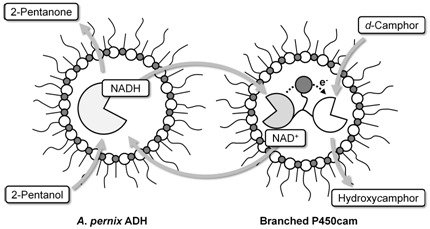
Artificial Self-Sufficient P450 in Reversed Micelles
Abstract
:1. Introduction
2. Results and Discussion
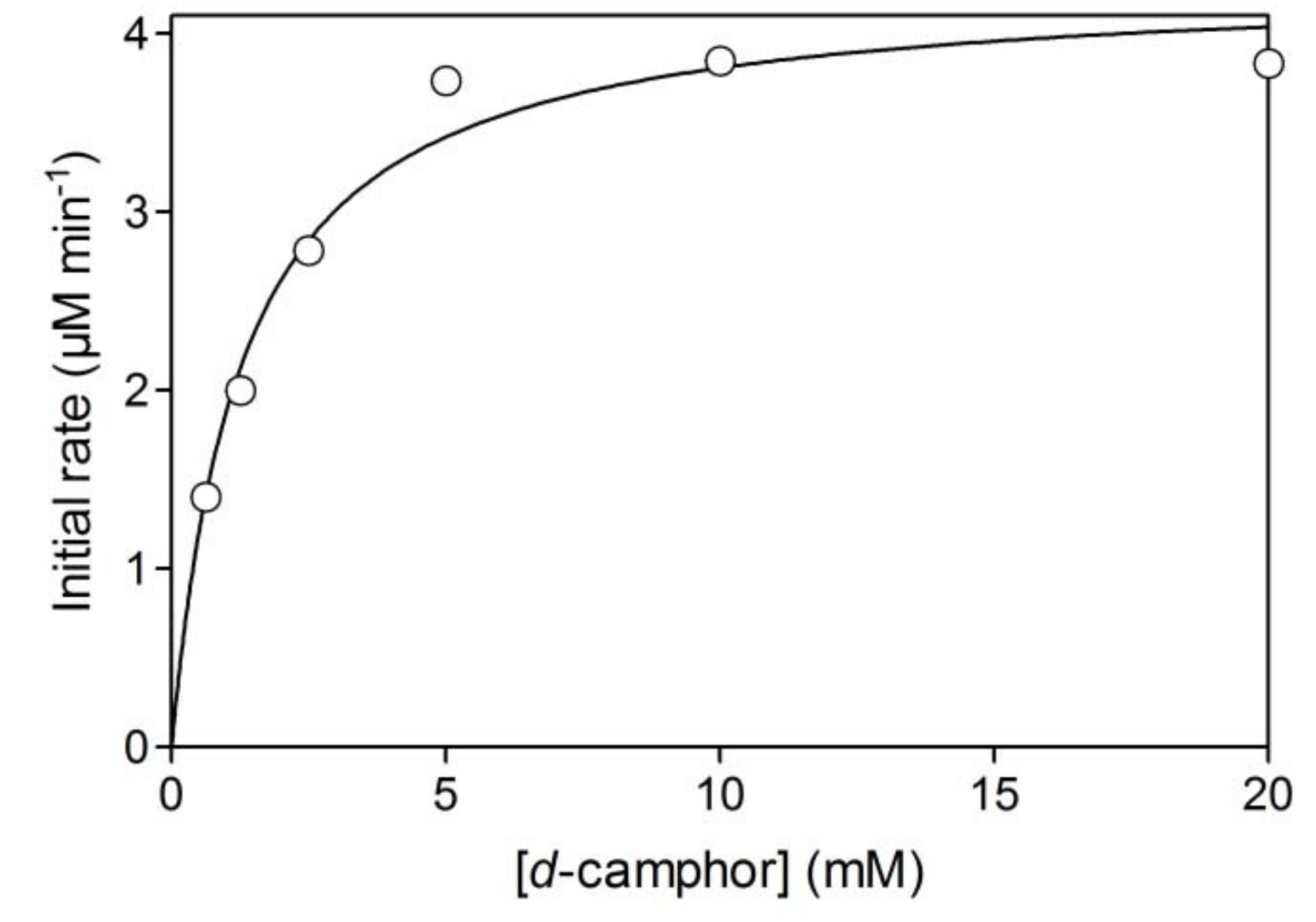
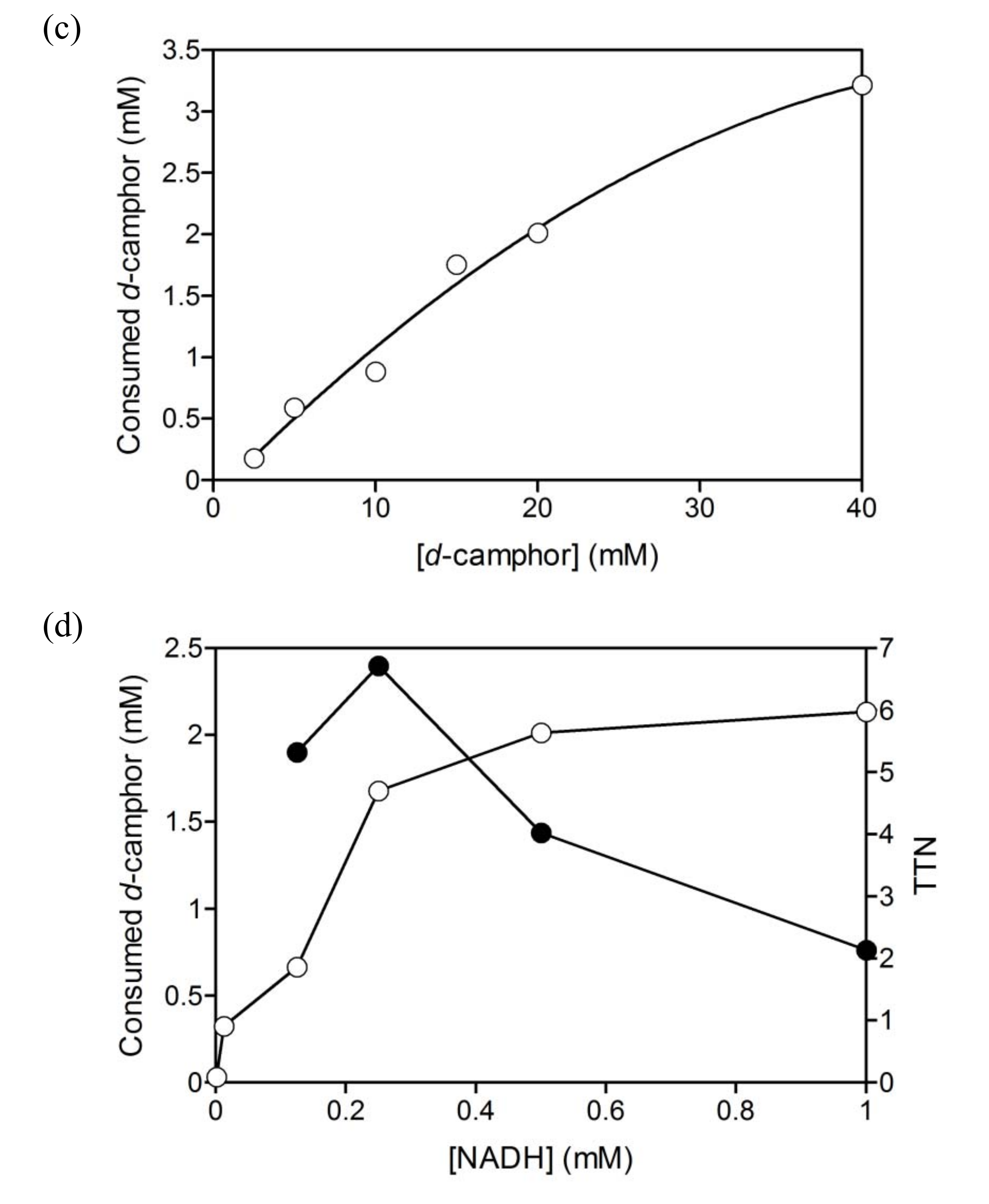
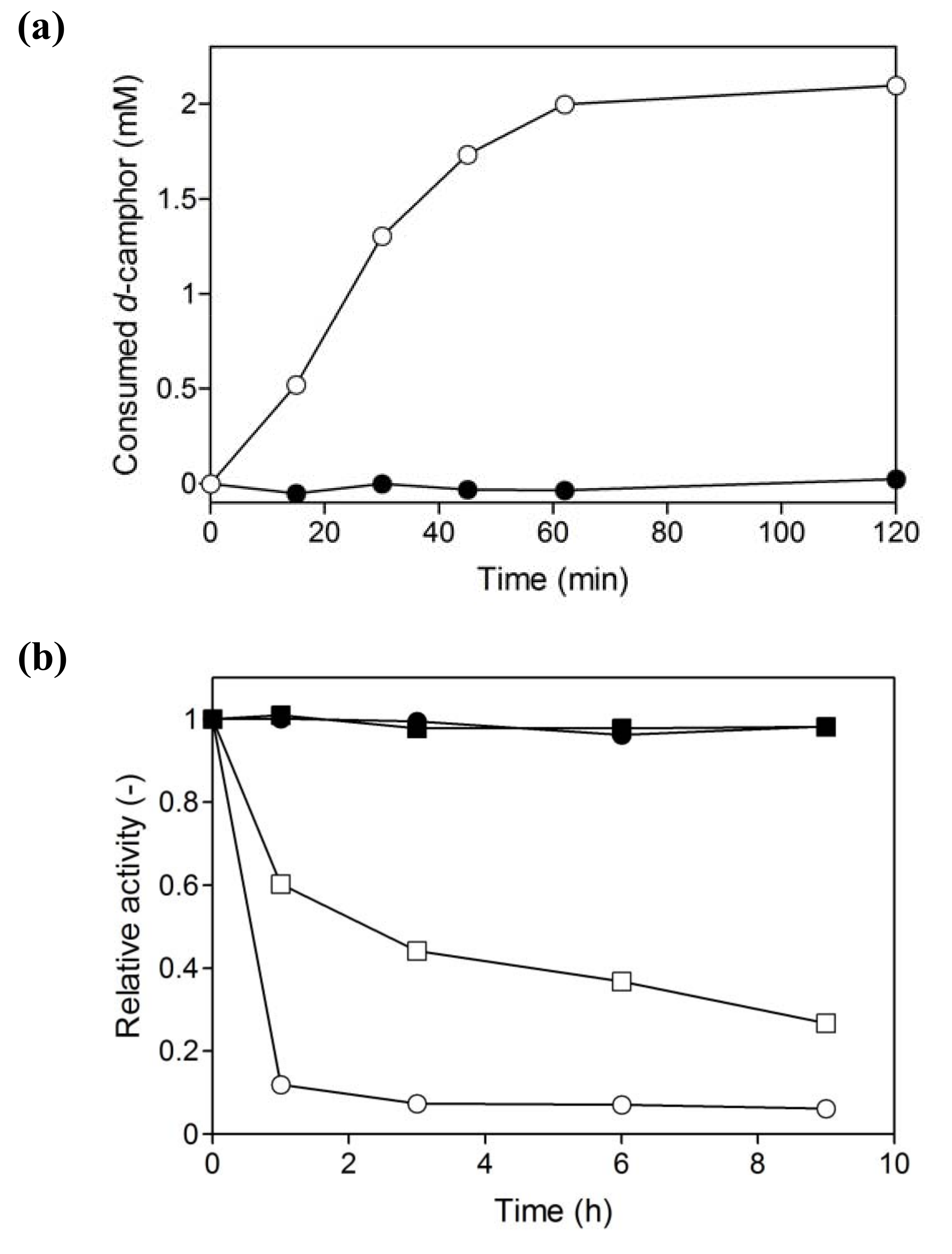
2.1. Hydroxylation of d-camphor by branched P450cam in reversed micelles
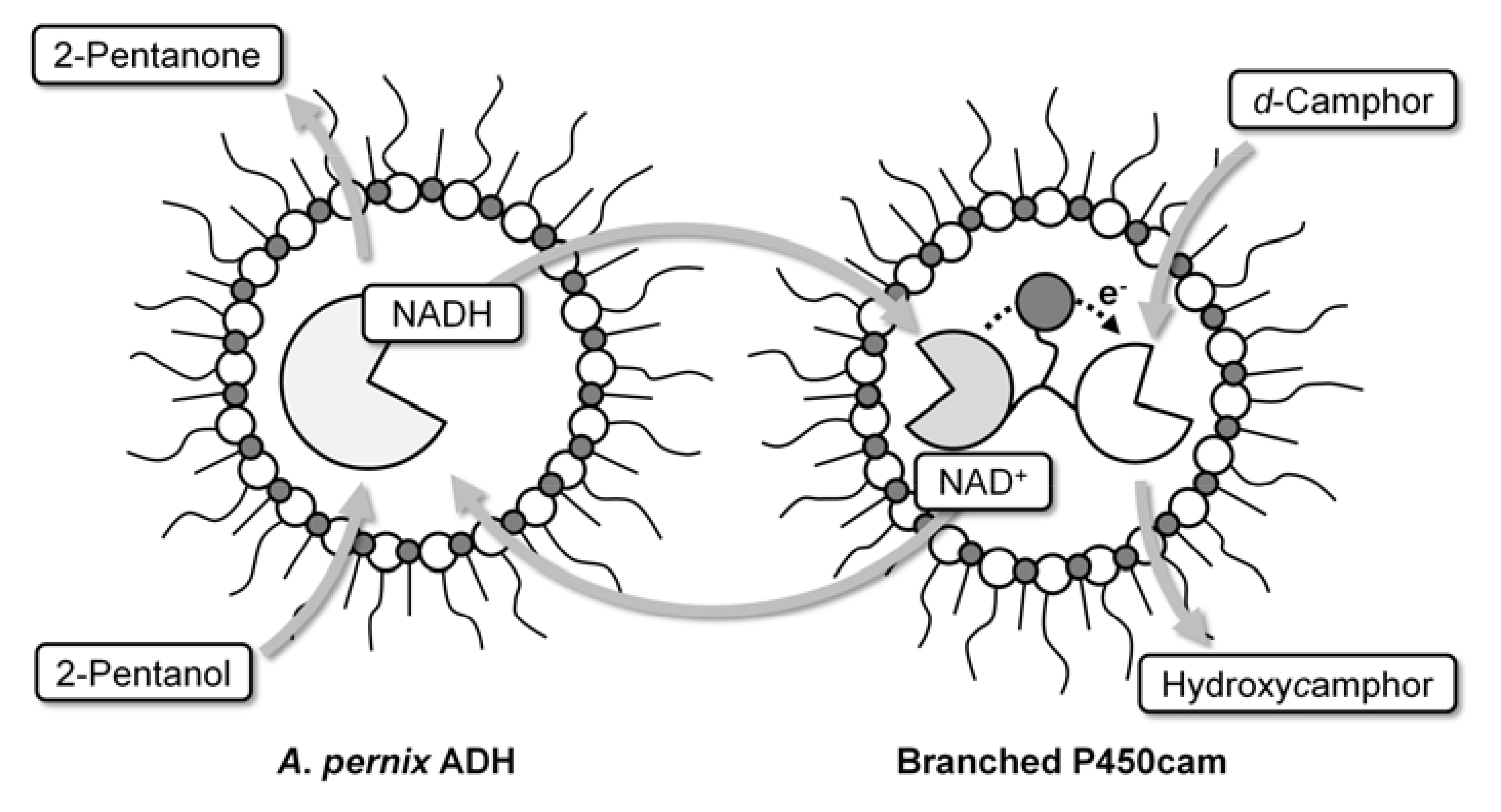
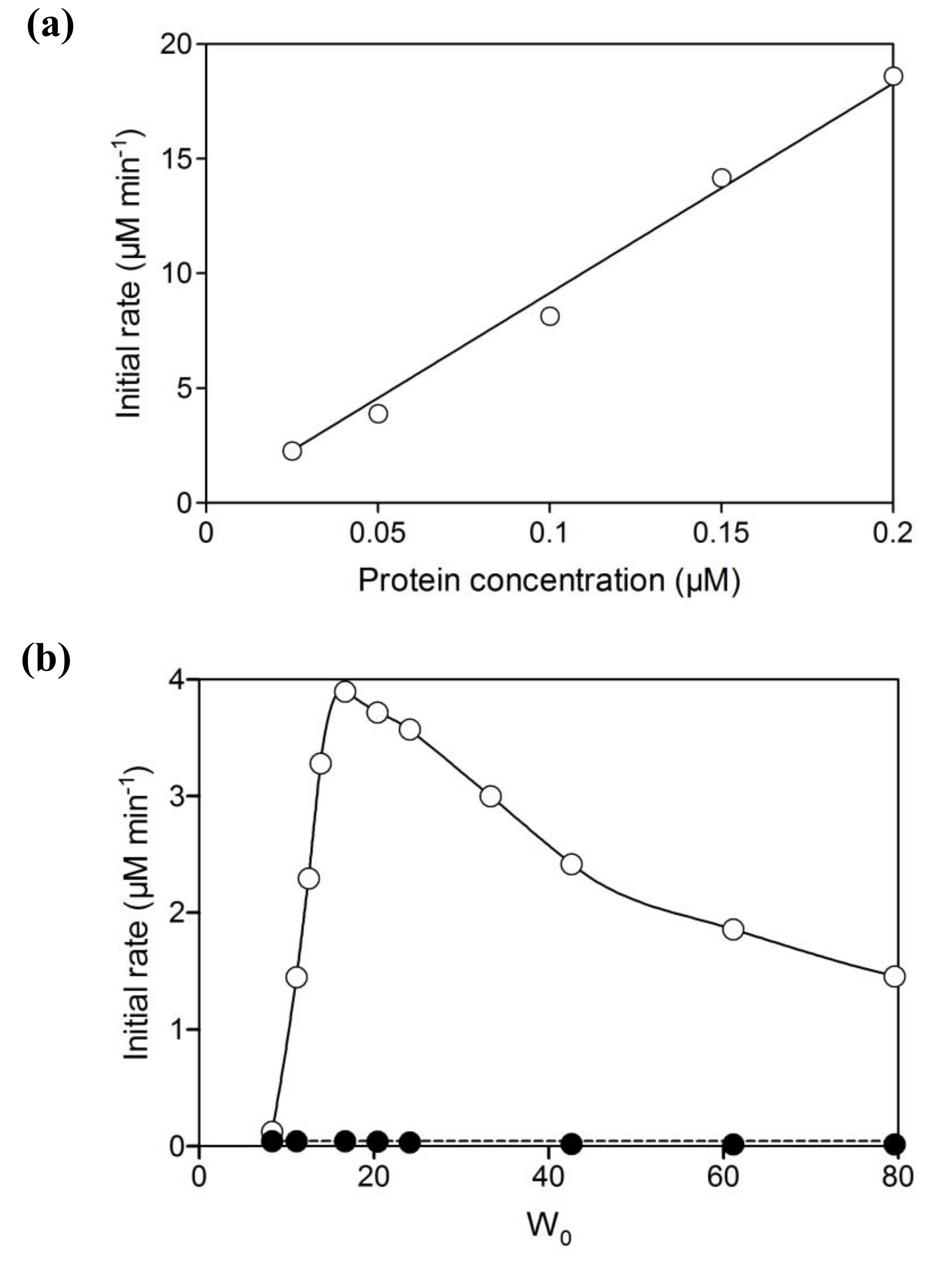
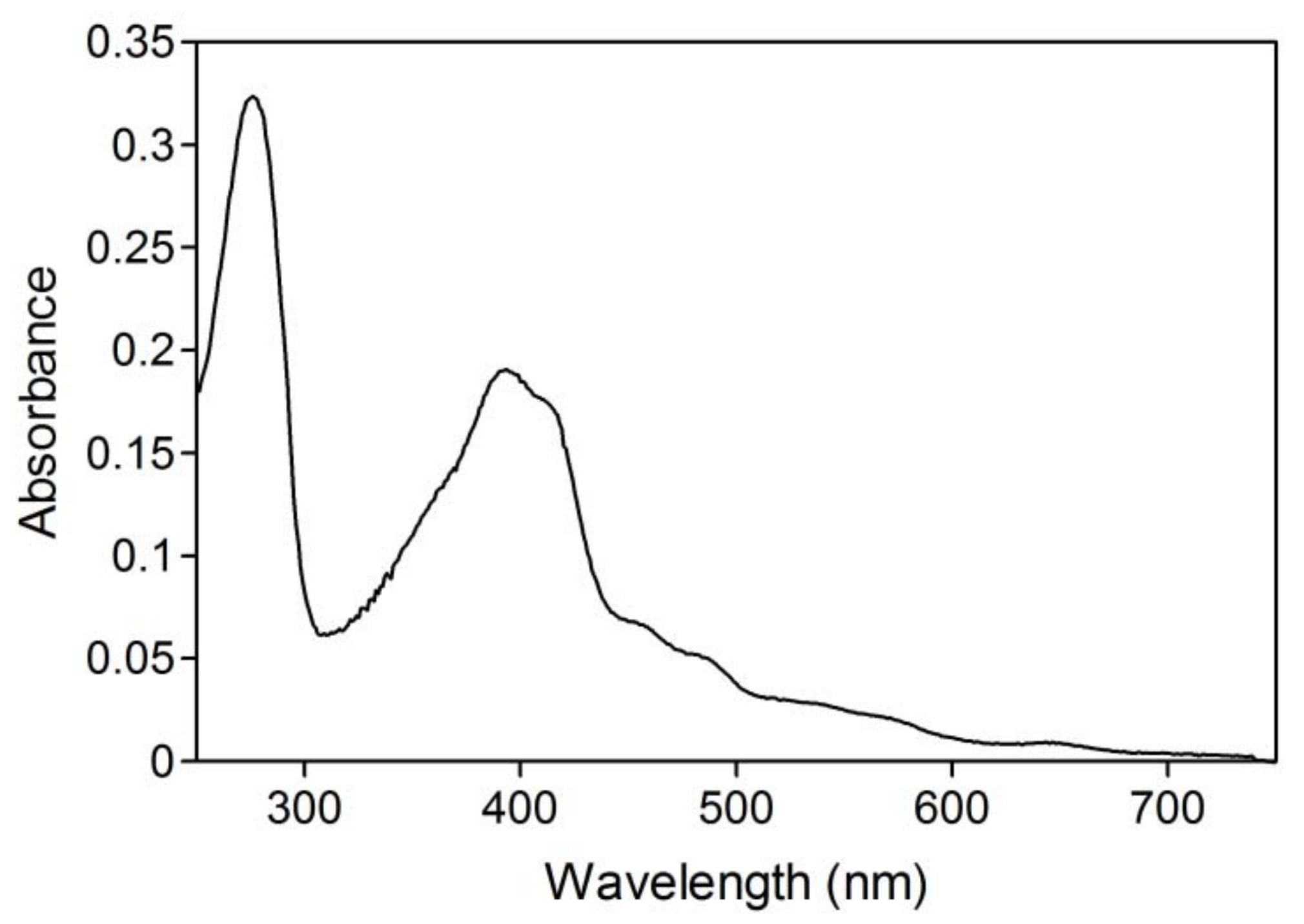
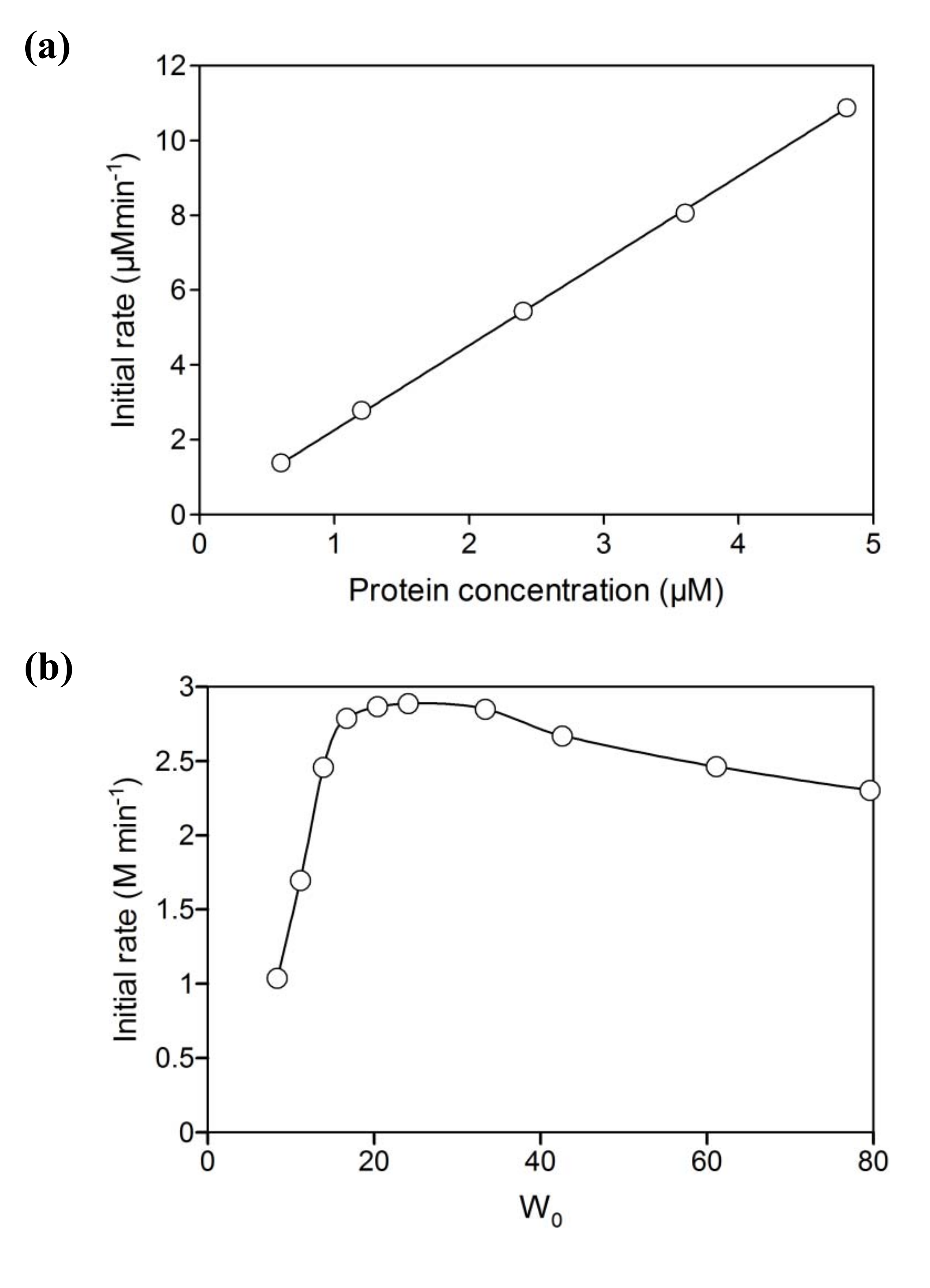
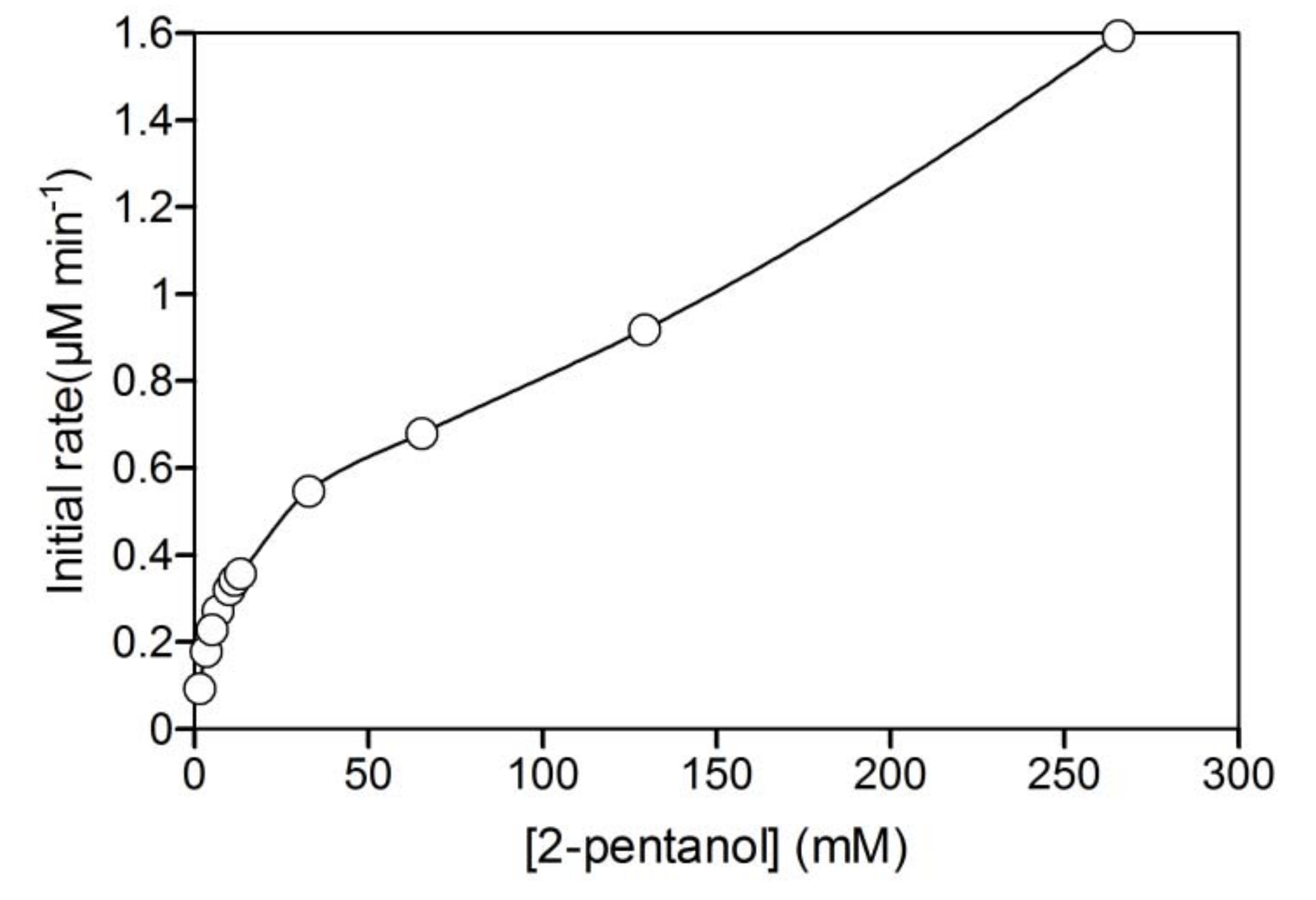
2.2. NAD+ reduction by A. pernix ADH in reversed micelles
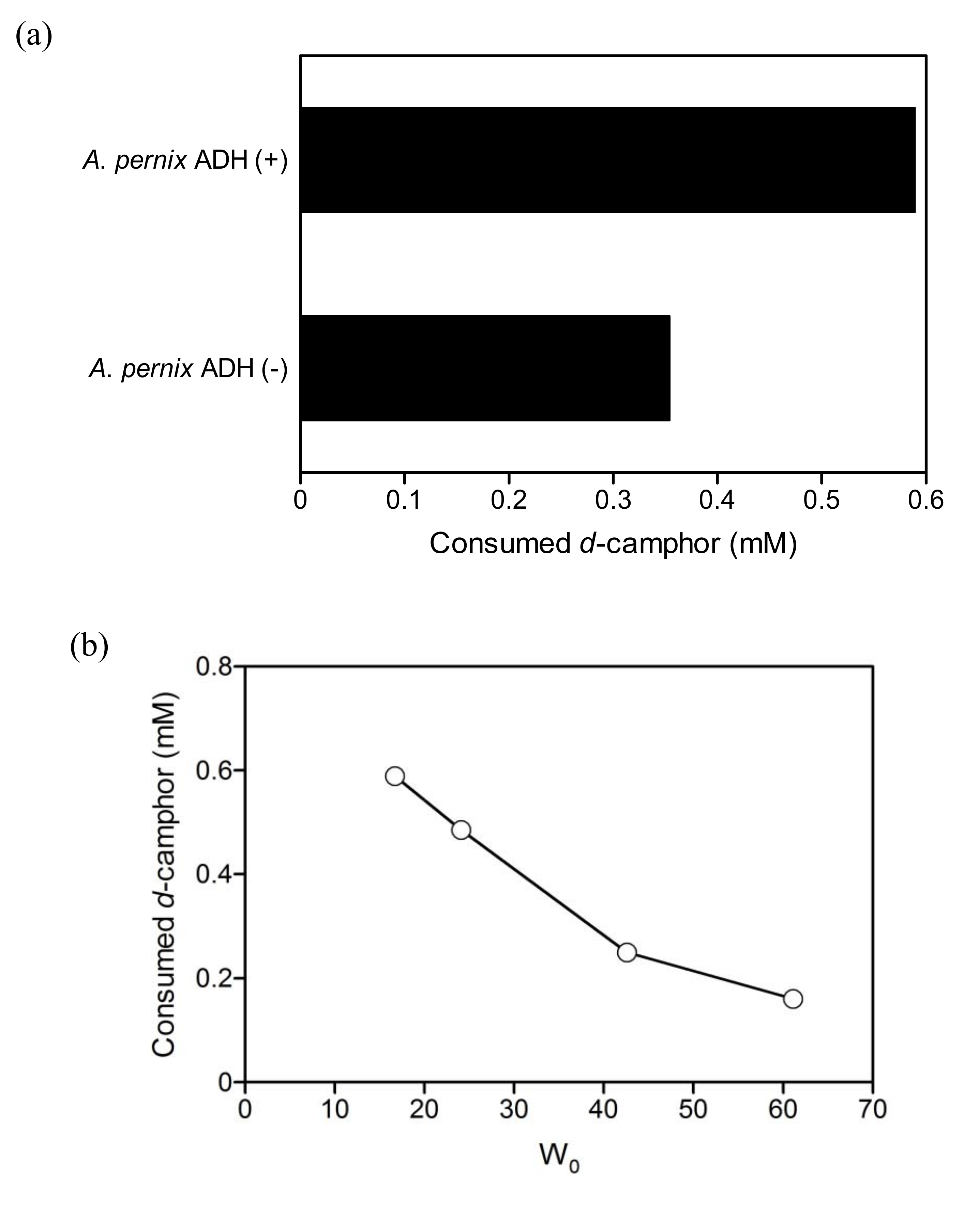
2.3. Coupled reaction by branched P450cam and A. pernix ADH
3. Experimental Section
3.1. Protein preparations
3.2. Preparation of reversed micelle solutions
3.3. Activity assays
3.4. Hydroxylation of d-camphor with cofactor regeneration
4. Conclusions
Acknowledgments
References
- Bell, G.; Halling, P.J.; Moore, B.D.; Partridge, J.; Rees, D.G. Biocatalyst Behaviour in Low-water Systems. Trends Biotechnol. 1995, 13, 468–473. [Google Scholar] [CrossRef]
- Eckstein, M.; Dauβmann, T.; Kragl, U. Recent Development in NAD(P)H Regeneration for Enzymatic Reductions in One- and Two-Phase Systems. Biocatal. Biotrans. 2004, 22, 89–96. [Google Scholar] [CrossRef]
