The Cinnamon-Derived Dietary Factor Cinnamic Aldehyde Activates the Nrf2-Dependent Antioxidant Response in Human Epithelial Colon Cells
Abstract
:Abbreviations
| ARE | antioxidant response element |
| CA | trans-cinnamic aldehyde |
| CE | ethanolic cinnamon extract |
| GC-MS | gas chromatography-mass spectrometry |
| γ-GCS | γ-glutamylcysteine synthetase |
| GSH | glutathione |
| HO-1 | heme oxygenase 1 |
| Nrf2 | NF-E2-related factor 2 |
| ROS | reactive oxygen species |
| SDS-PAGE | sodium dodecylsulfate polyacrylamide gel electrophoresis |
| tBHQ | tert-butylhydroquinone |
1. Introduction
2. Results and Discussion
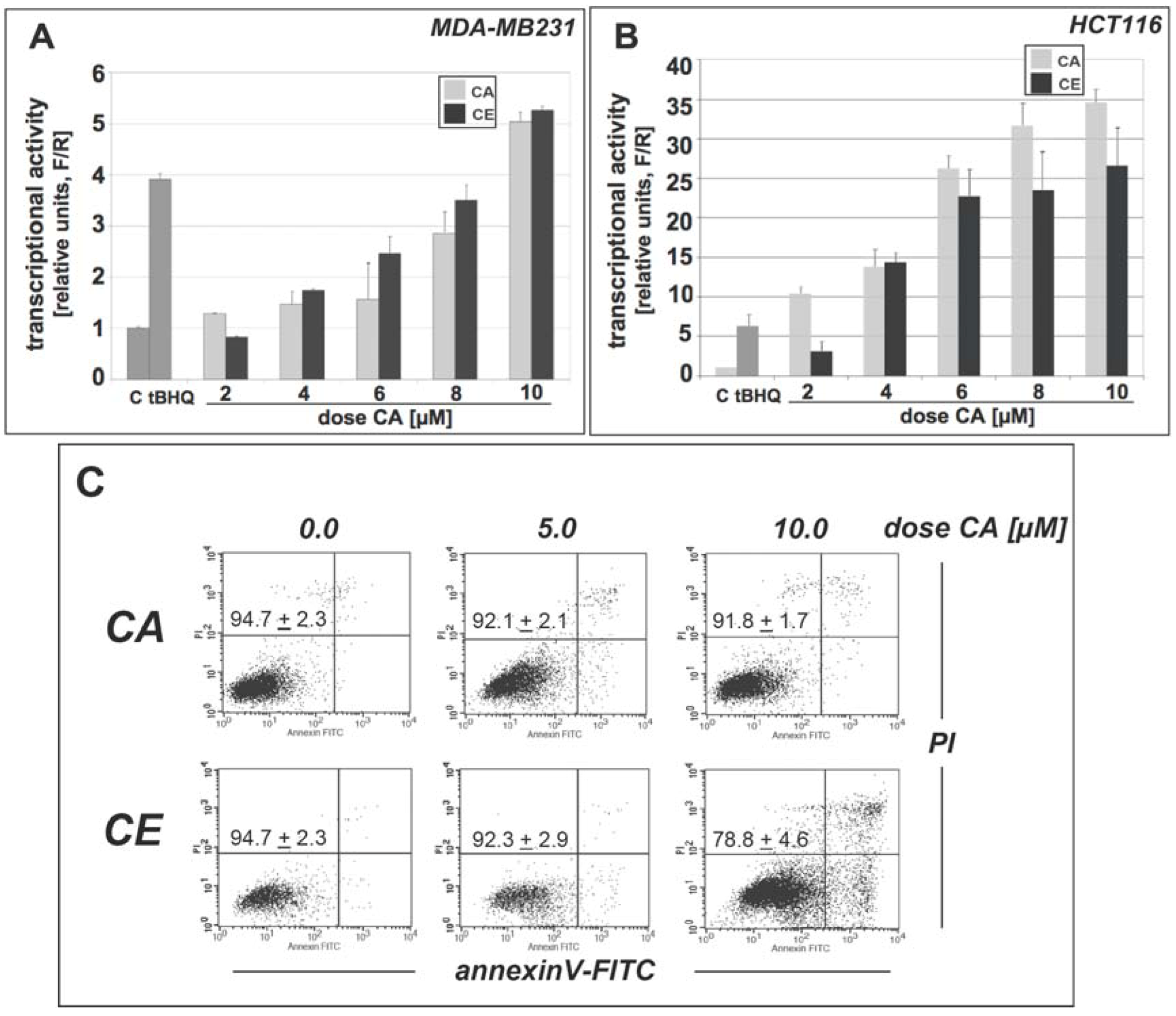
2.1. Cinnamic aldehyde and a total ethanolic extract of cinnamon powder, standardized for cinnamic aldehyde content, are equally potent inducers of Nrf2 transcriptional activity
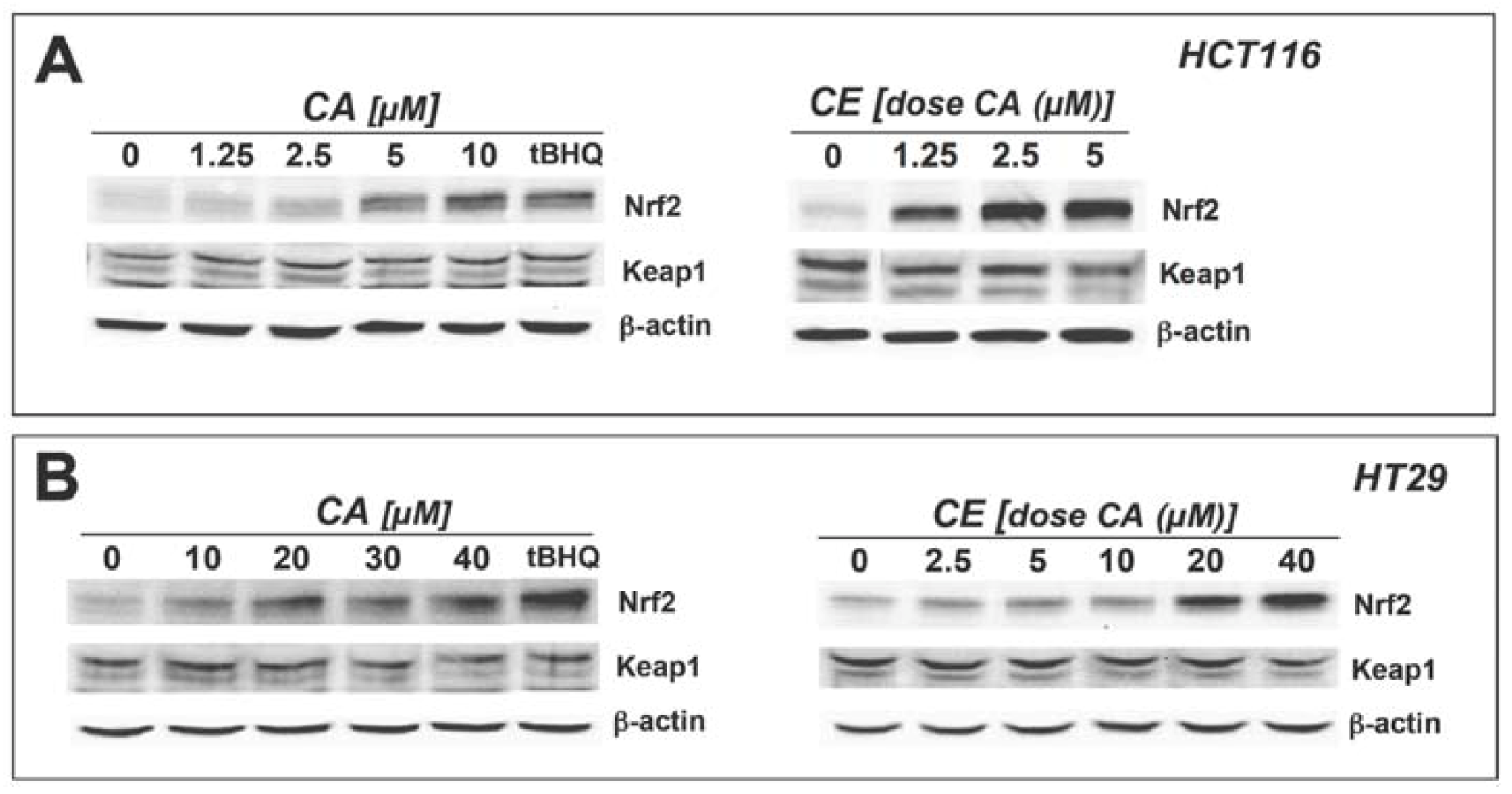
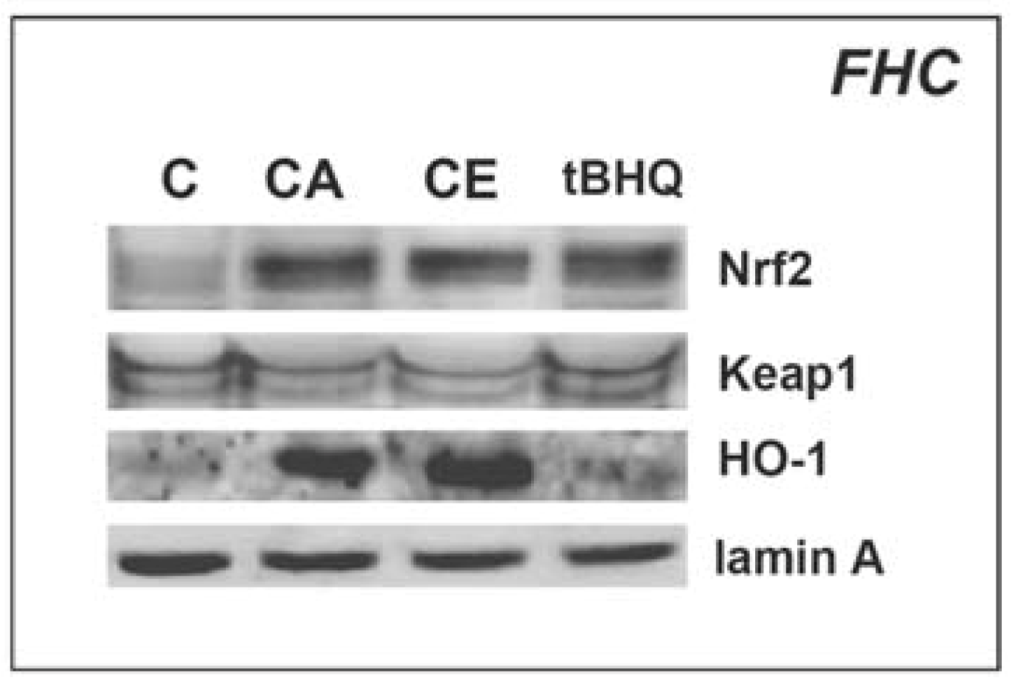
2.2. Cinnamic aldehyde and a total ethanolic extract of cinnamon powder upregulate protein levels of Nrf2 and Nrf2 downstream targets in cultured human colon cells

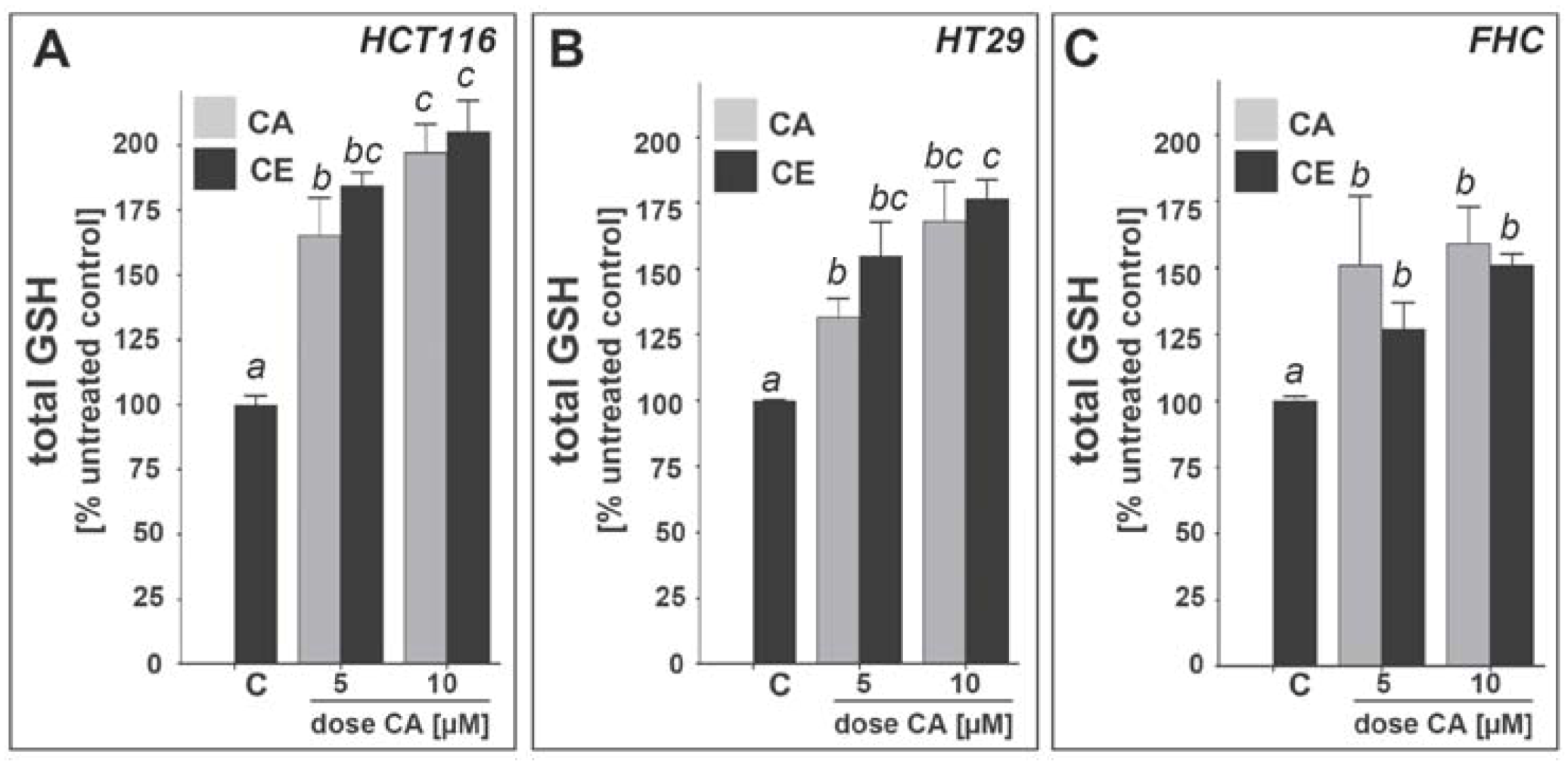
2.3. Cinnamic aldehyde and an ethanolic extract of cinnamon powder induce upregulation of cellular glutathione levels in HCT116, HT29, and FHC epithelial colon cells
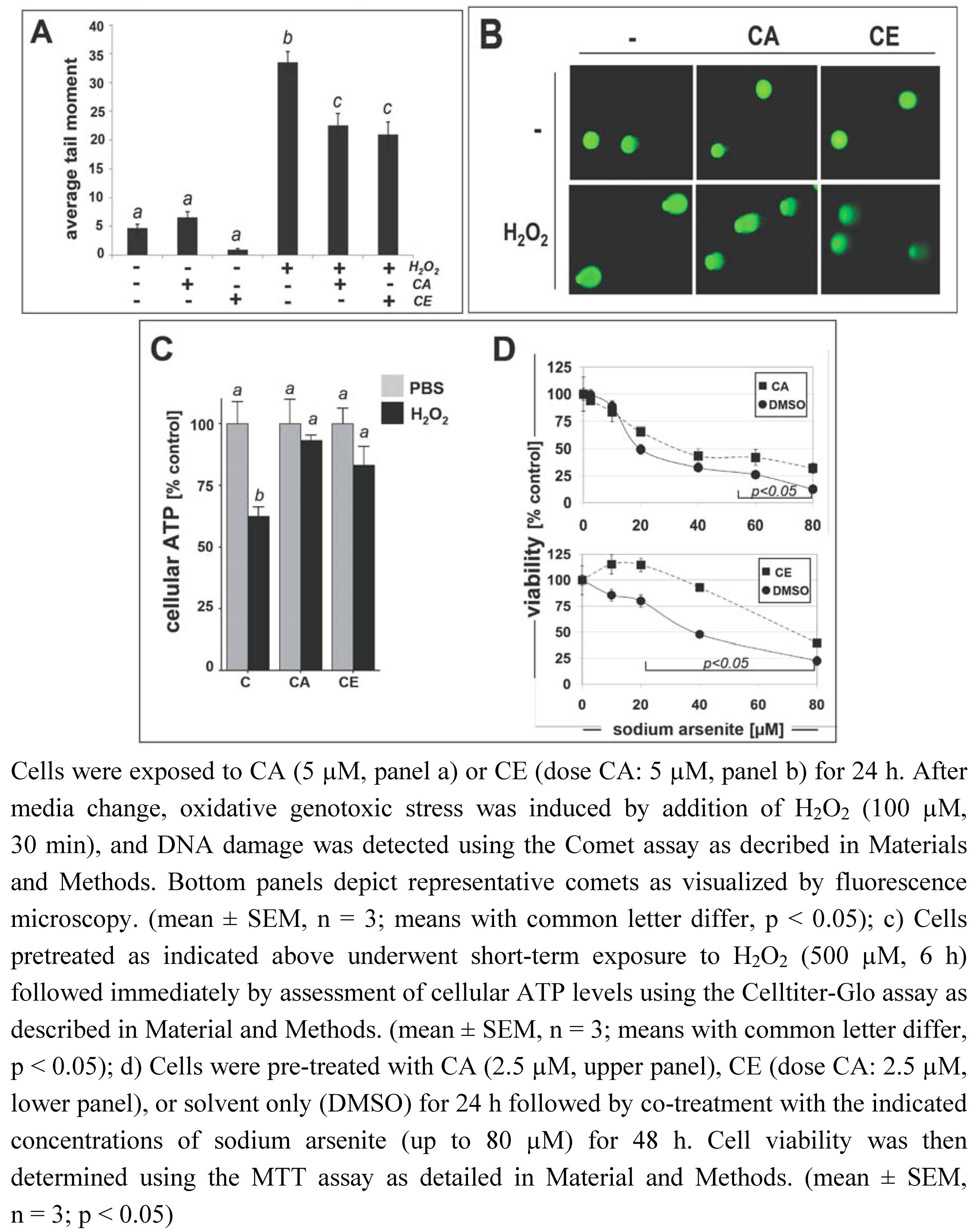
2.4. Protection of HCT116 colon cells against oxidative stress-induced genotoxicity and arsenic-induced oxidative insult
3. Experimental
3.1. Chemicals and dietary products
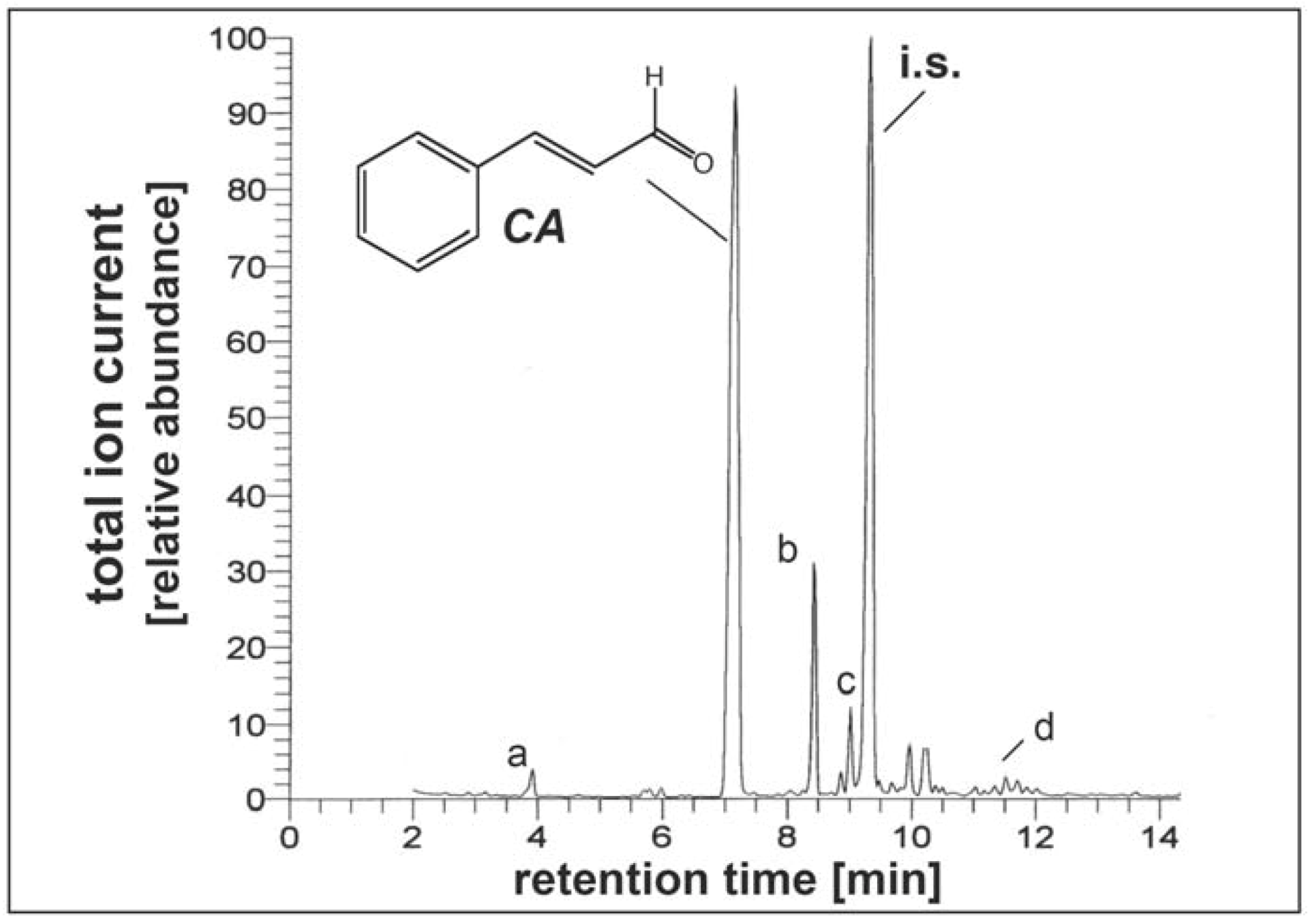
3.2. Ethanolic extraction of cinnamon powder and GC-MS quantification of cinnamic aldehyde content
3.3. General cell culture
3.4. Nrf2 reporter gene assay
3.5. Apoptosis and viability analysis
3.6. Immunoblot analysis
3.7. Determination of total cellular glutathione content
3.8. Comet assay (alkaline single cell gel electrophoresis)
3.9. Statistical Analysis
