Reactivity Ratios for Organotin Copolymer Systems
Abstract
:1. Introduction
2. Results and Discussion
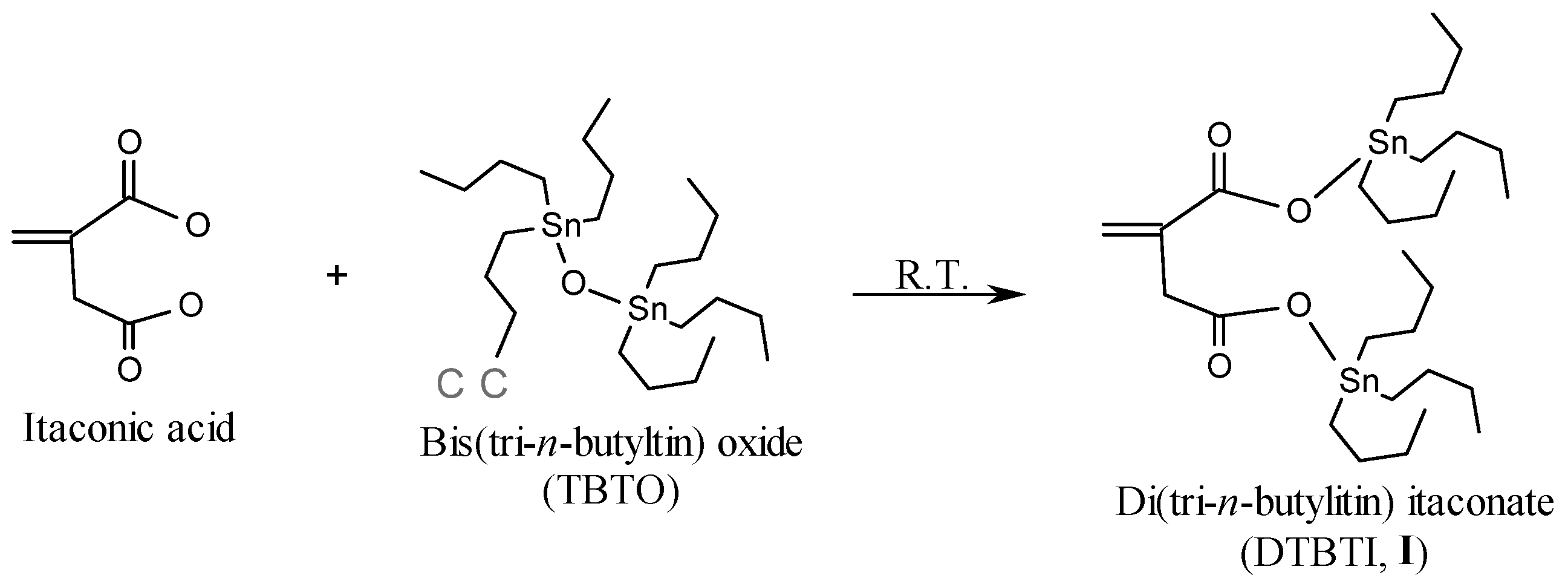
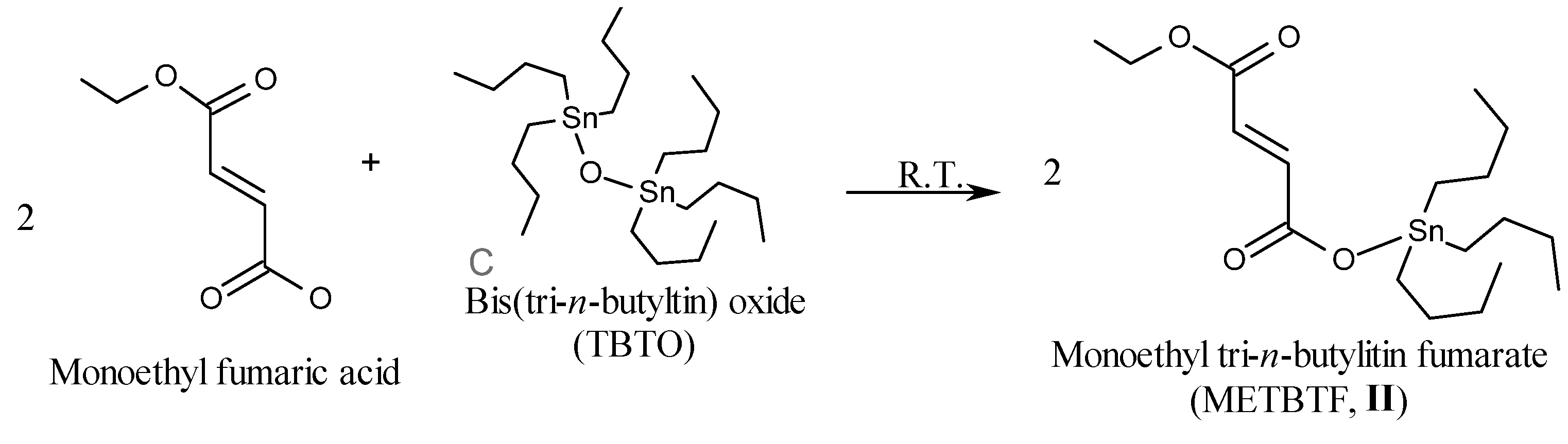
2.1. Synthesis of Organotin Monomers
| Monomer | Calc. | Found | ||||
|---|---|---|---|---|---|---|
| %C | %H | %Sn | %C | %H | %Sn* | |
| I | 49.19 | 8.26 | 33.52 | 48.39 | 8.41 | 33.49 |
| II | 49.99 | 7.91 | 27.40 | 50.23 | 8.23 | 27.30 |
2.2. Copolymer Synthesis


| Copolymer Ratio | %Sn | |||
|---|---|---|---|---|
| III a | IV b | V c | VI d | |
| 10/90 | 21.54 | 10.66 | 10.92 | 2.24 |
| 20/80 | 20.03 | 9.16 | 11.66 | 2.12 |
| 30/70 | 18.28 | 7.17 | 11.34 | 1.51 |
| 40/60 | 15.54 | 5.18 | 8.05 | 1.17 |
| 50/50 | 10.73 | 3.95 | 5.50 | 0.65 |
2.3. Structural Characterization of the Copolymers III-VI
2.4. Reactivity Ratio Determination
| Copolymer | % Sn | M1a | F b | m1c | F d | Conversion (wt/wt%) f | |
|---|---|---|---|---|---|---|---|
| Code | Ratio | ||||||
| III | 10/90 | 10.73 | 7.81 | 0.0689 | 0.0645 | 0.1111 | 0.1 |
| 20/80 | 15.54 | 9.07 | 0.1264 | 0.1122 | 0.25 | 0.2 | |
| 30/70 | 18.28 | 8.33 | 0.1754 | 0.1492 | 0.428 | 0.3 | |
| 40/60 | 20.03 | 6.62 | 0.2169 | 0.1782 | 0.667 | 0.4 | |
| 50/50 | 21.55 | 5.32 | 0.2626 | 0.20795 | 1.0 | 0.5 | |
| IV | 10/90 | 60 | 9.92 | 0.0257 | 0.0257 | 0.1111 | 0.11 |
| 20/80 | 85 | 7.27 | 0.0383 | 0.0383 | 0.1764 | 0.15 | |
| 30/70 | 108 | 5.94 | 0.0529 | 0.0530 | 0.25 | 0.20 | |
| 40/60 | 128 | 5.27 | 0.0656 | 0.0656 | 0.3333 | 0.25 | |
| V | 10/90 | 5.50 | 8.59 | 0.0587 | 0.0554 | 0.1111 | 0.1 |
| 20/80 | 8.05 | 6.39 | 0.0945 | 0.0864 | 0.250 | 0.2 | |
| 30/70 | 11.34 | 3.39 | 0.1528 | 0.1326 | 0.428 | 0.3 | |