- Kragl, U.; Eckstein, M.; Kaftzik, N. Enzyme Catalysis in Ionic Liquids. Curr. Opin. Biotechnol. 2002, 13, 565–571. [Google Scholar] [CrossRef]
- Zoumpanioti, M.; Stamatis, H.; Papadimitriou, V.; Xenakis, A. Spectroscopic and Catalytic Studies of Lipases in Ternary Hexane-1-propnanol-water Surfactantless Microemulsion Systems. Colloids Surf. B 2006, 47, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Klyachko, N.L.; Levashov, A.V. Bioorganic Synthesis in Reverse Micelles and Related Systems. Curr. Opin. Colloid Interf. Dci. 2003, 8, 179–186. [Google Scholar] [CrossRef]
- Sarcar, S.; Jain, T.K.; Maitra, A. Activity and Stability of Yeast Alcohol Dehydrogenase (YADH) Entrapped in Aerosol OT Revers Micelles. Biotechnol. Bioeng. 1992, 39, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Larsson, K.M.; Adlercreutz, P.; Mattiasson, B. Activity and Stability of Horse-liver Alcohol Dehydrogenase in Sodium Dioctylsulfosuccinate/cyclohexane Reversed Micelles. Eur. J. Biochem. 1987, 166, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A.; Katiyar, S.S. Activity and Kinetic Characteristics of Glutathione Reductase in Vitro in Reversed Micelle Pool. Biochim. Biophys. Acta 1989, 996, 1–6. [Google Scholar] [CrossRef]