4. Summary and Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds (cinnamic aldehyde) are available from the authors.
References and Notes
- Half, E.; Arber, N. Colon cancer: Preventive agents and the present status of chemoprevention. Expert Opin. Pharmacother. 2009, 10, 211–219. [Google Scholar] [CrossRef]
- Marshall, J.R. Prevention of colorectal cancer: Diet, chemoprevention, and lifestyle. Gastroenterol. Clin. North Am. 2008, 37, 73–82. [Google Scholar] [CrossRef]
- Tanaka, T. Colorectal carcinogenesis: Review of human and experimental animal studies. J. Carcinog. 2009, 8, 5. [Google Scholar] [CrossRef]
- Meyskens, F.L., Jr.; McLaren, C.E.; Pelot, D.; Fujikawa-Brooks, S.; Carpenter, P.M.; Hawk, E.; Kelloff, G.; Lawson, M.J.; Kidao, J.; McCracken, J.; Albers, C.G.; Ahnen, D.J.; Turgeon, D.K.; Goldschmid, S.; Lance, P.; Hagedorn, C.H.; Gillen, D.L.; Gerner, E.W. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev. Res. (Phila Pa) 2008, 1, 32–38. [Google Scholar] [CrossRef]
- Rudolf, E.; Andelova, H.; Cervinka, M. Polyphenolic compounds in chemoprevention of colon cancer - targets and signaling pathways. Anticancer Agents Med. Chem. 2007, 7, 559–575. [Google Scholar]
- Johnson, J.J.; Mukhtar, H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007, 255, 170–181. [Google Scholar] [CrossRef]
- Yuan, J.H.; Li, Y.Q.; Yang, X.Y. Protective effects of epigallocatechin gallate on colon preneoplastic lesions induced by 2-amino-3-methylimidazo[4,5-f ] quinoline in mice. Mol. Med. 2008, 14, 590–598. [Google Scholar]
- Yu, X.; Kensler, T. Nrf2 as a target for cancer chemoprevention. Mutat. Res. 2005, 591, 93–102. [Google Scholar] [CrossRef]
- McMahon, M.; Itoh, K.; Yamamoto, M.; Chanas, S.A.; Henderson, C.J.; McLellan, L.I.; Wolf, C.R.; Cavin, C.; Hayes, J.D. The Cap'n'Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001, 61, 3299–3307. [Google Scholar]