| 40/60 | 11.66 | 1.42 | 0.1595 | 0.1375 | 0.667 | 0.4 | |
| 50/50 | 10.92 | 0.43 | 0.1444 | 0.1262 | 1.000 | 0.5 | |
| VI | 10/90 | 0.65 | 7.24 | 0.0056 | 0.055 | 0.1111 | 0.1 |
| 20/80 | 1.17 | 6.26 | 0.0102 | 0.0101 | 0.25 | 0.2 | |
| 30/70 | 1.51 | 7.84 | 0.0134 | 0.0132 | 0.428 | 0.3 | |
| 40/60 | 2.12 | 5.65 | 0.0193 | 0.0189 | 0.667 | 0.4 | |
| 50/50 | 2.24 | 3.96 | 0.0204 | 0.0200 | 1.000 | 0.5 | |
| Copolymer | Monomer Ratio F = M1/M2 | M-Unit Ratio in Copolymer | Parameters of FR Eq. | ||
|---|---|---|---|---|---|
| Code | Ratio | F2 /f | F/f(f-1) | ||
| III | 10/90 | 0.1111 | 0.0645 | 0.1787 | -1.4987 |
| 20/80 | 0.25 | 0.1122 | 0.4943 | -1.7273 | |
| 30/70 | 0.428 | 0.1492 | 1.0446 | -2.0127 | |
| 40/60 | 0.667 | 0.1782 | 2.0516 | -2.4088 | |
| 50/50 | 1.0 | 0.2079 | 3.8088 | -2.8088 | |
| IV | 10/90 | 0.1111 | 0.0257 | 0.4789 | -4.2031 |
| 20/80 | 0.1674 | 0.0383 | 0.8124 | -4.429 | |
| 30/70 | 0.25 | 0.0529 | 1.1816 | -4.4765 | |
| 40/60 | 0.3333 | 0.0656 | 1.6932 | -4.7468 | |
| 50/50 | 0.1111 | 0.0257 | 0.4789 | -4.2031 | |
| V | 10/90 | 0.1111 | 0.0587 | 0.2103 | -1.7821 |
| 20/80 | 0.25 | 0.0945 | 0.6612 | -2.3946 | |
| 30/70 | 0.428 | 0.1528 | 1.1988 | -2.3729 | |
| 40/60 | 0.667 | 0.1595 | 2.7901 | -3.5161 | |
| 50/50 | 1.0 | 0.1444 | 6.9269 | -5.9269 | |
| VI | 10/90 | 0.111 | 0.0056 | 2.1960 | -19.654 |
| 20/80 | 0.250 | 0.0103 | 6.0860 | -24.094 | |
| 30/70 | 0.428 | 0.0134 | 13.6642 | -31.498 | |
| 40/60 | 0.667 | 0.0193 | 23.0147 | -33.838 | |
| 50/50 | 1.000 | 0.0209 | 48.8220 | -47.822 | |
3. Experimental
3.1. Materials
3.2. Characterization
3.3. Synthesis of Organotin Monomers
3.3.1. Synthesis of Di(tri-n-butyltin) Itaconate (DTBTI, I)
