest-coordination sites at channel walls. Chem. Commun. 2002, 222–223. [Google Scholar] [CrossRef]
- Lu, J.Y.; Babb, A.M. An unprecedented interpenetrating structure with two covalent-bonded open-framework of different dimensionality. Chem. Commun. 2001, 821–822. [Google Scholar] [CrossRef]
- Tong, M.L.; Li, L.J.; Mochizuki, K.; Chang, H.C.; Chen, X.M.; Li, Y.; Kitagawa, S. A novel three-dimensional coordination polymer constructed with mixed-valence dimeric copper(I,II) units. Chem. Commun. 2003, 428–429. [Google Scholar] [CrossRef]
- Kang, Y.; Yao, Y.G.; Qin, Y.Y.; Zhang, J.; Chen, Y.B.; Li, Z.J.; Wen, Y.H.; Cheng, J.K.; Hu, R.F. A novel ligand-unsupported 3D framework polymer of trimeric copper(I) and its NLO property. Chem. Commun. 2004, 1046–1047. [Google Scholar] [CrossRef] [PubMed]
- Eubank, J.F.; Walsh, R.D.; Eddaoudi, M. Terminal co-ligand directed synthesis of a neutral, non-interpenetrated (10,3)-a metal–organic framework. Chem. Commun. 2005, 2095–2097. [Google Scholar] [CrossRef] [PubMed]
- Lou, B.Y.; Jiang, F.L.; Wu, B.L.; Yuan, D.Q.; Hong, M.C. From helical array to porous architecture: exploring the use of side chains of amino acids to engineer 1D infinite coordination polymeric chain into porous frameworks. Cryst. Growth Des. 2006, 6, 989–993. [Google Scholar] [CrossRef]
- Zeng, Y.F.; Liu, F.C.; Zhao, J.P.; Cai, S.; Bu, X.H.; Ribas, J. An azido–metal–isonicotinate complex showing long-range ordered ferromagnetic interaction: synthesis, structure and magnetic properties. Chem. Commun. 2006, 21, 2227. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.C.; Zeng, Y.F.; Zhao, J.P.; Hu, B.W.; Hu, X.; Ribas, J.; Bu, X.H. Novel lanthanide–azido complexes: Hydrothermal syntheses, structures and magnetic properties. Dalton Trans. 2009, 12, 2074. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zeng, Y.F.; Chen, Z.; Saudo, E.C.; Liu, F.C.; Ribas, J.; Bu, X.H. 3d−4f coordination polymers containing alternating EE/EO azido chain synthesized by synergistic coordination of lanthanide and transition metal ions. Cryst. Growth Des. 2009, 9, 421–426. [Google Scholar] [CrossRef]
- Batten, S.R.; Robson, R. Interpenetrating nets: ordered, periodic entanglement. Angew. Chem. Int. Ed. 1998, 37, 1460–1494. [Google Scholar] [CrossRef]
- Qu, Z.R.; Zhao, H.; Wang, X.S.; Li, Y.H.; Song, Y.M.; Liu, Y.J; Ye, Q.; Xiong, R.G.; Abrahams, B.F.; Xue, Z.L.; You, X.Z. Homochiral Zn and Cd coordination polymers containing amino acid−tetrazole ligands. Inorg. Chem. 2003, 42, 7710–7712. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Tong, M.L.; Yu, X.L.; Chen, X.M. Controlled aggregation of heterometallic nanoscale Cu12Ln6 clusters (Ln = GdIII or NdIII) into 2D coordination polymers. Inorg. Chem. 2005, 44, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Huang, X.F.; Xiong, R.G. An unexpected intermediate or precipitate-novel 3D Cd-coordination polymer formed in the preparation of 5-substituted 1H-tetrazoles from nitrile in water. Chin. J. Inorg. Chem. 2005, 21, 1020–1024. [Google Scholar]
- Ye, Q.; Song, Y.M.; Wang, G.X.; Chen, K.; Fu, D.W.; Chan, W.H.; Zhu, J.S.; Huang, S.P.; Xiong, R.G. Ferroelectric metal-organic framework with a high dielectric constant. J. Am. Chem. Soc. 2006, 128, 6554–6555. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Dieguez, A.; Colacio, E. [Znn(polyox)(pmtz)n]: The first polyoxalate-containing coordination polymer from an unforeseen chemical rearrangement of 5-pyrimidyltetrazole under hydrothermal conditions. Chem. Commun. 2006, 4140–4142. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhao, Y.F.; Zhang, X.M. Blue-green photoluminescent 5-and 10-connected metal 5-(4′-carboxy-phenyl)tetrazolate coordination polymers. Inorg. Chem. Commun. 2007, 10, 1194–1197. [Google Scholar] [CrossRef]
- Yang, G.W.; Li, Q.Y.; Wang, J.; Yuan, R.X.; Xie, J.M. New CuII and CdII coordination polymers employing 5-[N-acetato(4-pyridyl)] tetrazolate as a bridging ligand. Chin. J. Inorg. Chem. 2007, 23, 1887–1894. [Google Scholar]
- Fu, D.W.; Zhao, H. Intermediate captured in the reaction of synthesizing valartan analogue (I). Chin. J. Inorg. Chem. 2007, 23, 122–123. [Google Scholar]
- Huang, X.H.; Sheng, T.L.; Xiang, S.C.; Fu, R.B.; Hu, S.M.; Li, Y.M.; Wu, X.T. Synthesis, structure and luminescence of a novel 2D cadmium coordination polymer with a ligand generated in situ. Chin. J. Struct. Chem. 2007, 26, 333–337. [Google Scholar]
- Bai, Y.L.; Tao, J.; Huang, R.B.; Zheng, L.S.; Zheng, S.L.; Oshida, K.; Einaga, Y. Pressure effects and mössbauer spectroscopic studies on a 3D mixed-valence iron spin-crossover complex with NiAs topology. Chem. Commun. 2008, 1753–1755. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhang, X.Q.; Bian, H.D.; Liang, H.; Zhao, B.; Yan, S.P.; Liao, D.Z. pH-Dependent Cu(II) coordination polymers with tetrazole-1-acetic acid: synthesis, crystal structures, EPR and magnetic properties. Cryst. Growth Des. 2008, 8, 1140–1146. [Google Scholar] [CrossRef]
- Yu, Z.P.; Xie, Y.; Wang, S.J.; Yong, G.P.; Wang, Z.Y. Synthesis, crystal structures and optical properties of two coordination polymers from 4-(1H-tetrazol-5-yl) benzoic acid. Inorg. Chem. Commun. 2008, 11, 372–376. [Google Scholar] [CrossRef]