- Zhang, D.D. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab. Rev. 2006, 38, 1–21. [Google Scholar]
- Osburn, W.O.; Kensler, T.W. Nrf2 signaling: an adaptive response pathway for protection against environmental toxic insults. Mutat. Res. 2008, 659, 31–39. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Kensler, T.W. The role of Keap1 in cellular protective responses. Chem. Res. Toxicol. 2005, 18, 1779–1791. [Google Scholar] [CrossRef]
- Wang, X.J.; Sun, Z.; Chen, W.; Eblin, K.E.; Gandolfi, J.A.; Zhang, D.D. Nrf2 protects human bladder urothelial cells from arsenite and monomethylarsonous acid toxicity. Toxicol. Appl. Pharmacol. 2007, 225, 206–213. [Google Scholar] [CrossRef]
- Du, Y.; Villeneuve, N.F.; Wang, X.J.; Sun, Z.; Chen, W.; Li, J.; Lou, H.; Wong, P.K.; Zhang, D.D. Oridonin confers protection against arsenic-induced toxicity through activation of the Nrf2-mediated defensive response. Environ. Health Perspect. 2008, 116, 1154–1161. [Google Scholar] [CrossRef]
- Juge, N.; Mithen, R.F.; Traka, M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol. Life Sci. 2007, 64, 1105–1127. [Google Scholar] [CrossRef]
- Lau, A.; Villeneuve, N.F.; Sun, Z.; Wong, P.K.; Zhang, D.D. Dual roles of Nrf2 in cancer. Pharmacol. Res. 2008, 58, 262–270. [Google Scholar] [CrossRef]
- Wondrak, G.T.; Cabello, C.M.; Villeneuve, N.F.; Zhang, S.; Ley, S.; Li, Y.; Sun, Z.; Zhang, D.D. Cinnamoyl-based Nrf2-activators targeting human skin cell photo-oxidative stress. Free Radic. Biol. Med. 2008, 45, 385–395. [Google Scholar] [CrossRef]
- Cabello, C.M.; Bair, W.B., 3rd; Lamore, S.D.; Ley, S.; Bause, A.S.; Azimian, S.; Wondrak, G.T. The cinnamon-derived Michael acceptor cinnamic aldehyde impairs melanoma cell proliferation, invasiveness, and tumor growth. Free Radic. Biol. Med. 2009, 46, 220–231. [Google Scholar] [CrossRef]