- Piera-Velázquez, S.; Marhuenda-Egea, F.; Cadenas, E. The Dependence of a Halophilic Malate Dehydrogenase on ωo and Surfactant Concentration in Reversed Micelles. J. Mol. Catal. B-Enzym. 2001, 13, 49–55. [Google Scholar] [CrossRef]
- Hirakawa, H.; Kamiya, N.; Yata, T.; Nagamune, T. Regioselective Reduction of a Steroid in a Reversed Micelle System with Enzymatic NADH-regeneration. Biochem. Eng. J. 2003, 16, 35–40. [Google Scholar] [CrossRef]
- Sono, M.; Roach, M.P.; Coulter, E.D.; Dawson, J.H. Heme-Containing Oxygenases. Chem. Rev. 1996, 96, 2841–2887. [Google Scholar] [CrossRef] [PubMed]
- Hannemann, F.; Bichet, A.; Ewen, K.M.; Bernhardt, R. Cytochrome P450 Systems - Biological Variations of Electron Transport Chains. Biochim. Biophys. Acta 2007, 1770, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Bru, R.; Sánchez-Ferrer, A.; García-Carmona, F. Kinetic Models in Reversed Micelles. Biochem. J. 1995, 310, 721–739. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, H.; Michizoe, J.; Maruyama, T.; Kamiya, N.; Goto, M. Electron-Transfer Reactions and Functionalization of Cytochrome P450cam Monooxygenase System in Reversed Micelles. Langmuir 2004, 20, 5564–5568. [Google Scholar] [CrossRef] [PubMed]