- Dong, W.W.; Zhao, J.; Xu, L. Syntheses, crystal structure and properties of two novel coordination polymers with the flexible tetrazole-1-acetic acid (Htza). J. Solid State Chem. 2008, 181, 1149–1154. [Google Scholar] [CrossRef]
- Yang, G.W.; Li, Q.Y.; Zhou, Y.; Sha, P.; Ma, Y.S.; Yuan, R.X. Mn and Cu-Na coordination compounds containing the tetrazole-5-acetato anion (tza) ligands. Inorg. Chem. Commun. 2008, 11, 723–726. [Google Scholar] [CrossRef]
- Jia, Q.X.; Wang, Y.Q.; Yue, Q.; Wang, Q.L.; Gao, E.Q. Isomorphous Co-II and Mn-II materials of tetrazolate-5-carboxylate with an unprecedented self-penetrating net and distinct magnetic behaviours. Chem. Commun. 2008, 4894–4896. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.W.; Zhao, J.; Xu, L. Remarkable structural transformation of [Zn(tza)(2)] during recrystallization, syntheses and crystal structures of [M(tza)(2)] (M = Zn, Cd, Mn, Co; Htza = tetrazole-1-acetic acid. Cryst. Growth Des. 2008, 8, 2882–2886. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Wang, Y.Q.; Peng, H.Q.; Cheng, A.L.; Gao, E.Q. Synthesis, structure, and photoluminescence of a zinc(II) coordination polymer with 4-(tetrazol-5-yl)benzoate. Struct. Chem. 2008, 19, 535–539. [Google Scholar] [CrossRef]
- Li, Y.; Xu, G.; Zou, W.Q.; Wang, M.S.; Zheng, F.K.; Wu, M.F.; Zeng, H.Y.; Guo, G.C.; Huang, J.S. A novel metal-organic network with high thermal stability: Nonlinear optical and photoluminescent properties. Inorg. Chem. 2008, 47, 7945–7947. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.W.; Li, Q.Y.; Zhou, Y.; Gu, G.Q.; Ma, Y.S.; Yuan, R.X. Two copper(II) coordination polymers containing atza ligand [atza = 5-aminotetrazole-1-acetato]. Inorg. Chem. Commun. 2008, 11, 1239–1242. [Google Scholar] [CrossRef]
- Nouar, F.; Eubank, J.F.; Bousquet, T.; Wojtas, L.; Zaworotko, M.J.; Eddaoudi, M. Supermolecular building blocks (SBBs) for the design and synthesis of highly porous metal-organic frameworks. J. Am. Chem. Soc. 2008, 130, 1833–1835. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Yang, G.W.; Yuan, R.X; Wang, J.P.; Cui, P.F. Bis(5-aminotetrazole-1-acetato-κO)tetraaquacobalt(II) and catena-Poly[[cadmium(II)]-bis(μ-5-aminotetrazole-1-acetato-κ3N4:O,O′)]. Acta Crystallogr. 2008, C64, m26–m29. [Google Scholar]
- Keene, T.D.; Deng, Y.H.; Li, F.G.; Ding, Y.F.; Wu, B.; Liu, S.X.; Ambrus, C.; Waldmann, O.; Decurtins, S.; Yang, X.J. Magnetostructural investigations into an S = 1/2 sheet and a tetranuclear butterfly cluster. Inorg. Chim. Acta 2009, 362, 2265–2269. [Google Scholar] [CrossRef]
- Aromi, G.; Roubeau, O.; Helliwell, M.; Teat, S.J.; Winpenny, R.E.P. Novel topologies in NiII cluster chemistry: Incorporation of alkaline-earth metals in the new [NiII6MgII2] and [NiII8MII](M = Sr, Ba) cages. Dalton Trans. 2003, 3436–3442. [Google Scholar] [CrossRef]
- Liu, Y.L.; Kravtsov, V.; Walsh, R.D.; Poddar, P.; Srikanth, H.; Eddaoudi, M. Directed assembly of metal–organic cubes from deliberately predesigned molecular building blocks. Chem. Commun. 2004, 2806–2807. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.Q.; Jiang, L.; Senoh, H.; Takeichi, N.; Xu, Q. Rational assembly of a 3D metal–organic framework for gas adsorption with predesigned cubic building blocks and 1D open channels. Chem. Commun. 2005, 3526–3528. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zou, R.Q.; Zhong, R.Q.; Kachi-Terajima, C.; Takamizawa, S. Cubic metal−organic polyhedrons of Nickel(II) imidazole dicarboxylate depositing protons or alkali metal ions. Cryst. Growth Des. 2008, 8, 2458–2463. [Google Scholar] [CrossRef]
- Allen, F. The Cambridge Structure Database: A quarter of a million crystal structures and rising. Acta Crystallogr. 2002, B58, 380–388. [Google Scholar] [CrossRef]
- Allen, F.; Motherwell, W.D.S. Applications of the Cambridge Structural Database in organic and crystal chemistry. Acta Crystallogr. 2002, B58, 407–422. [Google Scholar] [CrossRef]
- Zorkii, P.M.; Oleinikov, P.N. Crystal-chemical classes of “Cambridge” crystal structures: Statistical analysis of topology. J. Struct. Chem. 2001, 42, 24–31. [Google Scholar] [CrossRef]
- Blatov, V.A. Multipurpose crystallochemical analysis with the program package TOPOS. IUCr CompComm Newsletter 2006, 7, 4–38. [Google Scholar]
- Peresypkina, E.V.; Blatov, V.A. Topology of molecular packings in organic crystals. Acta Crystallogr 2000, B56, 1035–1045. [Google Scholar]
- Wells, A.F. Further studies of three-dimensional nets; Monograph. 8, American Crystallographic Association, Polycrystal Book Service: Pittsburgh, PA, USA, 1979. [Google Scholar]
- Smith, J.V. Enumeration of 4-connected 3-dimensional nets and classification of framework silicates, II. Perpendicular and near-perpendicular linkages from 4.82, 3.122 and 4.6.12 nets. Amer. Mineral. 1978, 960–969. [Google Scholar]
