Abstract
Two novel organic–inorganic compounds based on tetrahexylammonium (THA) and tetraheptylammonium (THPA) ions and the Preyssler anion, [NaP5W30O110]14-, were synthesized and formulated as (THA)7.7H6.3 [NaP5W30O110] (A) and (THPA)7.5 H6.5[N aP5W30O110] (B). The synthesized compounds were characterized by IR, UV, and TGA and used for the catalytic synthesis of 4-aminopyrazolo[3,4,-d]pyrimidine derivatives 2a-2d. Our findings showed efficient catalytic activities for A and B.
1. Introduction
Interest in the synthesis of large polytungstates has attracted much attention. In this area, some examples for species containing more than 18 tungsten atoms have been reported [1]. One of the largest polytungstates is [NaP5W30O110]14-, the so-called Preyssler anion. This heteropolyanion consists of a cyclic assembly of five [PW6O22] units, each derived from the Keggin anion, [PW12O40]3-. This anion can be obtained by the removal of two sets of three corner shared WO6 octahedra. We have shown that the pure acid form of this polyanion, H14[NaP5W30O110], has excellent potential applications in catalytic reactions [2,3,4,5,6]. Usually, acid-catalyzed reactions are carried out by use of diverse conventional mineral acids such as H2SO4, HF, HCl, H3PO4, etc. The replacement of these conventional hazardous and polluting corrosive liquid acid catalysts by solid acid catalysts is one of the key current demands in the field of catalysis. Cleaner technologies could be possible by making use of environmentally friendly catalysts involving the use of solid acids. It was shown that heteropolyacids in the solid state are pure Bronsted acids and stronger acids that conventional solid acids such as SiO2–Al2O3, H3PO4, HNO3, H2SO4, and HX and HY zeolites [7,8]. These compounds have several advantages as catalysts which make them economically and environmentally attractive [9,10,11,12,13]. If one applies the proposed principles of Green Chemistry we see that the Preyssler catalyst can be considered a promising green catalyst candidate. This solid acid catalyst is “green” with respect to corrosiveness, safety, quantity of waste, and separability. Moreover, while some of the acidic catalysts such as HCl, HNO3, H2SO4, etc, can produce chlorated, nitrated, sulfated, etc, by products, Preyssler acid does not produce any of these by products. Thus, it can reduce quantity of waste formed. In our studies it has been found that Preyssler’s anion catalyzes oxidations of organic substances without any structural degradation. This leads to the recovery and recyclibility of this catalyst, which is very important in catalytic processes, especially in industry.
We are interested in development of applications of the Preyssler anion in other forms such as organic-inorganic forms. Recently, we have developed a series of reactions catalyzed by Preyssler and nano Preyssler acidic or inorganic salt forms [14,15,16,17].
Research based on new type organic-inorganic molecular multifunctional materials is continuously attracting the considerable interest in organic synthesis [18]. Along this line, heteropolyacids are excellent molecular acceptors and can form novel organic-inorganic complexes with a number of organic substrates containing N, S and O atoms. Such organic substrates often include several types of cationic organic species [19].
Tetrahexyammonium (THA) and tetraheptylammonium (THPA) cations are examples of excellent electron donors that possess high charge and suitable size which makes them very good inorganic blocks for the construction of organic-inorganic hybrid compounds with the Preyssler anion. In some cases heteropolyacids can be partly reduced, which offers the opportunity to prepare organic donor-inorganic acceptor materials with a mixed valance state in the organic and inorganic counterparts. The presence of strong electron delocalization in the organic chains suggests electron transfer between organic donors and inorganic acceptors [20].
To the best of our knowledge, there are no reports of the reaction products of the Preyssler heteropolyacid with THA or THPA as organic cations. Herein, we wish to report two novel organic-inorganic compounds based on THA and THPA and the Preyssler anion that display good catalytic activity in organic media.
2. Results and Discussion
Synthesis of two novel compounds were carried out based on the reactions of the Preyssler anion with THA and THPA, by neutralization of the Preyssler acid. In this case, diluted solutions were generally required to avoid the precipitation of mixed salts. The products were studied and characterized by potentiometeric and conductometeric titrations, IR, and UV spectroscopy and thermogravimetric method (TGA).