- Michizoe, J.; Ichinose, H.; Kamiya, N.; Maruyama, T.; Goto, M. Functionalization of the Cytochrome P450cam Monooxygenase System in the Cell-like Aqueous Compartments of Water-in-Oil Emulsions. J. Biosci. Bioeng. 2005, 99, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.D.; Clark, D.S. P450cam Biocatalysis in Surfactant-Stabilized Two-Phase Emulsions. Biotechnol. Bioeng. 2008, 99, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, H.; Kamiya, N.; Tanaka, T.; Nagamune, T. Intramolecular Electron Transfer in a Cytochrome P450cam System with a Site-specific Branched Structure. Protein Eng. Des. Sel. 2007, 20, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Haandrikman, G.; Daane, G.J.; Kerkhof, F.J.; van Os, N.M.; Rupert, L.A. Microcalorimetric Investigation of the Solubilization of Water in Reversed Micelles and Water-in-Oil Microemulsions. J. Phys. Chem. 1992, 96, 9061–9068. [Google Scholar] [CrossRef]
- Pileni, M.P. Reverse Micelles as Microreactors. J. Phys. Chem. 1993, 97, 6961–6973. [Google Scholar] [CrossRef]
- OuYang, B.; Pochapsky, S.S.; Pagani, G.M.; Pochapsky, T.C. Specific Effects of Potassium Ion Binding on Wild-Type and L358P Cytochrome P450cam. Biochemistry 2006, 45, 14379–14388. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, P. Cofactor Regeneration for Sustainable Enzymatic Biosynthesis. Biotechnol. Adv. 2007, 25, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Kroutil, W.; Mang, H.; Edegger, K.; Faber, K. Recent Advances in the Biocatalytic Reduction of Ketones and Oxidation of Sec-alcohols. Curr. Opin. Chem. Biol. 2004, 8, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; van der Donk, W.A. Regeneration of Cofactors for Use in Biocatalysis. Curr. Opin. Biotechnol. 2003, 14, 583–589. [Google Scholar] [CrossRef] [PubMed]
- van der Donk, W.A.; Zhao, H. Recent Developments in Pyridine Nucleotide Regeneration. Curr. Opin. Biotechnol. 2003, 14, 421–426. [Google Scholar] [CrossRef]
- Hollmann, F.; Hofstetter, K.; Schmid, A. Non-enzymatic Regeneration of Nicotinamide and Flavin Cofacors for Monooxygenase Catalysis. Trends Biotechnol. 2006, 24, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Kohlmann, C.; Mäkle, W.; Lütz, S. Electroenzymatic Synthesis. J. Mol. Catal. B-Enzym. 2008, 51, 57–72. [Google Scholar] [CrossRef]