- O’Keeffe, M.; Peskov, M.A.; Ramsden, S.J.; Yaghi, O.M. The reticular chemistry structure resource (RCSR) database of, and symbols for, crystal nets. Acc. Chem. Res. 2008, 41, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Colacio, E.; Dominguez-Vera, J.M.; Ghazi, M.; Kivekas, R.; Klinga, M.; Moreno, J.M. Singly anti-anti carboxylate-bridged zig-zag chain complexes from a carboxylate-containing tridentate schiff base ligand and M(hfac)2 [M = MnII, NiII, and CuII]: Synthesis, crystal structure, and magnetic properties. Eur. J. Inorg. Chem. 1999, 441–445. [Google Scholar] [CrossRef]
- Liu, Y.Y. catena-Poly[[triaquamanganese(II)]-μ-1,2,4-triazole-3,5-dicarboxylato-κ3O3:N4,O5]. Acta Crystallogr. 2007, E63, m1605. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Zhang, Y.W.; Zhang, G.; Cheng, L. catena-Poly[[triaquazinc(II)]-μ-1H-1,2,4-triazole-3,5-dicarboxylato]. Acta Crystallogr. 2008, E64, m1113. [Google Scholar] [CrossRef] [PubMed]
- King, P.; Clerac, R.; Anson, C.E.; Powell, A.K. The building block approach to extended solids: 3,5-pyrazoledicarboxylate coordination compounds of increasing dimensionality. Dalton Trans. 2004, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Santillan, G.A.; Carrano, C.J. Cobalt, Zinc, and Nickel complexes of a diatopic heteroscorpionate ligand: building blocks for coordination polymers. Inorg. Chem. 2008, 47, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Hammes, B.S.; Kieber-Emmons, M.T.; Letizia, J.A.; Shirin, Z.; Carrano, C.J.; Zakharov, L.N.; Rheingold, A.L. Synthesis and characterization of several zinc(II) complexes containing the bulky heteroscorpionate ligand bis(5-tert-butyl-3-methylpyrazol-2-yl)acetate: Relevance to the resting states of the zinc(II) enzymes thermolysin and carboxypeptidase A. Inorg. Chim. Acta 2003, 346, 227–238. [Google Scholar] [CrossRef]
- Dou, Q.Q.; He, Y.K.; Zhang, L.T.; Han, Z.B. catena-Poly[4,4′-bipyridinium [bis(μ3-pyrazole-3,5-dicarboxylato-κ5O5,N1:N2,O3:O3)dicopper(II)]]. Acta Crystallogr. 2007, E63, m2908–m2909. [Google Scholar] [CrossRef]
- An, C.X.; Lu, Y.C.; Shang, Z.F.; Zhang, Z.H. Syntheses and crystal structures of the metal complexes based on pyrazolecarboxylic acid ligands. Inorg. Chim. Acta 2008, 361, 2721–2730. [Google Scholar] [CrossRef]
- Tian, J.L.; Yan, S.P.; Liao, D.Z.; Jiang, Z.H.; Cheng, P. Syntheses, structures and properties of two one-dimensional chain complexes: [Mn(Hpdc)(H2O)2]n and [Cu2(Hpdc)2][4,4′-dpdo] (Hpdc=3,5-pyrazoledicarboxylic acid group, dpdo=4,4′-dipyridyl-N,N′-dioxide hydrate). Inorg. Chem. Commun. 2003, 6, 1025–1029. [Google Scholar] [CrossRef]
- Branzea, D.G.; Guerri, A.; Fabelo, O.; Ruiz-Perez, C.; Chamoreau, L.-M.; Sangregorio, C.; Caneschi, A.; Andruh, M. Heterobinuclear complexes as tectons in designing coordination polymers. Cryst. Growth Des. 2008, 8, 941–949. [Google Scholar] [CrossRef]
- Gu, C.S.; Gao, S.; Huo, L.H.; Zhao, H.; Zhao, J.G. catena-Poly[[(1,10-phenanthroline-κ2N,N′)copper(II)]-μ-4-carboxyimidazole-5-carboxylato(2-)-κ4N,O:N′,O′]. Acta Crystallogr. 2004, E60, m1852–m1854. [Google Scholar] [CrossRef]
- Wang, X.; Qin, C.; Wang, E.; Xu, L. New one-dimensional imidazole-bridged cadmium(II) coordination polymers-syntheses, crystal structures and photoluminescence. J. Mol. Struct. 2005, 749, 45–50. [Google Scholar] [CrossRef]
- Mijangos, E.; Costa, J.S.; Roubeau, O.; Teat, S.J.; Gamez, P.; Reedijk, J.; Gasque, L. Self-assembly of an infinite Copper(II) chiral metallohelicate. Cryst. Growth Des. 2008, 8, 3187–3192. [Google Scholar] [CrossRef]
- Hao, L.J.; Bao, Z.M.; Yu, T.L. catena-Poly[[(2,2-bipyridine)cobalt(II)]-μ-imidazole-4,5-dicarboxylato]. Acta Crystallogr. 2007, E63, m1871. [Google Scholar] [CrossRef]
- Chen, H.M.; Yang, S.P.; Zhang, F.; Yu, X.B. Synthesis, crystal structure and properties of aquacopper(II) N-[(1-Methylimidazole-2-yl) methylene]-β-alaninate hexafluoraphosphate and copper(II)[N-(1-methylimidaz ole-2-yl) methyl-β-alanine superchlorate. Synth. React. Inorg. Met. Org. Chem. 2003, 33, 1787–1800. [Google Scholar] [CrossRef]
- Gao, S.; Liu, J.W.; Huo, L.H. catena-Poly[[aquacadmium(II)]bis(μ-4,5-diphenyl-1H-imidazole-1-acetate)- κ3N:O,O′; κ3O,O′:N]. Acta Crystallogr. 2005, E61, m1012. [Google Scholar] [CrossRef]
- Long, L.S.; Yang, S.P.; Tong, Y.X.; Mao, Z.W.; Chen, X.M.; Ji, L.N. Synthesis, crystal structures and properties of copper(II) complexes of Schiff base derivatives containing imidazole and β-alanine groups. J. Chem. Soc. Dalton Trans. 1999, 1999–2004. [Google Scholar] [CrossRef]
- Landaverry, Y.R.; White, K.N.; Olmstead, M.M.; Einarsdottir, O.; Konopelski, J.P. Cytochrome c oxidase active site mimics: New ligands for copper and an unexpected oxidative c-c bond formation. Heterocycles 2006, 70, 147–152. [Google Scholar]
- Deng, Q.J.; Zeng, M.H.; Liang, H.; Ng, S.W.; Huang, K.L. catena-Poly[[[diaquamanganese(II)]bis(μ-1H-benzimidazole-5-carboxylato)-κ2N3:O; κ2O:N3] dihydrate]. Acta Crystallogr. 2006, E62, m1293–m1295. [Google Scholar] [CrossRef]
- Wang, L.; Cai, J.W.; Mao, Z.W.; Feng, X.L.; Huang, J.W. Dinickel complexes bridged by unusual (N,O,O′)-coordinated α-amino acids: syntheses, structural characterization and magnetic properties. Transit. Metal Chem. 2004, 29, 411–418. [Google Scholar] [CrossRef]