- Chew, E.H.; Nagle, A.A.; Zhang, Y.; Scarmagnani, S.; Palaniappan, P.; Bradshaw, T.D.; Holmgren, A.; Westwell, A.D. Cinnamaldehydes inhibit thioredoxin reductase and induce Nrf2: Potential candidates for cancer therapy and chemoprevention. Free Radic. Biol. Med. 2010, 48, 98–111. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Rana, T.; Sengupta, A. Inhibition of lipid peroxidation and enhancement of GST activity by cardamom and cinnamon during chemically induced colon carcinogenesis in Swiss albino mice. Asian Pac. J. Cancer Prev. 2007, 8, 578–582. [Google Scholar]
- Singh, G.; Maurya, S.; DeLampasona, M.P.; Catalan, C.A. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem. Toxicol. 2007, 45, 1650–1661. [Google Scholar] [CrossRef]
- Matan, N.; Rimkeeree, H.; Mawson, A.J.; Chompreeda, P.; Haruthaithanasan, V.; Parker, M. Antimicrobial activity of cinnamon and clove oils under modified atmosphere conditions. Int. J. Food Microbiol. 2006, 107, 180–185. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, C.H.; Kim, M.S.; Kim, J.Y.; Jung, K.J.; Chung, J.H.; An, W.G.; Lee, J.W.; Yu, B.P.; Chung, H.Y. Suppression of age-related inflammatory NF-kappaB activation by cinnamaldehyde. Biogerontology 2007, 8, 545–554. [Google Scholar] [CrossRef]
- Anderson, R.A.; Broadhurst, C.L.; Polansky, M.M.; Schmidt, W.F.; Khan, A.; Flanagan, V.P.; Schoene, N.W.; Graves, D.J. Isolation and characterization of polyphenol type-A polymers from cinnamon with insulin-like biological activity. J. Agric. Food Chem. 2004, 52, 65–70. [Google Scholar] [CrossRef]
- Peng, X.; Cheng, K.W.; Ma, J.; Chen, B.; Ho, C.T.; Lo, C.; Chen, F.; Wang, M. Cinnamon bark proanthocyanidins as reactive carbonyl scavengers to prevent the formation of advanced glycation endproducts. J. Agric. Food Chem. 2008, 56, 1907–1911. [Google Scholar]
- Shan, B.; Cai, Y.Z.; Brooks, J.D.; Corke, H. Antibacterial properties and major bioactive components of cinnamon stick (Cinnamomum burmannii): activity against foodborne pathogenic bacteria. J. Agric. Food Chem. 2007, 55, 5484–5490. [Google Scholar]