2.1. IR Spectrocopy
The Preyssler anion is made up of five PW6 units arranged in a crown, so that the whole anion has an internal fivefold symmetry axis. Perpendicular to this axis is a mirror plane that contains the five planes containing the five phosphorus atoms. The tungsten atoms are distributed in four parallel planes perpendicular to the axis. A PW6 unit consists of two groups of three corner-shared WO6 octahedra. Two pairs of octahedral of each group are joined together by sharing on edge located in the mirror plane. Each WO6 octahedron shares a vertex with the central PO4 tetrahedron [21]. All tungsten atoms are octahedrally surrounded by oxygen atoms.
The anion contains W=O double bonds which are directed toward the exterior of the polyanion, W-Ob-W bonds (inter bridges between corner-sharing octahedral), W-Oc-W bonds (intra bridges between edge- sharing octahedral) and one XO4 tetrahedron. The XO4 tetrahedron is surrounded by MO6 octahedron sets linked together through oxygen atoms.
The IR spectra for A and B exhibited prominent bands for the polyoxoanion and THA and THPA at 600-1200 cm-1 and 1,300-3,000 cm-1, respectively (Figure 1 and Figure 2). The symmetric and asymmetric stretching for M-O bonds are observed in the following spectral regions for Preyssler anion: W=Od bonds (960 cm-1), W-Ob-W bridges (920 cm-1), and W-Oc-W bridges (795 cm-1). The P-O stretchings are observed in 1,000-1,165 cm-1. The sharp peak in 1,165 cm−1 is a characteristic signal of the Preyssler anion that cannot be observed in other heteropolyanions. With respect to the fingerprint region of the Preyssler anion (600-1,200 cm-1), the IR spectra showed that the polyanion retains the Preyssler structure in both A and B, and has electronic interactions with the organic moieties in the solid state.
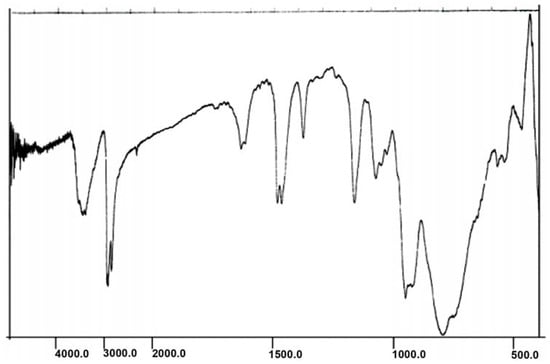
Figure 1.
IR spectrum of compound A.
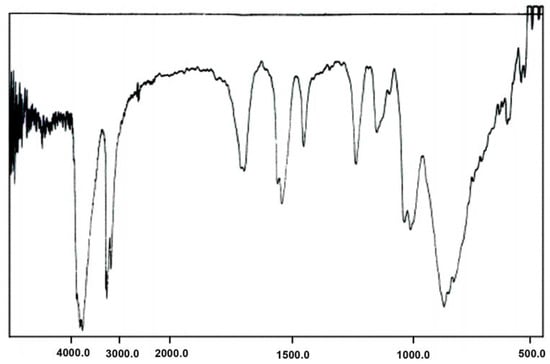
Figure 2.
IR spectrum of compound B.
2.2. UV spectrum
The UV spectra of A and B exhibit two peaks at 217 and 280 nm, ascribed to Ot→W and Ob,c→W charge transfer bands, respectively (Figure 3). The maximum wavelength in 280 nm confirms that the polyanion retains the Preyssler structure in both A and B.
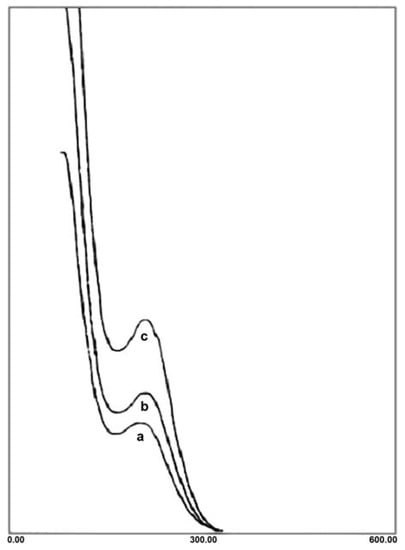
Figure 3.
UV spectra of Preyssler (a), A (b) and B (c).
2.3. Thermogravimetric analysis
Thermogravimetric analysis (TGA), coupled with spectroscopic measurements could unambiguously elucidate the structure of the compounds. The TGA of the two compounds was performed in the range of 50–600 °C. The TG curves generally show two steps. The first one occurs at temperatures lower than 120 °C, and corresponds to the loss of crystallization water. This leads to the corresponding anhydrous heteropolycompound. The second step occurs at temperatures higher than 200 °C, dependoing on the metal, heteroatom and the counterion. It corresponds to the decomposition of the heteropolycompound.