- Gao, S.; Gu, C.S.; Huo, L.H.; Zhao, H.; Zhao, J.G. catena-Poly[[(1,10-phenanthroline-κ2N,N′)cadmium(II)]-μ-imidazole-4,5-dicarboxylato-κ4N,O:N,O′]. Acta Crystallogr. 2004, E60, m1672–m1674. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Y.; Liu, P.; Wang, J.; Huang, M.H. Cadmium(II) and cobalt(II) complexes generated from benzimidazole-5-carboxylate: Self-assembly by hydrogen bonding and π–π interactions. J. Solid State Chem. 2005, 178, 2306–2312. [Google Scholar] [CrossRef]
- Mahata, P.; Natarajan, S. Pyridine- and imidazoledicarboxylates of zinc: Hydrothermal synthesis, structure, and properties. Eur. J. Inorg. Chem. 2005, 2156–2163. [Google Scholar] [CrossRef]
- Colacio, E.; Ghazi, M.; Kivekas, R.; Moreno, J.M. Helical-chain copper(II) complexes and a cyclic tetranuclear copper(II) complex with single syn−anti carboxylate bridges and ferromagnetic exchange interactions. Inorg.Chem. 2000, 39, 2882–2890. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.H.; Zhou, Y.L.; Ng, S.W. catena-Poly[[diaqua[(Z)-3-(1H-benzimidazol-2-yl)prop-2-enoato-κ2N,O]cobalt(II)]-μ-(Z)-3-(1H-benzimidazol-2-yl)prop-2-enoato-κ2O:O′]. Acta Crystallogr. 2006, E62, m2099–m2100. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, Y.A.; Okamura, T.; Zou, Z.H.; Ueyama, N.; Sun, W.Y. Synthesis and crystal structure of a one-dimensional coordination polymer of nickel(II) with 4′-(imidazol-1-ylmethyl)benzoate anion. Inorg. Chem. Commun. 2001, 4, 501–503. [Google Scholar] [CrossRef]
- Qin, C.; Wang, E.B. catena-Poly[[aqua(4,4′-bipyridine-κN)manganese(II)]-μ-imidazole-4,5-dicarboxylato-κ4N3,O4:O4′,O5]. Acta Crystallogr. 2007, E63, m2876. [Google Scholar] [CrossRef]
- Fang, R.Q.; Zhang, X.M. Diversity of coordination architecture of metal 4,5-dicarboxyimidazole. Inorg. Chem. 2006, 45, 4801–4810. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Q.; Zhang, J.; Yang, G.Y. catena-Poly[[diaquacadmium(II)]-μ-5-carboxyimidazole-4-carboxyl-ato-κ4N1,O5:O4,N3]. Acta Crystallogr. 2004, C60, m590–m591. [Google Scholar]
- Yao, Y.L.; Che, Y.X.; Zheng, J.M. Structural and fluorescent characterizations of one-and two-dimensional Cd(II)metal-organic frameworks. Inorg. Chem. Commun. 2008, 11, 883–885. [Google Scholar] [CrossRef]
- Guo, Z.G.; Cao, R.; Li, X.J.; Yuan, D.Q.; Bi, W.H.; Zhu, X.D.; Li, Y.F. A Series of cadmium(II) coordination polymers synthesized at different pH. Eur. J. Inorg. Chem. 2007, 5, 742–748. [Google Scholar] [CrossRef]
- Bai, Y.L.; Tao, J.; Huang, R.B.; Zheng, L.S. A three-dimensional supramolecular network built with the zigzag chain complex bis(5-carboxy-1H-imidazole-4-carboxylato)copper(II). Acta Crystallogr. 2005, C61, m98–m100. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.F. catena-Poly[[aqua(μ-5-carboxyimidazole-4-carboxylato-κ4N3,O4:N1,O5)zinc(II)] hemi-hydrate]. Acta Crystallogr. 2006, E62, m2039–m2040. [Google Scholar]
- Hao, L.J.; Yu, T.L. catena-Poly[[(2,2′-bipyridine)nickel(II)]-μ-imidazole-4,5-dicarboxylato]. Acta Crystallogr. 2007, E63, m2374. [Google Scholar] [CrossRef]
- Bruijnincx, P.C.A.; Lutz, M.; den Breejen, J.P.; Spek, A.L.; van Koten, G.; Gebbink, R.J.M.K. Zinc complexes of the biomimetic N,N,O ligand family of substituted 3,3-bis(1-alkylimidazol-2-yl)propionates: The formation of oxalate from pyruvate. J. Biol. Inorg. Chem. 2007, 12, 1181–1196. [Google Scholar] [CrossRef] [PubMed]
- Drozdzewski, P.; Pawlak, B.; Glowiak, T. Unusual coordination behavior of imidazole-4-acetic acid. Synthesis, crystal structure and vibrational studies of one-dimensional co-ordination polymer of zinc(II) with two different ligand forms. Polyhedron 2002, 21, 2819–2825. [Google Scholar] [CrossRef]
- Li, X.M.; Dong, Y.H.; Wang, Q.W.; Liu, B. catena-Poly[[(2,2′-bipyridine-κ2N,N′)zinc(II)]-μ-imidazole-4,5-dicarboxylato-κ4N1,O5:N3,O4]. Acta Crystallogr. 2007, E63, m1274–m1276. [Google Scholar] [CrossRef]
- Li, Z.F.; Wang, S.W.; Zhang, Q.; Yu, X.J. catena-Poly[[(2,2′-bipyridine-κ2N,N′)iron(II)]-μ-5-carboxy-4-carboxylatoimidazol-1-ido-κ4N3,O4:N1,O5]. Acta Crystallogr. 2007, E63, m2445. [Google Scholar] [CrossRef]
- Akhriff, Y.; Server-Carrio, J.; Sancho, A.; Garcia-Lozano, J.; Escriva, E.; Soto, L. Two polymeric compounds built from mononuclear and tetrameric squarate−copper(II) complexes by deprotonation of 3,3-Bis(2-imidazolyl)propionic acid (HBIP). Synthesis, crystal structure, and magnetic characterization of [Cu(HBIP)(BIP)](C4O4)1/2·2H2O and [{Cu(BIP)(OH2)}4(μ-C4O4)](ClO4)2·4H2O. Inorg. Chem. 2001, 40, 6832–6840. [Google Scholar] [PubMed]
- Liu, G.F.; Ren, Z.G.; Chen, Y.; Liu, D.; Li, H.X.; Zhang, Y.; Lang, J.P. Solvothermal synthesis, structure and luminescent properties of a new 3D coordination polymer [K2Cd(Htda)2]n (Htda = 1,2,3-triazole-4,5-dicarboxylate). Inorg. Chem. Commun. 2008, 11, 225–229. [Google Scholar] [CrossRef]
- Chandrasekhar, V.; Thilagar, P.; Senapati, T. Transition metal-assisted hydrolysis of pyrazole-appended organooxotin carboxylates accompanied by ligand transfer. Eur. J. Inorg. Chem. 2007, 1004–1009. [Google Scholar] [CrossRef]
- Abdeljalil, E.F.; Najib, B.L.; Abdelali, K.; Bali, B.E.; Bolte, M. catena-Poly[[[(3,5-dimethyl-1H-pyrazole-κN2) copper(II)]-μ-[(3,5-dimethyl-1H-pyrazol-1-yl)methylamino]acetato] nitrate monohydrate]. Acta Crystallogr. 2006, E62, m551–m552. [Google Scholar] [CrossRef]