- PROGRAM, N.T. NTP Technical Report on the Toxicology and Carcinogenesis Studies of Trans-Cinnamaldehyde. Technical Report 514 2004. NIH Publication No 04-4448. [Google Scholar]
- Friedman, M.; Kozukue, N.; Harden, L.A. Cinnamaldehyde content in foods determined by gas chromatography-mass spectrometry. J. Agric. Food Chem. 2000, 48, 5702–5709. [Google Scholar] [CrossRef]
- Lazarus, S.A.; Adamson, G.E.; Hammerstone, J.F.; Schmitz, H.H. High-performance liquid Chromatography/Mass spectrometry analysis of proanthocyanidins in foods and beverages. J. Agric. Food Chem. 1999, 47, 3693–3701. [Google Scholar] [CrossRef]
- Dick, R.A.; Yu, X.; Kensler, T.W. NADPH alkenal/one oxidoreductase activity determines sensitivity of cancer cells to the chemotherapeutic alkylating agent irofulven. Clin. Cancer Res. 2004, 10, 1492–1499. [Google Scholar] [CrossRef]
- Yu, X.; Egner, P.A.; Wakabayashi, J.; Wakabayashi, N.; Yamamoto, M.; Kensler, T.W. Nrf2-mediated induction of cytoprotective enzymes by 15-deoxy-Delta12,14-prostaglandin J2 is attenuated by alkenal/one oxidoreductase. J. Biol. Chem. 2006, 281, 26245–26252. [Google Scholar]
- Dickinson, D.A.; Levonen, A.L.; Moellering, D.R.; Arnold, E.K.; Zhang, H.; Darley-Usmar, V.M.; Forman, H.J. Human glutamate cysteine ligase gene regulation through the electrophile response element. Free Radic. Biol. Med. 2004, 37, 1152–1159. [Google Scholar] [CrossRef]
- Chang, D.K.; Goel, A.; Ricciardiello, L.; Lee, D.H.; Chang, C.L.; Carethers, J.M.; Boland, C.R. Effect of H(2)O(2) on cell cycle and survival in DNA mismatch repair-deficient and -proficient cell lines. Cancer Lett. 2003, 195, 243–251. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Cabello, C.M.; Bair, W.B., 3rd; Ley, S.; Lamore, S.D.; Azimian, S.; Wondrak, G.T. The experimental chemotherapeutic N(6)-furfuryladenosine (kinetin-riboside) induces rapid ATP depletion, genotoxic stress, and CDKN1A (p21) upregulation in human cancer cell lines. Biochem. Pharmacol. 2009, 77, 1125–1138. [Google Scholar]