For the acids, the protons come off with oxygen, as n/2H2O. In the case of a mixed salt with acidic hydrogen and tetraalkylammonium cations, the weight loss is expressed as x (tetraalkylammonium)2O+n/2 H2O to take into account the departure of the organic material and the protons.
From room temperature to 130 °C, A remains stable and there is no weight loss (Figure 4). This means that this compound is anhydrous and contains no crystallization water. In the range of 180–400 °C, the total weight loss of A is 28.15% (calculated value: 28.21%), corresponding to 7.7/2 (THA)2O+ 6.3/2 H2O. Thus the synthesized compound A can be formulated as (THA)7.7 H 6.3[NaP5W30O110].
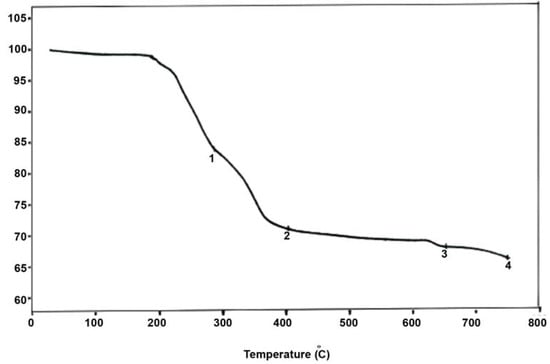
Figure 4.
Thermogravimetric data for compound A.
From room temperature to 130 °C, there was no weight loss for compound B (Figure 5). Thus, this compound is anhydrous. In the range of 200–440 °C, the weight loss was 30.30% (calculated value: 30.35%), corresponding to 7.5/2 (THPA)2O+ 6.5/2 H2O. Therefore the compound B is formulated as (THPA)7.5 H6.5[NaP5W30O110].
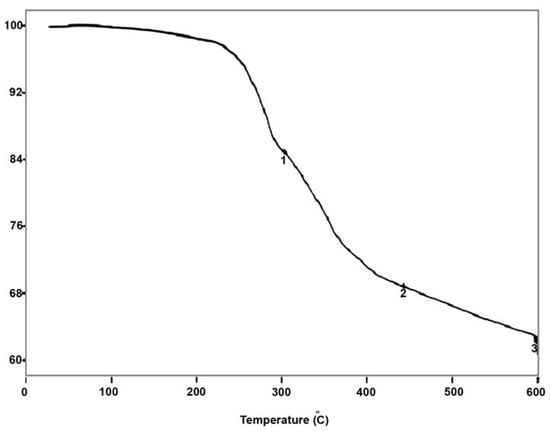
Figure 5.
Thermogravimetric data for compound B.
2.4. Potentiometeric titrations
The pH scale in an aqueous solution is governed by kw (10-14) and the equilibrium in water predicts a potential change of 0.059 V for each unit change in pH for the linear region of equation E = k + 0.059 pH [22]. In a similar way, the pH scale in non-aqueous solvents is governed by the autoprotolysis constants of the solvent. The solvents with small autoprotolysis constants were thought to be advantageous for titrations. They provide a better opportunity for precise titrations, because of their longer milivolt scales. In our study, we used solvents such as acetone, N,N-dimethylformamide and acetonitrile with longer milivolt ranges for the potentiometric titrations [22].
However in every case, the potentiometeric titration curves of A and B showed two or three protons were dissociated. This is attributed to the behavior of the glass electrode. A glass electrode is widely used as the indicator electrode in non-aqueous as well as in aqueous titrations. Its response to pH has been shown to be Nernstian in various non-aqueous solvents [23] and many acid dissociation constants in aprotic solvents have been obtained by using a glass electrode as pH sensor [24].
In non-aqueous solutions media, these electrodes show certain undesirable features. For example the potential response of a glass electrode in non-aqueous solutions is often very slow, so in some cases, it takes many hours before the equilibrium potential is reached. Some efforts have been made to improve the response speed, but without sufficient success [25]. In addition, the solvents dehydrate the glass membrane, thereby reducing its affinity for, or response to, hydrogen ions.
Thus, it is difficult to obtain reliable information concerning the number of acidic protons with a glass electrode. It is suggested that solvent as well as glass electrode can affect the number of titrated protons.