- Gao, S.; Liu, J.W.; Huo, L.H.; Zhao, H. catena-Poly[[(2,2′-bipyridine-2N,N′)cadmium(II)]--5-carboxyimidazole-4-carboxylato-4N3,O4:N1,O5]. Acta Crystallogr. 2004, E60, m1728–m1729. [Google Scholar]
- Cheng, A.L.; Liu, N.; Zhang, J.Y.; Gao, E.Q. Assembling the cage-based metal−organic network from a cubic metalloligand. Inorg. Chem. 2007, 46, 1034–1035. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Zheng, S.L.; Yu, X.L.; Chen, X.M. Syntheses, structures, and photoluminescent properties of three silver(I) cluster-based coordination polymers with heteroaryldicarboxylate. Cryst. Growth Des. 2004, 4, 831–836. [Google Scholar] [CrossRef]
- Han, Z.B.; Ma, Y. Poly[di-μ2-aqua-μ-pyrazole-3,5-dicarboxylato-copper(II)]. Acta Crystallogr. 2006, E62, m2236–m2237. [Google Scholar] [CrossRef]
- Chen, H.; Ma, C.B.; Xiang, S.C.; Hu, M.Q.; Si, Y.T.; Chen, C.N.; Liu, Q.T. Synthesis and characterization of vanadium(III) and vanadium(IV) polymers containing 3,5-pyrazoledicarboxylato. J. Coord. Chem. 2008, 61, 3556–3567. [Google Scholar] [CrossRef]
- Kasuga, N.C.; Tsuruta, S.; Amano, A.; Nomiya, K. Poly[(μ3-N-acetyl-L-histidinato-κ4N,O:O:O′)silver(I)]. Acta Crystallogr. 2007, E63, m2440. [Google Scholar] [CrossRef]
- Akhriff, Y.; Server-Carrio, J.; Sancho, A.; Garcia-Lozano, J.; Escriva, E.; Folgado, J.V.; Soto, L. Synthesis, crystal structure, and magnetic properties of oxalato−copper(II) complexes with 3,3-bis(2-imidazolyl)propionic acid, an imidazole−carboxylate polyfunctional ligand: From mononuclear entities to ladder-like chains. Inorg. Chem. 1999, 38, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Gong, Y.Q.; Yuan, D.Q.; Hong, M.C. Luminescent 2D supramolecular network constructed from tubular coordination polymer based on H-bonding and pi-pi interactions. J. Mol. Struct. 2006, 789, 128–132. [Google Scholar] [CrossRef]
- Meng, W.W.; Chen, J.X. Synthesis and crystal structures of new nickel(Ⅱ) and manganese(Ⅱ) coordination polymers containing 5-benzimidazolecarboxylate ligand. Chin. J. Inorg. Chem. 2008, 24, 1610–1615. [Google Scholar]
- Xu, K.; Yu, L.P. catena-Poly[[di-μ-aqua-bis[aquacobalt(II)]]-bis(μ3-1H-benzimidazole-5,6-dicarboxylato). Acta Crystallogr. 2009, E65, m295. [Google Scholar] [CrossRef] [PubMed]
- van Koningsbruggen, P.J.; van Hal, J.W.; Muller, E.; de Graaff, V.; G.Haasnoot, J.; Reedijk, J. A novel type of twisted antiparallel double-chain structure with stacking between the two strands. Structure, synthesis and magnetic properties of [{[Cu3L2(dien)(H2O)2]·3H2O}∞][L = 1H-1,2,4-triazole-3,5-dicarboxylate(3–), dien = 3-Azapentane-1,5-diamine]. J. Chem. Soc. Dalton Trans. 1993, 1371–1376. [Google Scholar] [CrossRef]
- Wang, J.J.; Zhang, B.; Shu, H.M.; Du, C.Q.; Hu, H.M. A two-dimensional coordination polymer containing linear trinuclear copper (II) clusters. Acta Crystallogr. 2007, E63, m2190. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, R.H.; Lou, B.Y.; Han, L.; Hong, M.C. Poly[iron(II)-di-μ-imidazole-4,5-dicarboxylato-κ3N3,O4:O5]. Acta Crystallogr. 2004, C60, m296–m298. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.G.; Yuan, D.Q.; Bi, W.H.; Li, X.J.; Cao, R. A novel antiferromagnetic nickel coordination framework with 1-H-benzimidazole-5-carboxylic acid. J. Mol. Struct. 2006, 782, 106–109. [Google Scholar] [CrossRef]
- Wang, Y.T.; Tang, G.M.; Qin, D.W. Metal-controlled assembly tuning coordination polymers with flexible 2-(1H-imidazole-1-yl)acetic acid (Hima). Aust. J. Chem. 2006, 59, 647–652. [Google Scholar] [CrossRef]
- Deng, Q.J.; Zeng, M.H.; Liang, H.; Huang, K.L. Hydrothermal synthesis and crystal structure of a new 2D layered cadmium(II) coordination polymer: [Cd(bimc)2]n (bimc = 1H-Benzimidazole-5-carboxylate). Chin. J. Struct. Chem. 2006, 25, 975–978. [Google Scholar]
- Zhang, J.Z.; Cao, W.R.; Pan, J.X.; Chen, Q.W. A novel two-dimensional square grid cobalt complex: Synthesis, structure, luminescent and magnetic properties. Inorg. Chem. Commun. 2007, 10, 1360–1364. [Google Scholar] [CrossRef]
- Guo, Z.G.; Li, X.J.; Gao, S.Y.; Li, Y.F.; Cao, R. A new three-dimensional supramolecular network, [Cd(Hbic)2(H2O)]·(4,4′-bpy) ·H2O (H2bic=1-H-benzimidazole-5carboxylic acid; 4,4-bpy=4,4′-bipyridine): Synthesis, crystal structure and luminescence property. J. Mol. Struct. 2007, 846, 123–127. [Google Scholar] [CrossRef]
- Wei, Y.Q.; Yu, Y.F.; Wu, K.C. Highly stable five-coordinated Mn(II) polymer [Mn(Hbidc)]n (Hbidc=1H-Benzimidazole-5,6-dicarboxylate): Crystal structure, antiferromegnetic property, and strong long-lived luminescence. Cryst. Growth Des. 2008, 8, 2087–2089. [Google Scholar] [CrossRef]
- Yao, Y.L.; Che, Y.X.; Zheng, J.M. The coordination chemistry of benzimidazole-5,6-dicarboxylic acid with Mn(II), Ni(II), and Ln(III) complexes (Ln = Tb, Ho, Er, Lu). Cryst. Growth Des. 2008, 8, 2299–2306. [Google Scholar] [CrossRef]
- Martinez-Lorente, M.-A.; Tuchagues, J.-P.; Petrouleas, V.; Savariault, J.-M.; Poinsot, R.; Drillon, M. Bis(4-imidazoleacetato)iron.bis(methanol): a 2D antiferromagnetic iron(II) system exhibiting 3D long-range ordering with a net magnetic moment at 15 K. Inorg. Chem. 1991, 30, 3587–3589. [Google Scholar] [CrossRef]