3.3.2. Synthesis of Monoethyl Tri-n-butyltin Fumarate (METBTF, II)
3.4. General Procedure for Copolymerization
3.5. Reactivity Ratios Determination
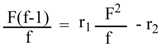
4. Conclusions
References
- Milovanovic, M.B.; Trifunovic, S.S.; Katsikas, L.; Popovic, I.G. Preparation and modification of itaconic anhydride–methyl methacrylate copolymers. J. Serb. Chem. Soc. 2007, 72, 1507–1514. [Google Scholar] [CrossRef]
- Al-Deyab, S.S.; Al-Hazmi, A.M.; El-Newehy, M.H. Synthesis and characterization of organotin containing copolymers: reactivity ratio studies. Molecules 2010, 15, 1784–1797. [Google Scholar]
- Erol, I.; Sen, O.; Dedelioglu, A.; Cifci, C. Synthesis and characterization of novel fluorine-containing methacrylate copolymers: Reactivity ratios, thermal properties, and antimicrobial activity. J. Appl. Polym. Sci. 2009, 114, 3351–3359. [Google Scholar] [CrossRef]
- Hou, C.; Liu, J.; Ji, C.; Ying, L.; Sun, H.; Wang, C. Monomer apparent reactivity ratios for acrylonitrile/methyl vinyl ketone copolymerization system. J. Appl. Polym. Sci. 2006, 102, 4045–4048. [Google Scholar] [CrossRef]
- Habibi, A.; Vasheghani-Farahani, E.; Semsarzadeh, M.A.; Sadaghiani, K. Monomer reactivity ratios for lauryl methacrylate–isobutyl methacrylate in bulk free radical copolymerization. Polym. Int. 2003, 52, 1434–1443. [Google Scholar] [CrossRef]
- Wessling, R.A. Kinetics of continuous addition emulsion polymerization. J. Appl. Polym. Sci. 1968, 12, 309–319. [Google Scholar] [CrossRef]
- Dimitratos, J.; Elicabe, G.; Georgakis, C. Control of Emulsion Polymerization Reactors. AIChE J. 1994, 40, 1993–2021. [Google Scholar]