2.5. Conductometric titration
For A and B, determination of acidic hydrogens was carried out via conductometric titration in acetone, N,N-dimethylformamide and acetonitrile. In all cases the conductometric curves showed two or three protons were dissociated. It is well known that the proton conductivity of heteropolyacids is strictly related to the number of coordinated water molecules to the heteropolyanions. According to Kreuer, the heteropolyacid acts as a Bronsted acid toward the hydration water, which is loosely bound in the structure, resulting in proton conductivity [26].
Consequently, the conductivity of heteropolyacid is strictly related to the number of water molecules coordinated to the anion and a large uptake of water is essential for fast proton conduction. As indicated by the obtained thermogravimetric results, there are no water molecules in A and B. This leads to the absence of proton conduction.
Another reason can be explained based on hydrogen bonding. We have recently developed a systematic vibrational study of Keggin heteropolyacids with aminoacids [27]. Our study showed that intermolecular interactions such as hydrogen bonds between external oxygens and water molecules and aminoacids led to a change and split in the frequencies of the metal-oxygen stretching for polyanion. In Figure 1 and Figure 2 we can see the characteristic bands of Preyssler anion are unchanged. This is attributed to absence of water molecules, resulted in absence of hydrogen bonding, so can lead to absence of proton conductivity.
2.6. Catalytic activity
In connection with our earlier work using Keggin heteropolyacids in the synthesis of 4-amino[3,4-d] pyrimidines [28], we wish to report the results of our study on the use of A and B in this reaction. When a mixture of 5-amino-4-cyano-1 substituted-1H-pyrazoles and formamide in acetic acid was refluxed in the presence of A and B as catalyst, 4-amino[3,4-d]pyrimidines were obtained (Scheme 1). The results are summarized in Table 1. The results show that the catalysts can catalyze this reaction in mild conditions. All compounds were characterized by mass, IR, and 1H-NMR spectra (Table 2).
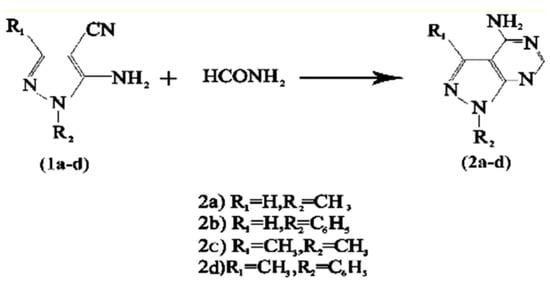
Scheme 1.
Catalytic synthesis of 4-amino[3,4-d]pyrimidines (2a-d) in the presence of A and B.

Table 1.
Catalytic synthesis of 4-amino[3,4-d ]pyrimidines 2a-d.

Table 2.
Spectral data for 2a-d.
3. Experimental
3.1. Chemicals and apparatus
Preyssler anion was synthesized according to our earlier work [6]. Pyrazole derivatives were prepared according to literature procedures [29,30]. All of the reagents used were obtained from commercial sources. All yields were calculated from crystallized products. IR spectra were obtained with a Bruker 500 scientific spectrometer. 1H-NMR spectra were recorded on a Bruker 100 MHz Aspect 3000 FT NMR spectrometer. Mass spectra were obtained with a Varian CH-7 instrument. The UV spectra were obtained with a double beam UV-Vis spectrophotometer (Philips 8800). The TG curves were recorded using a TGA-1500 instrument. Melting points were obtained on an Electrothermal type 9100 apparatus. All of the titrations were performed with tetrabutylammonium hydroxide.
3.2. General procedure for synthesis of compounds A and B
To a stirred aqueous solution of H14[NaP5W30O110]·25.5 H2O was added dropwise a solution of THABr or THPABr (molar ratio: 1:14). The mixture was stirred for about 6 hours and induced the formation of a viscous solid and an oil for THA and THPA, respectively. The separated compounds were purified in a mixture of water and acetonitrile. Recrystalization was performed in water and acetonitrile.
3.3. Catalytic test
An appropriate pyrazole (0.1 mmole) mixed with formamide (0.2 mmol) and catalyst (0.05 mmol) in acetic acid (10 mL) and refluxed for 6 hours. After the reaction was completed, the catalyst was separated. The residue was evaporated and water was added. The product was filtered and purified from a mixture of water and ethanol (Table 1). All compounds were characterized by mass, IR, and 1H-NMR spectra (Table 2).