- Keinan, E.; Hafeli, E.K.; Setg, K.K.; Lamed, R. Thermostable Enzymes in Organic Synthesis. 2. Asymmetric Reduction of Ketones with Alcohol Dehydrogenase from Thermoanaerobium brokii. J. Am. Chem. Soc. 1986, 108, 162–169. [Google Scholar] [CrossRef]
- Kosjek, B.; Stampfer, W.; Pogorevc, M.; Goessler, W.; Faber, K.; Kroutil, W. Purification and Characterization of a Chemotolerant Alcohol Dehydrogenase Applicable to Coupled Redox Reactions. Biotechnol. Bioeng. 2004, 86, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Hummel, W. Large-scale Applications of NAD(P)-dependent Oxidoreductases: Recent Developments. Trends Biotechnol. 1999, 17, 487–492. [Google Scholar] [CrossRef]
- Wong, C.-H.; Whitesides, G.M. Enzyme-Catalyzed Organic Synthesis: NAD(P)H Cofactor Regeneration by Using Glucose 6-Phosphate and the Glucose-6-phosphate Dehydrogenase from Leuconostoc mesenteroides. J. Am. Chem. Soc. 1981, 103, 4890–4899. [Google Scholar] [CrossRef]
- Mouri, T.; Shimizu, T.; Kamiya, N.; Goto, M.; Ichinose, H. Design of a Cytochrome P450BM3 Reaction System Linked by Two-Step Cofactor Regeneration Catalyzed by a Soluble Transhydrogenase and Glycerol Dehydrogenase. Biotechnol. Prog. 2009, 25, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Tishkov, V.I.; Popov, V.O. Catalytic Mechanism and Application of Formate Dehydrogenase. Biochemistry (Moscow) 2004, 69, 1537–1554. [Google Scholar] [CrossRef]
- Vrtis, J.M.; Wite, A.K.; Metcalf, W.W.; van der Donk, W.A. Phosphite Dehydrogenase: A Versatile Cofactor-Regeneration Enzyme. Angew. Chem. Int. Ed. 2002, 41, 3257–3259. [Google Scholar] [CrossRef]
- Relyea, H. A.; van der Donk, W. A. Mechanism and Applications of Phosphite Dehydrogenase. Bioorg. Chem. 2005, 33, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, H.; Kamiya, N.; Kawarabayashi, Y.; Nagamune, T. Properties of an Alcohol Dehydrogenase from the Hyperthermophilic Archaeon Aeropyrum pernix K1. J. Biosci. Bioeng. 2004, 97, 202–206. [Google Scholar] [CrossRef]
- Hirakawa, H.; Kamiya, N.; Kawarabayashi, Y.; Nagamune, T. Log P Effect of Organic Solvents on a Thermophilic Alcohol Dehydrogenase. Biochim. Biophys. Acta 2005, 1748, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Dalziel, K.; Dickinson, F.M. The Kinetics and Mechanism of Liver Alcohol Dehydrogenase with Primary and Secondary Alcohols as Substrates. Biochem. J. 1966, 100, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Dalziel, K.; Dickinson, F.M. Substrate