- Yang, C.Y.; Chang, C.C.; Ho, S.C.; Chiu, H.F. Is colon cancer mortality related to arsenic exposure? J. Toxicol. Environ. Health A 2008, 71, 533–538. [Google Scholar] [CrossRef]
- Wang, X.J.; Sun, Z.; Chen, W.; Li, Y.; Villeneuve, N.F.; Zhang, D.D. Activation of Nrf2 by arsenite and monomethylarsonous acid is independent of Keap1-C151: Enhanced Keap1-Cul3 interaction. Toxicol. Appl. Pharmacol. 2008, 230, 383–389. [Google Scholar] [CrossRef]
- Jiang, T.; Huang, Z.; Chan, J.Y.; Zhang, D.D. Nrf2 protects against As(III)-induced damage in mouse liver and bladder. Toxicol. Appl. Pharmacol. 2009, 240, 8–14. [Google Scholar] [CrossRef]
- Zhang, D.D.; Hannink, M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell Biol. 2003, 23, 8137–8151. [Google Scholar] [CrossRef]
- Zhang, D.D.; Lo, S.C.; Cross, J.V.; Templeton, D.J.; Hannink, M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol. Cell Biol. 2004, 24, 10941–10953. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, S.; Chan, J.Y.; Zhang, D.D. Keap1 controls postinduction repression of the Nrf2-mediated antioxidant response by escorting nuclear export of Nrf2. Mol. Cell Biol. 2007, 27, 6334–6349. [Google Scholar] [CrossRef]
- Wang, X.J.; Sun, Z.; Villeneuve, N.F.; Zhang, S.; Zhao, F.; Li, Y.; Chen, W.; Yi, X.; Zheng, W.; Wondrak, G.T.; Wong, P.K.; Zhang, D.D. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis 2008, 29, 1235–1243. [Google Scholar] [CrossRef]
- Wondrak, G.T.; Roberts, M.J.; Cervantes-Laurean, D.; Jacobson, M.K.; Jacobson, E.L. Proteins of the Extracellular Matrix Are Sensitizers of Photo-oxidative Stress in Human Skin Cells. J. Invest. Dermatol. 2003, 121, 578–586. [Google Scholar]