4. Conclusions
Two novel organic- norganic heteropolyanions were prepared for the first time and showed catalytic activity in the synthesis of pyrazolopyrimidines. Thermogravimetric analysis showed that A and B consist of 7.7 and 7.5 tetraalkylammonium cations, respectively. This work demonstrates the applicability of the Preyssler anion for some reactions that require bifunctional catalysts with hydrogen ions and organic section along with catalytic activity and functionality over a wide range of pH.
References
- Muller, A.; Peters, F.; Pope, M.T.; Gatteschi, D. Polyoxometalates: Very large clusters-nanoscale magnets. Chem. Rev. 1998, 98, 239–271. [Google Scholar] [CrossRef] [PubMed]
- Bamoharram, F.F.; Heravi, M.M.; Roshani, M.; Jahangir, M. Gharib, An effective direct esterification of butanol by eco-friendly Preyssler catalyst. [NaP5W30O110]14-. J. Mol. Catal. A Chem. 2007, 271, 126–130. [Google Scholar] [CrossRef]
- Bamoharram, F.F.; Heravi, M.M.; Roshani, M.; Akbarpour, M. Catalytic performance of Preyssler heteropolyacid as a green and recyclable catalyst in oxidation of primary aromatic amines. J. Mol. Catal. A Chem. 2006, 255, 193–198. [Google Scholar] [CrossRef]
- Bamoharram, F.F.; Heravi, M.M.; Roshani, M.; Tavakoli, N. N-oxidation of pyridine carboxylic acids using hydrogen peroxide catalyzed by a green heteropolyacid catalyst: Preyssler's anion, [NaP5W30O110]14−. J. Mol. Catal. A Chem. 2006, 252, 219–225. [Google Scholar] [CrossRef]
- Bamoharram, F.F.; Heravi, M.M.; Roshani, M.; Jahangir, M.; Gharib, A. A catalytic method for synthesis of γ-butyrolactone, -caprolactone and 2-cumaranone in the presence of Preyssler's anion, [NaP5W30O110]14−, as a green and reusable catalyst. J. Mol. Catal. A Chem. 2006, 252, 90–95. [Google Scholar] [CrossRef]
- Bamoharram, F.F.; Heravi, M.M.; Roshani, M.; Jahangir, M.; Gharib, A. Preyssler catalyst, [NaP5W30O110]14−: A green, efficient and reusable catalyst for esterification of salicylic acid with aliphatic and benzylic alcohols. Appl. Catal. A Gen. 2006, 302, 42–47. [Google Scholar] [CrossRef]
- Kapustin, G.I.; Brueva, T.R.; Klyachko, A.L.; Timofeeva, M.N.; Kulikov, S.M.; Kozhevnikov, I.V. A study of the acidity of heteropoly acids. Kinet. Katal. 1990, 31, 1017–1020. [Google Scholar]
- Kozhevnikov, I.V. Catalysis by heteropoly acids and multicomponent polyoxometalates in liquid-phase reactions. Chem. Rev. 1998, 98, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Urabe, K.; Onaka, M. Zeolite, Clay and Heteropoly Acid in Organic Reactions; Kodansha/VCH: Tokyo, Japan, 1992; p. 99. [Google Scholar]
- Pope, M.T. Heteropoly and Isopoly Oxometalates; Springer: Berlin, Germany, 1983. [Google Scholar]
- Kozhevnikov, I.V. Catalysis by Polyoxometalates. In Catalysts for fine chemical synthesis; Wiley & Sons: Chichester, UK, 2002; Volume 2, p. 216. [Google Scholar]
- Kozhevnikov, I.V. Sustainable Heterogeneous Acid Catalysis by Heteropoly Acids, In Handbook of Green Chemistry – Green Catalysis, Vol. 2: Heterogeneous Catalysis; Paul, T.A., Robert, H.C., Eds.; Wiley-VCH: Weinheim, Germany, 2009; pp. 153–174. [Google Scholar]
- Misono, M.; Ono, I.; Koyano, G.; Aoshima, A. Heteropolyacids. Versatile green catalysts usable in a variety of reaction media. Pure Appl. Chem. 2000, 72, 1305–1311. [Google Scholar] [CrossRef]
- Bamoharram, F.F.; Heravi, M.M.; Roshani, M. Gharib, A Catalytic method for synthesis of aspirin by a green, efficient and recyclable solid acid catalyst (Preyssler's anion) at room temperature. J. Chin. Chem. Soc. 2006, 17, 505–509. [Google Scholar]