- Manski, C.F.; Tamer, E.T. Inference on regressions with interval data on a regressor or outcome. Econometrica 2002, 70, 519–547. [Google Scholar] [CrossRef]
- Canegallo, S.; Canu, P.; Morbidelli, M.; Storti, G. Composition control in emulsion copolymerization. II. Application to binary and ternary systems. J. Appl. Polym. Sci. 1994, 54, 1919–1935. [Google Scholar] [CrossRef]
- Al-Deyab, S.S.; El-Newehy, M.H. Synthesis and Characterization of Novel Organotin-Phosphorous Compounds II. Molecules 2010, 15, 1425–1432. [Google Scholar] [CrossRef]
- Rehman, S-ur.; Shahid, K.; Ali, S.; Mazhar, M.; Badshah, A.; Eng, G.; Song, X.; Ryczkowski, J. Synthesis, spectroscopic characterization, and in vitro biological activity of organotin(IV) complexes of (E)-3-(4-methoxyphenyl)-2-phenyl-2-propenoic acid. Heter. Chem. 2005, 16, 175–183. [Google Scholar] [CrossRef]
- Garg, B.K.; Corredor, J.; Subramanian, R.V. Copolymerization of Tri-n-butyltin Acrylate and Tri-n-butyltin Methacrylate Monomers with Vinyl Monomers Containing Functional Groups. J. Macromol. Sci. Part A: Pure Appl. Chem. 1977, 11, 1567–1601. [Google Scholar]
- Al-Diab, S.S. Synthesis of novel organotin copolymers. J. Chem. Res. 1986, (S), 306–307. [Google Scholar]
- Gaina, C.; Gaina, V. Synthesis and characterization of novel organotin carboxylate maleimide monomers and polymers. eXPRESS Polym. Lett. 2009, 3, 352–358. [Google Scholar] [CrossRef]
- Tawfik, S.Y.; Messiha, N.N.; El-Hamouly, S.H. Effect of substitution on the reactivity of some new p-phenylacrylamide derivatives with organotin monomers. J. Polym. Sci. 1993, 31, 427–433. [Google Scholar]
- Ghanem, N.A.; Messiha, N.N.; Abd-Elmalek, M.M.; Ikladious, N.E.; Shaaban, A.F. J. Coat. Tech. 1981, 53, 57–60.