- Shen, G.; Khor, T.O.; Hu, R.; Yu, S.; Nair, S.; Ho, C.T.; Reddy, B.S.; Huang, M.T.; Newmark, H.L.; Kong, A.N. Chemoprevention of familial adenomatous polyposis by natural dietary compounds sulforaphane and dibenzoylmethane alone and in combination in ApcMin/+ mouse. Cancer Res. 2007, 67, 9937–9944. [Google Scholar]
- Khor, T.O.; Huang, M.T.; Prawan, A.; Liu, Y.; Hao, X.; Yu, S.; Cheung, W.K.; Chan, J.Y.; Reddy, B.S.; Yang, C.S.; Kong, A.N. Increased susceptibility of Nrf2 knockout mice to colitis-associated colorectal cancer. Cancer Prev. Res. (Phila Pa) 2008, 1, 187–191. [Google Scholar] [CrossRef]
- Khor, T.O.; Yu, S.; Kong, A.N. Dietary cancer chemopreventive agents - targeting inflammation and Nrf2 signaling pathway. Planta Med. 2008, 74, 1540–1547. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Cole, R.N.; Itoh, K.; Wakabayashi, N.; Katoh, Y.; Yamamoto, M.; Talalay, P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Nat. Acad. Sci. USA 2002, 99, 11908–11913. [Google Scholar]
- Kobayashi, A.; Kang, M.I.; Watai, Y.; Tong, K.I.; Shibata, T.; Uchida, K.; Yamamoto, M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol. Cell Biol. 2006, 26, 221–229. [Google Scholar] [CrossRef]
- Spencer, S.R.; Xue, L.A.; Klenz, E.M.; Talalay, P. The potency of inducers of NAD(P)H:(quinone-acceptor) oxidoreductase parallels their efficiency as substrates for glutathione transferases. Structural and electronic correlations. Biochem. J. 1991, 273, 711–717. [Google Scholar]
- Dinkova-Kostova, A.T.; Abeygunawardana, C.; Talalay, P. Chemoprotective properties of phenylpropenoids, bis(benzylidene)cycloalkanones, and related Michael reaction acceptors: correlation of potencies as phase 2 enzyme inducers and radical scavengers. J. Med. Chem. 1998, 41, 5287–5296. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Fahey, J.W.; Talalay, P. Chemical Structures of Inducers of Nicotinamide Quinone Oxidoreductase 1 (NQO1). Methods Enzymol. 2004, 382, 423–449. [Google Scholar] [CrossRef]