- Sun, W.Y.; Zhang, Y.A.; Okamura, T.; Ye, N.; Ueyama, N. Synthesis and crystal structure of a new two-dimensional coordination polymer, {[CoII(imbz)2]·H2O}n [imbz- = 4-(Imidazol-1-ylmethyl)benzoate anion]. Chem. Lett. 2000, 1222–1223. [Google Scholar] [CrossRef]
- Zhao, X.X.; Ma, J.P.; Dong, Y.B.; Huang, R.Q.; Lai, T.S. Construction of metal−organic frameworks (M = Cd(II), Co(II), Zn(II), and Cu(II)) based on semirigid oxadiazole bridging ligands by solution and hydrothermal reactions. Cryst. Growth Des. 2007, 7, 1058–1068. [Google Scholar] [CrossRef]
- Ding, D.G.; Xu, H.; Fan, Y.T.; Hou, H.W. Anion-dependent assemblies of two unprecedented copper(II) polymers with four-fold screw axes and trapped sodium chains. Inorg. Chem. Commun. 2008, 11, 1280–1283. [Google Scholar] [CrossRef]
- Wang, D.E.; Wang, F.; Meng, X.G.; Ding, Y.; Wen, L.L.; Li, D.F.; Lan, S.M. Syntheses, crystal structures and luminescent properties of three inorganic-organic hybrid frameworks constructed from 4,5-imidazoledicarboxylate. Z. Anorg. Allg. Chem. 2008, 634, 2643–2648. [Google Scholar] [CrossRef]
- Gao, S.; Huo, L.H.; Zhao, H.; Liu, J.W. Poly[aquamanganese(II)-μ3-1H-imidazole-4,5-dicarboxylato]. Acta Crystallogr. 2005, E61, m155–m157. [Google Scholar] [CrossRef]
- Lu, W.G.; Gu, J.Z.; Jiang, L.; Tan, M.Y.; Lu, T.B. Achiral and chiral coordination polymers containing helical chains: the chirality transfer between helical chains. Cryst. Growth Des. 2008, 8, 192–199. [Google Scholar] [CrossRef]
- Lu, J.Y.; Ge, Z.H. Synthesis and structures of two new metal–organic polymers containing imidazoldicarboxylate ligands for hydrogen bonding networks, one with a covalent pleated sheet conformation. Inorg. Chim. Acta 2005, 358, 828–833. [Google Scholar] [CrossRef]
- Chen, L.; Bu, X.H. Histidine-controlled two-dimensional assembly of zinc phosphite four-ring units. Chem. Mater. 2006, 18, 1857–1860. [Google Scholar] [CrossRef]
- Shi, W.; Chen, X.Y.; Xu, N.; Song, H.B.; Zhao, B.; Cheng, P.; Liao, D.Z.; Yan, S.P. Synthesis, crystal structures, and magnetic properties of 2D manganese(II) and 1D gadolinium(III) coordination polymers with 1H-1,2,3-triazole-4,5-dicarboxylic acid. Eur. J. Inorg. Chem. 2006, 4931–4937. [Google Scholar] [CrossRef]
- Yue, Y.F.; Liang, J.; Gao, E.Q.; Fang, C.J.; Yan, Z.G.; Yan, C.H. Supramolecular engineering of a 2D Kagomé lattice: Synthesis, structures, and magnetic properties. Inorg. Chem. 2008, 47, 6115–6117. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Ma, J.P.; Liu, L.L.; Huang, R.Q.; Dong, Y.B. A novel two-dimensional framework based on unprecedented cadmium(II) chains. Acta Crystallogr. 2009, C65, m66–m68. [Google Scholar]
- Zhang, X.F.; Gao, S.; Huo, L.H.; Zhao, H.; Zhao, J.G. Synthesis and crystal structure of 2D coordination polymer [Mn(HIDC)(H2O)]n constructed by 1H-imidazole-4,5-dicarboxylate ligand. Chin. J. Inorg. Chem. 2006, 22, 139–141. [Google Scholar]
- Zhang, X.F.; Gao, S.; Huo, L.H.; Zhao, H. Poly[[aquazinc(II)]-μ3-imidazole-4,5-dicarboxylato]. Acta Crystallogr. 2007, E63, m299–m301. [Google Scholar] [CrossRef]
- Zhang, X.F.; Gao, S.; Huo, L.H.; Zhao, H. A two-dimensional cadmium(II) coordination polymer with unusual 4.82 topology: poly[aqua(μ3-1H-imidazole-4,5-dicarboxylato)cadmium(II)]. Acta Crystallogr. 2007, E63, m1314–m1316. [Google Scholar]
- Wang, Y.T.; Tang, G.M.; Wu, Y.; Qin, X.Y.; Qin, D.W. Metal-controlled assembly tuning the topology and dimensionality of coordination polymers of Ag(I), Cd(II) and Zn(II) with the flexible 2-(1H-imidazole-1-yl)acetic acid (Hima). J. Mol. Struct. 2007, 831, 61–68. [Google Scholar] [CrossRef]
- Wu, C.D.; Ayyappan, P.; Evans, O.R.; Lin, W.B. Synthesis and x-ray structures of cadmium coordination polymers based on new pyridine−carboxylate and imidazole−carboxylate linkers. Cryst. Growth Des. 2007, 7, 1690–1694. [Google Scholar] [CrossRef]
- Hu, T.L.; Du, W.P.; Hu, B.W.; Li, J.R.; Bu, X.H.; Cao, R. Novel Ag(I) complexes with azole heterocycle ligands bearing acetic acid group: synthesis, characterization and crystal structures. CrystEngComm. 2008, 10, 1037–1043. [Google Scholar] [CrossRef]
- Li, X.Z.; Qu, Z.R. Poly[aqua[μ3-5-(2-carboxylatophenyl)-1H-tetrazolato]zinc(II)]. Acta Crystallogr. 2008, E64, m808. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Wu, B.Z.; Qu, Z.R. Poly[diaqua-1κ2O-bis[μ3-2-(1H-tetrazol-5-yl)benzoate-(2)]dicadmium(II)]. Acta Crystallogr. 2008, E64, m1008. [Google Scholar]