Activation and Inhibition in Coenzyme-Substrate Reactions. Biochem. J. 1966, 100, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.K.; Mazumdar, S. Role of Threonine 101 on the Stability of the Heme Active Site of Cytochrome P450cam: Multiwavelength Circular Dichroism Studies. Biochemistry 2006, 45, 12715–12722. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hirakawa, H.; Kamiya, N.; Kawarabayasi, Y.; Nagamune, T. Artificial Self-Sufficient P450 in Reversed Micelles. Molecules 2010, 15, 2935-2948. https://doi.org/10.3390/molecules15052935
Hirakawa H, Kamiya N, Kawarabayasi Y, Nagamune T. Artificial Self-Sufficient P450 in Reversed Micelles. Molecules. 2010; 15(5):2935-2948. https://doi.org/10.3390/molecules15052935
Chicago/Turabian StyleHirakawa, Hidehiko, Noriho Kamiya, Yutaka Kawarabayasi, and Teruyuki Nagamune. 2010. "Artificial Self-Sufficient P450 in Reversed Micelles" Molecules 15, no. 5: 2935-2948. https://doi.org/10.3390/molecules15052935
APA StyleHirakawa, H., Kamiya, N., Kawarabayasi, Y., & Nagamune, T. (2010). Artificial Self-Sufficient P450 in Reversed Micelles. Molecules, 15(5), 2935-2948. https://doi.org/10.3390/molecules15052935