- Shimizu, M.; Hochadel, J.F.; Fulmer, B.A.; Waalkes, M.P. Effect of glutathione depletion and metallothionein gene expression on arsenic-induced cytotoxicity and c-myc expression in vitro. Toxicol. Sci. 1998, 45, 204–211. [Google Scholar]
- Bredfeldt, T.G.; Kopplin, M.J.; Gandolfi, A.J. Effects of arsenite on UROtsa cells: Low-level arsenite causes accumulation of ubiquitinated proteins that is enhanced by reduction in cellular glutathione levels. Toxicol. Appl. Pharmacol. 2004, 198, 412–418. [Google Scholar] [CrossRef]
© 2010 by the authors;
Share and Cite
Wondrak, G.T.; Villeneuve, N.F.; Lamore, S.D.; Bause, A.S.; Jiang, T.; Zhang, D.D. The Cinnamon-Derived Dietary Factor Cinnamic Aldehyde Activates the Nrf2-Dependent Antioxidant Response in Human Epithelial Colon Cells. Molecules 2010, 15, 3338-3355. https://doi.org/10.3390/molecules15053338
Wondrak GT, Villeneuve NF, Lamore SD, Bause AS, Jiang T, Zhang DD. The Cinnamon-Derived Dietary Factor Cinnamic Aldehyde Activates the Nrf2-Dependent Antioxidant Response in Human Epithelial Colon Cells. Molecules. 2010; 15(5):3338-3355. https://doi.org/10.3390/molecules15053338
Chicago/Turabian StyleWondrak, Georg Thomas, Nicole F. Villeneuve, Sarah D. Lamore, Alexandra S. Bause, Tao Jiang, and Donna D. Zhang. 2010. "The Cinnamon-Derived Dietary Factor Cinnamic Aldehyde Activates the Nrf2-Dependent Antioxidant Response in Human Epithelial Colon Cells" Molecules 15, no. 5: 3338-3355. https://doi.org/10.3390/molecules15053338
APA StyleWondrak, G. T., Villeneuve, N. F., Lamore, S. D., Bause, A. S., Jiang, T., & Zhang, D. D. (2010). The Cinnamon-Derived Dietary Factor Cinnamic Aldehyde Activates the Nrf2-Dependent Antioxidant Response in Human Epithelial Colon Cells. Molecules, 15(5), 3338-3355. https://doi.org/10.3390/molecules15053338