- Shaaban, A.F.; Hilmym, N.H.; Wakid, A.M.; El-Monairy, O.M.; Mohammed, A.A. Structure-performance relationships in organotin mercaptide stabilizers. Pure Appl. Chem. 1981, 53, 577–582. [Google Scholar] [CrossRef]
- Eng, G.; Tierney, E.J.; Bellama, J.M.; Brinckman, F.E. Correlation of molecular total surface area with organotin toxicity for biological and physicochemical applications. Appl. Organomet. Chem. 1988, 2, 171–175. [Google Scholar]
- Finemann, M.; Ross, S.D. Linear method for determining monomer reactivity ratios in copolymerization. J. Polym. Sci. 1950, 5, 259–262. [Google Scholar] [CrossRef]
- Gilman, H.; Rosenberg, D. Reaction of Triphenyltin Hydride with Methyllithium. J. Am. Chem. Soc. 1953, 75, 3592–3593. [Google Scholar] [CrossRef]
- Pekel, N.; Sahiner, N.; Guven, O.; Rzaev, Z.M.O. Synthesis and characterization of N-vinylimidazole-ethyl methacrylate copolymers and determination of monomer reactivity ratios. Eur. Polym. J. 2001, 37, 2443–2451. [Google Scholar] [CrossRef]
- Minora, Y.; Tadokoro, T.; Susuki, Y. Radical copolymerization of crotonyl compounds with styrene. J. Polym. Sci. Part A-1: Polym. Chem. 1967, 5, 2641–2654. [Google Scholar]
- Nair, C.P.R.; Dona, M.; Ninan, K.N. Free radical copolymerisation of N-(4-hydroxy phenyl) maleimide with vinyl monomers: solvent and penultimate-unit effects. Eur. Polym. J. 1999, 35, 1829–1840. [Google Scholar]
- Florjanczyk, Z.; Krawiec, W.; Such, K. A study of the relative reactivity of maleic anhydride and some maleimides in free radical copolymerization and terpolymerization. J. Polym. Sci. 1990, 28, 795–801. [Google Scholar]
- Miller, A.; Szafko, J.; Turska, E. Reactivity ratios for acrylonitrile-vinyl chloroacetate copolymerization systems. J. Polym. Sci. 1977, 15, 51–63. [Google Scholar]
- Cummins, R.A.; Dunn, P. Organotin carboxylates. Aust. J. Chem. 1964, 17, 185–191. [Google Scholar] [CrossRef]
- Stanely, R.S.; Dannin, J.; Tsou, K.S. Copolymerization of p-triphenyltinstyrene and p-triphenylleadstyrene with styrene or vinyltoluene. J. Polym. Sci. Part A: Gen. Pap. 1965, 3, 3199–3207. [Google Scholar]
- Kreisel, M.; Garbatski, U.; David, H.K. Copolymerization of styrene. I. Copolymerization with styrene derivatives containing nitrile groups in the side-chain. J. Polym. Sci. 1964, 2, 105–121. [Google Scholar]
- Shaaban, A.F.; Arief, M.M.; Mahmoud, A.A.; Messiha, N.N. Organotin polymers. XI. Radical copolymerization reactions of di-(tri-n-butyltin) itaconate with 2-chloroethyl acrylate n-butyl acrylate and allyl methacrylate. Acta Polym. 1987, 38, 492–495. [Google Scholar]
- Shaaban, A.F.; Arief, M.M.; Mahmoud, A.A.; Messiha, N.N. Organotin polymers: 10. Copolymerization parameters for di-(tri-n-butyltin) itaconate with methyl acrylate, ethyl acrylate, N-vinyl pyrrolidone and acrylonitrile. Polymer 1987, 28, 1423–1425. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
El-Newehy, M.H.; Al-Deyab, S.S.; Al-Hazmi, A.M.A. Reactivity Ratios for Organotin Copolymer Systems. Molecules 2010, 15, 2749-2758. https://doi.org/10.3390/molecules15042749
El-Newehy MH, Al-Deyab SS, Al-Hazmi AMA. Reactivity Ratios for Organotin Copolymer Systems. Molecules. 2010; 15(4):2749-2758. https://doi.org/10.3390/molecules15042749
Chicago/Turabian StyleEl-Newehy, Mohamed H., Salem S. Al-Deyab, and Ali Mohsen Ali Al-Hazmi. 2010. "Reactivity Ratios for Organotin Copolymer Systems" Molecules 15, no. 4: 2749-2758. https://doi.org/10.3390/molecules15042749
APA StyleEl-Newehy, M. H., Al-Deyab, S. S., & Al-Hazmi, A. M. A. (2010). Reactivity Ratios for Organotin Copolymer Systems. Molecules, 15(4), 2749-2758. https://doi.org/10.3390/molecules15042749