- Frisch, M.; Cahill, C.L. Syntheses, structures and fluorescent properties of two novel coordination polymers in the U–Cu–H3pdc system. Dalton Trans. 2005, 1518–1523. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.Q.; Wang, M.S.; Li, Y.; Wu, A.Q.; Zheng, F.K.; Chen, Q.Y.; Guo, G.C.; Huang, J.S. Unprecedented (3,10)-connected 2-D metal-organic framework constructed from octanuclear cobalt(II) clusters and a new bifunctional ligand. Inorg. Chem. 2007, 46, 6852–6854. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.X.; Zeng, M.H.; Zou, H.H.; Zhou, Y.L.; Liang, H. A unique 2D framework containing linear trimeric cobalt(II) of mixed Td–Oh–Td geometries linked by two different single-carboxylate-aromatic amine ligands: structure and magnetic properties. Dalton Trans. 2008, 2428–2432. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.D.; Cheng, J.W.; Du, S.W. Five d10 3D metal−organic frameworks constructed from aromatic polycarboxylate acids and flexible imidazole-based ligands. Cryst. Growth Des. 2008, 8, 3345–3353. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wu, H.C.; Lin, H.M.; Hou, W.H.; Lu, K.L. Crystal engineering toward intersecting channels in a interpenetrated diamondoid network based on a net-to-net H-bonding interaction. Chem. Commun. 2003, 60–61. [Google Scholar] [CrossRef]
- Zou, R.Q.; Zhong, R.Q.; Jiang, L.; Yamada, Y.; Kuriyama, N.; Xu, Q. Tuning the formation of cadmium(II) urocanate frameworks by control of reaction conditions: crystal structure, properties, and theoretical investigation. Chem. Asian J. 2006, 1, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.Q.; Yamada, Y.; Xu, Q. Strong fluorescent emission of a new fourfold-interpenetrated diamondoid metal-organic framework of zinc(II) urocanate with one-dimensional open channels. Microporous Mesoporous Mater. 2006, 91, 233–237. [Google Scholar] [CrossRef]
- Pan, L.; Huang, X.Y.; Li, J. Assembly of new coordination frameworks in a pH-controlled medium: Syntheses, structures, and properties of 3∞[Cd(Hpdc)(H2O)] and 3∞[Cd3(pdc)2(H2O)2]. J. Solid State Chem. 2001, 152, 236–246. [Google Scholar] [CrossRef]
- Li, J.T.; Tao, J.; Huang, R.B.; Zhang, L.S. Poly[μ4-5-(3-carboxylatophenyl)-1H-tetrazolato-zinc(II)]. Acta Crystallogr. 2005, E61, m984–m985. [Google Scholar] [CrossRef]
- Du, M.; Zhang, Z.H.; Tang, L.F.; Wang, X.G.; Zhao, X.J.; Batten, S.R. Molecular tectonics of metal-organic frameworks (MOFs): A rational design strategy for unusual mixed-connected network topologies. Chem. Eur. J. 2007, 13, 2578–2586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Gao, S.; Huo, L.H.; Zhao, H. A three-dimensional porous cadmium(II) coordination polymer: poly[[(pyridine-κN)cadmium(II)]-μ3-imidazole-4,5-dicarboxylato-κ6N,O:N′,O′:O′,O′′]. Acta Crystallogr. 2006, E62, m3233–m3235. [Google Scholar] [CrossRef]
- Zhang, W.X.; Xue, W.; Lin, J.B.; Zheng, Y.Z.; Chen, X.M. 3D geometrically frustrated magnets assembled by transition metal ion and 1,2,3-triazole-4,5-dicarboxylate as triangular nodes. CrystEngComm. 2008, 10, 1770–1776. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.Z. A novel three-dimensional heterometallic coordination polymer: poly[[hexaaquabis[μ3-3,5-dicarboxylatopyrazolato-κ5O3,N2:N1,O5:O5′](μ2-oxalato-κ4O1,O2:O1′, O2′) copper(II)dierbium(III)] trihydrate]. Acta Crystallogr 2008, C64, m283–m285. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Yuan, D.Q.; Bi, W.H.; Li, X.; Li, X.J.; Li, F.; Cao, R. Syntheses and characterizations of two 3D cobalt−organic frameworks from 2D honeycomb building blocks. Cryst. Growth Des. 2005, 5, 1849–1855. [Google Scholar] [CrossRef]
- King, P.; Clérac, R.; Anson, C.E.; Coulon, C.; Powell, A.K. Antiferromagnetic three-dimensional order induced by carboxylate bridges in a two-dimensional network of [Cu3(dcp)2(H2O)4] trimers. Inorg.Chem. 2003, 42, 3492–3500. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.L.; Ye, L.H.; Liu, X.F.; Yuan, L.M.; Lu, X.L.; Jiang, J.X. Rapid synthesis of a novel cadmium imidazole-4,5-dicarboxylate metal-organic framework under microwave-assisted solvothermal condition. Inorg. Chem. Commun. 2008, 11, 1250–1252. [Google Scholar] [CrossRef]
- Sang, R.L.; Xu, L. Unprecedented helix-based microporous metal–organic frameworks constructed from a single ligand. Chem.Commun. 2008, 6143–6145. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Long, L.S.; Huang, R.B.; Zheng, L.S. A lanthanide-based metal–organic framework with a dynamic porous property. Dalton Trans. 2008, 4714–4716. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Hu, S.; Li, W.; Lam, C.K.; Zheng, Y.Z.; Tong, M.L. Rational design and control of the dimensions of channels in three-dimensional, porous metal-organic frameworks constructed with predesigned hexagonal layers and pillars. Eur. J. Inorg. Chem. 2006, 1931–1935. [Google Scholar] [CrossRef]
- Lu, W.G.; Jiang, L.; Feng, X.L.; Lu, T.B. Three 3D coordination polymers constructed by Cd(II) and Zn(II) with imidazole-4,5-dicarboxylate and 4,4′-bipyridyl building blocks. Cryst. Growth Des. 2006, 6, 564–571. [Google Scholar] [CrossRef]
- Zhang, M.B.; Chen, Y.M.; Zheng, S.T.; Yang, G.Y. A 3D manganese coordination polymer [Mn3(IMDC)2(H2O)4] constructed from [Mn2(IMDC)2(H2O)2] layers and [Mn(H2O)2] pillars (IMDC = 4,5-imidazoledicarboxylate). Eur. J. Inorg. Chem. 2006, 1423–1428. [Google Scholar] [CrossRef]
- Yao, Y.L.; Che, Y.X.; Zheng, J.M. A new eight-connected CsCl-type net using bicadmium cores as nodes. Inorg. Chem. Commun. 2008, 11, 1253–1255. [Google Scholar] [CrossRef]
- Zhong, R.Q.; Zou, R.Q.; Xu, Q. Microporous metal-organic framework zinc(II) imidazole- 4,5-dicarboxylate: Four-fold helical structure and strong fluorescent emission. Microporous Mesoporous Mater. 2007, 102, 122–127. [Google Scholar] [CrossRef]