- Bamoharram, F.F.; Heravi, M.M.; Roushani, M.; Toosi, M. Synthesis and characterization of silica-supported Preyssler nanoparticles and its catalytic activity for photodegradation of methyl orange. Green Chem. Lett. Rev. 2009, 2, 35–41. [Google Scholar] [CrossRef]
- Bamoharram, F.F.; Heravi, M.M.; Heravi, M.M.; Meraji, M. Synthesis of silver nanoparticles in the presence of a green heteropolyacid, H14[NaP5W30O110 ], and their catalytic activity for photo degradation of methylen blue and methyl orange. Int. J. Green Nanotech. 2009, 1, 26–31. [Google Scholar] [CrossRef]
- Bamoharram, F.F.; Heravi, M.M.; Heravi, M.H.; Dehghan, M. Photocatalytic oxidation of benzyl alcohols in the presence of H14[ NaP5W30O110] as a green and reusable catalyst”, Syn. React. Inorg. Metal-Org. Nano-Metal Chem. 2009, 39, 394–399. [Google Scholar]
- Rombaut, G.; Turner, S.S.; Pévelen, D.L.; Mathonière, C.; Day, P.; Prout, K. An unusual phase transition in the crystal structure and physical properties of (TTF)9[Mo(CN)8]2·4H2O, where TTF = tetrathiafulvalene. J. Chem. Soc. Dalton Trans. 2001, 3244–3249. [Google Scholar] [CrossRef]
- Bi, L.; Wang, E.B.; Xu, L.; Huang, R.D. Synthesis, properties and crystal structure of some polyoxometallates containing the tris(hydroxymethyl)aminomethane cation. Inorg. Chim. Acta 2000, 305, 163–171. [Google Scholar] [CrossRef]
- Ouahab, L.; Bencharif, M.; Mhanni, A.; Pelloquin, D.; Halet, J.F.; Pena, O.; Padiou, J.; Grandjean, D.; Garrigou-Lagrange, C. Preparations, x-ray crystal structures, EH band calculations, and physical properties of [(TTF)6(H)(XM12O40)(Et4N)] (M = tungsten, molybdenum; X = phosphorus, silicon): Evidence of electron transfer between organic donors and polyoxometalates. Chem. Mater. 1992, 4, 666–674. [Google Scholar] [CrossRef]
- Alizadeh, M.H.; Harmalker, S.P.; Jeannin, Y.; Martin-Frere, J.; Pope, M.T. A heteropolyanion with fivefold molecular symmetry that contains a nonlabile encapsulated sodium ion. The structure and chemistry of [NaP5W30O110]14−. J. Am. Chem. Soc. 1985, 107, 2662–2669. [Google Scholar] [CrossRef]
- Walter, H. Titrations in nonaqueous solvents; Academic Press: New York, NY, USA/London, UK, 1967; p. 74. [Google Scholar]
- Safaric, L.; Stransky, Y. Titrimetric Analysis in Organic Solvents; Elsevier: Amsterdam, The Netherlands, 1986; p. 71. [Google Scholar]
- Izutsu, K. Acid–Base Dissociation Constants in Dipolar Aprotic Solvents; Blackwell Scientific Publications: Oxford, UK, 1990. [Google Scholar]
- Bates, R.G. Detemination of pH, Theory and Practice; Wiley: New York, NY, USA, 1973; p. 372. [Google Scholar]
- Kreuer, K.D. Proton conductivity: Materials and applications. Chem. Mater. 1996, 8, 610–641. [Google Scholar] [CrossRef]
- Bamoharram, F.F. Study of the interaction of Keggin heteropoly anions and amino acids by vibrational spectra. Molecules 2009, 14, 3214–3221. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Rajabzadeh, G.; Bamoharram, F.F.; Seifi, N. An eco-friendly catalytic route for synthesis of 4-amino-pyrazolo[3,4-d]pyrimidine derivatives by Keggin heteropolyacids under classical heating and microwave irradiation. J. Mol. Catal. A Chem. 2006, 256, 238–241. [Google Scholar] [CrossRef]
- Cheng, C.C.; Robins, R.K. Potential furine an- tagonists. VI. synthesis of 1-alkyl- and l-aryl-4-substituted pyrazolo(3,4-d)pyrimidines). J. Org. Chem. 1956, 21, 1240–1256. [Google Scholar] [CrossRef]
- Press, J.B.; Eudy, N.H.; Lovell, F.M.; Morton, G.O.; Siegel, M.M. A remarkable fragmentation reaction of a pyrimidyl radical. J. Am. Chem. Soc. 1982, 104, 4013–4014. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds of A and B and the pyrazolopyrimidines in Table 1 are available from the author. |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).