- Lu, W.G.; Jiang, L.; Feng, X.L.; Lu, T.B. four 3d porous metal−organic frameworks with various layered and pillared motifs. Cryst. Growth Des. 2008, 8, 986–994. [Google Scholar] [CrossRef]
- Cahill, C.L.; de Lill, D.T.; Frisch, M. Homo- and heterometallic coordination polymers from the f elements. CrystEngComm 2007, 9, 15–26. [Google Scholar] [CrossRef]
- Gu, J.Z.; Lu, W.G.; Jiang, L.; Zhou, H.C.; Lu, T.B. 3D porous metal-organic framework exhibiting selective adsorption of water over organic solvents. Inorg. Chem. 2007, 46, 5835–5837. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, L.R.; Li, G.H; Huo, Q.S.; Liu, Y.L. Assembly of two 3-D metal–organic frameworks from Cd(II) and 4,5-imidazoledicarboxylic acid or 2-ethyl-4,5-imidazole-dicarboxylic acid. CrystEngComm 2008, 10, 1662–1666. [Google Scholar] [CrossRef]
- Pan, L.; Huang, X.Y.; Li, J.; Wu, Y.G.; Zheng, N.W. Novel single- and double-layer and three-dimensional structures of rare-earth metal coordination polymers: The effect of lanthanide contraction and acidity control in crystal structure formation. Angew. Chem., Int. Ed. 2000, 39, 527–530. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Yang, G.Y. Organic–inorganic hybrid materials constructed from inorganic lanthanide sulfate skeletons and organic 4,5-imidazoledicarboxylic acid. Dalton Trans. 2007, 3771–3781. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Q.; Zhang, J.; Yang, G.Y. A series of luminescent lanthanide–cadmium–organic frameworks with helical channels and tubes. Chem. Commun. 2006, 4700–4702. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available |



| CSD Refcode | Topology Demonstration | Vertex Symbol* | Nodal Connectivity | Node Types |
|---|---|---|---|---|
| UFETEF[143] |  | {43.63} | 4-c | uninodal |
| BIZVEM[144] GIZSIS[145] RANBAK[146] |  | {4.62}2{4.6.4.6} | 3, 4-c | 2-nodal |
| LILYIP[147] (Considering an octacobalt cluster as a single node) |  | {32.4}2{36.4.32.4} | 3,10-c | 2-nodal |
| JOCGIS[148] |  | {3.4.5.62.7}2{3.6.7}2{32.42.52.62.76.8}* | 3,4,6-c | 3-nodal |
| KOBHEP[40] |  | {43.6}{44}{43}{42,62} | 3,4,4,4-c | 4-nodal |
| KOBHAL[40] |  | {3.42.6}{32.43}{3.42}{32.4.62} | 3,4,5,5-c | 4-nodal |
| CSD Refcode | Topology Demonstration | Point Symbol (Schläfli Symbol) | Nodal Connectivity | Node Types |
|---|---|---|---|---|
| UFETAB[143] |  | {66} | 4,4-c | uninodal |
| POLDOK[129] POLDUQ[129] |  | {42.84} | 4-c | uninodal |
| MOFTIL[46] MOFTIL01[47] |  | {66} | 4,4-c | uninodal |
| ACUXAY[166] |  | {4.82}{4.85} | 3,4-c | binodal |
| BOHVEA[44] BOHVIE[44] |  | {4.82.103}{4.82} | 3,4-c | 2-nodal |
| XECBOX[165] |  | {103} | 3,3-c | uninodal |
| MEVBIZ[151] |  | {4.6.8}{4.62.8.102} | 3,4-c | 2-nodal |
| PEXSOB[93] PEXSOB01[167] |  | {4.62}{4.67.82} | 3,5-c | 2-nodal |
| QEYWOH[155] |  | {42.6}2{44.62.87.102} | 3,6-c | 2-nodal |
| YELYUK[92] |  | {42.84}{46.66.83}{48.62}2 | 4,5,6-c | 3-nodal |
| GIDKOU[168] |  | {42.52.72.84} {42.52.72}2{42.84} | 4,4,4,5-c | 3-nodal |
| UFARUP[169] |  | {42.52.72.84} {42.52.72}2{42.84} | 4,4,4,5,5-c | 3-nodal |
| UFASAW[169] |  | {4.5.63.7}4 {42.52.64.72}2{52.84} | 4,4,4,5,5-c | 3-nodal |
| DIXVUC[39] |  | {4.82}6{43}{46.64}3{812.123} | 3,3,5,6-c | 4-nodal |
| CETGEO[170] |  | {4.84.10}{4.92}{8.92}{93} | 3,3,3,4-c | 4-nodal |
| KOCWEF[41] |  | {3.4.62.72}{3.43.52.6.72.8}{32.44.52.62}{32.45.5.64.73} | 4,4,5,5,5,5,6,6-c | 4-nodal |
| WIJDAV[171] |  | {6.102}{6.8.10}2{8.102} | 3,3,3,3-c | 4-nodal |
| LIZWEX[49] |  | {4.6.82.102}6{42.6}6 {43}2{62.84}3 | 3,3,4,4-c | 4-nodal |
| XECBIR[165] |  | {4.62.82.9}2{4.82}2{42.52.6.72.83}{42.52.72}2 {42.6.83} | 3,4,4,4,5-c | 5-nodal |
| KOLWUE[172] |  | {4.62}{4.64.84.10}{4.64.8}{4.82}{63} | 3,3,3,4,5-c | 5-nodal |
| KEPMIB[173] |  | {4.6.83.10}{4.6.8}2{43.66.86}{43.83} | 3,3,4,4,6-c | 5-nodal |
| GIKBOS[174] |  | {4.6.8}{42.63.8}{43.62.8}{44.65.86}{45.64.8} | 3,4,4,5,6-c | 5-nodal |
| YELYOE[92] |  | {42.52.62}2{42.52.72.84} {42.54.84}{43.5.62.72.82}2 {47.5.62}2 | 4,5,5,5,5-c | 5-nodal |
| NEVHEC[175] NEVHIG[175] NEVHOM[175] NEVHUS[175] NEVJAA[175] NEVJEE[175] NEVJII[175] NEVJOO[175] NEVJUU[175] |  | {4.82}{4.85}{42.88}{46} | 3,4,4,5-c | 4-nodal |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Deng, Y.; Liu, H.; Yu, B.; Yao, M. Supramolecular Coordination Assemblies Constructed From Multifunctional Azole-Containing Carboxylic Acids. Molecules 2010, 15, 3478-3506. https://doi.org/10.3390/molecules15053478
Deng Y, Liu H, Yu B, Yao M. Supramolecular Coordination Assemblies Constructed From Multifunctional Azole-Containing Carboxylic Acids. Molecules. 2010; 15(5):3478-3506. https://doi.org/10.3390/molecules15053478
Chicago/Turabian StyleDeng, Yuheng, Hao Liu, Bo Yu, and Min Yao. 2010. "Supramolecular Coordination Assemblies Constructed From Multifunctional Azole-Containing Carboxylic Acids" Molecules 15, no. 5: 3478-3506. https://doi.org/10.3390/molecules15053478
APA StyleDeng, Y., Liu, H., Yu, B., & Yao, M. (2010). Supramolecular Coordination Assemblies Constructed From Multifunctional Azole-Containing Carboxylic Acids. Molecules, 15(5), 3478-3506. https://doi.org/10.3390/molecules15053478