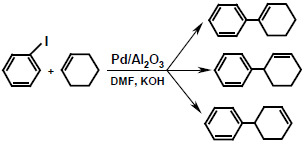
Selective Heck Arylation of Cyclohexene with Homogeneous and Heterogeneous Palladium Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Homogeneous catalysts

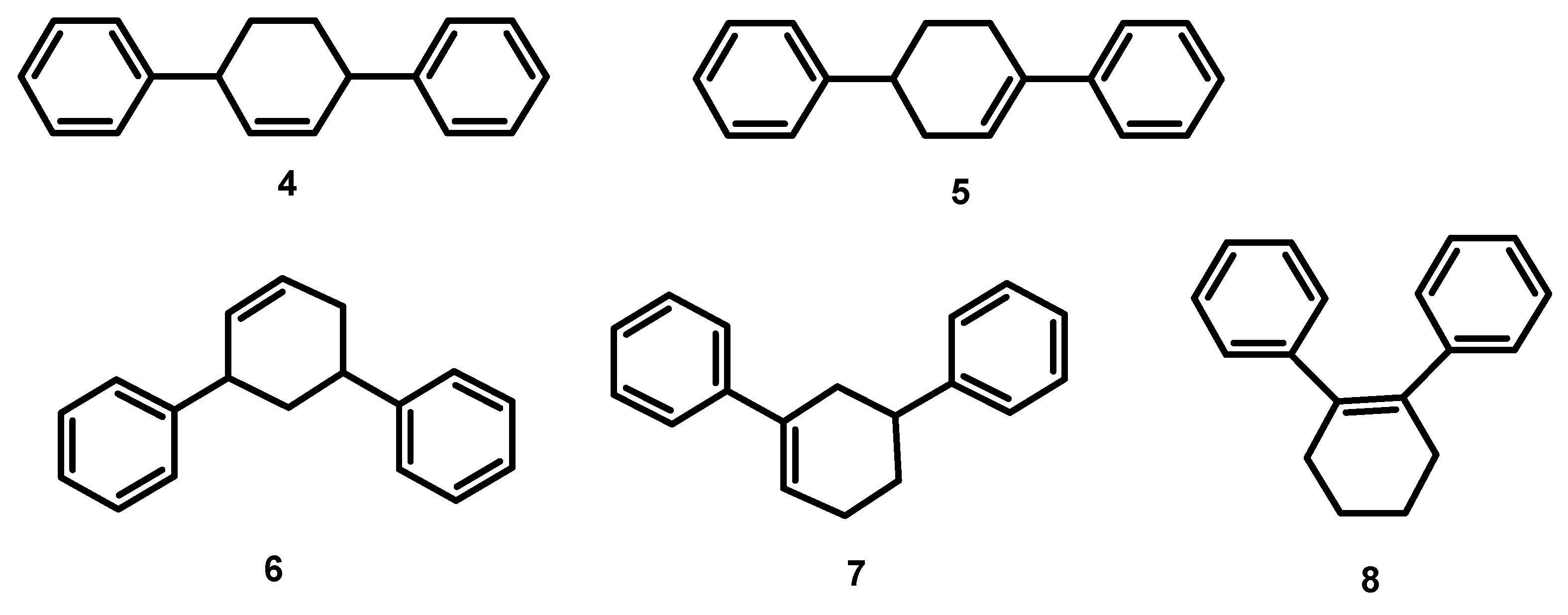
| Catalyst | Base | PhI conversion (%) | Yield
1+2+3 (%) | Selectivity 1:2:3 | Yield
4+5+6+7+8 (%) | Biphenyl
(%) |
|---|---|---|---|---|---|---|
| Pd(OAc)2 | NaHCO3 | 74 | 55 | 16:20:64 | 16 | 4 |
| NaOAc | 90 | 53 | 17:21:62 | 27 | 9 | |
| KOH | 100 | 67 | 13:3:84 | 23 | 10 | |
| PdCl2(PhCN)2 | NaHCO3 | 81 | 53 | 19:19:62 | 17 | 11 |
| NaOAc | 88 | 53 | 18:28:54 | 30 | 15 | |
| KOH | 100 | 60 | 15:5:80 | 20 | 20 |
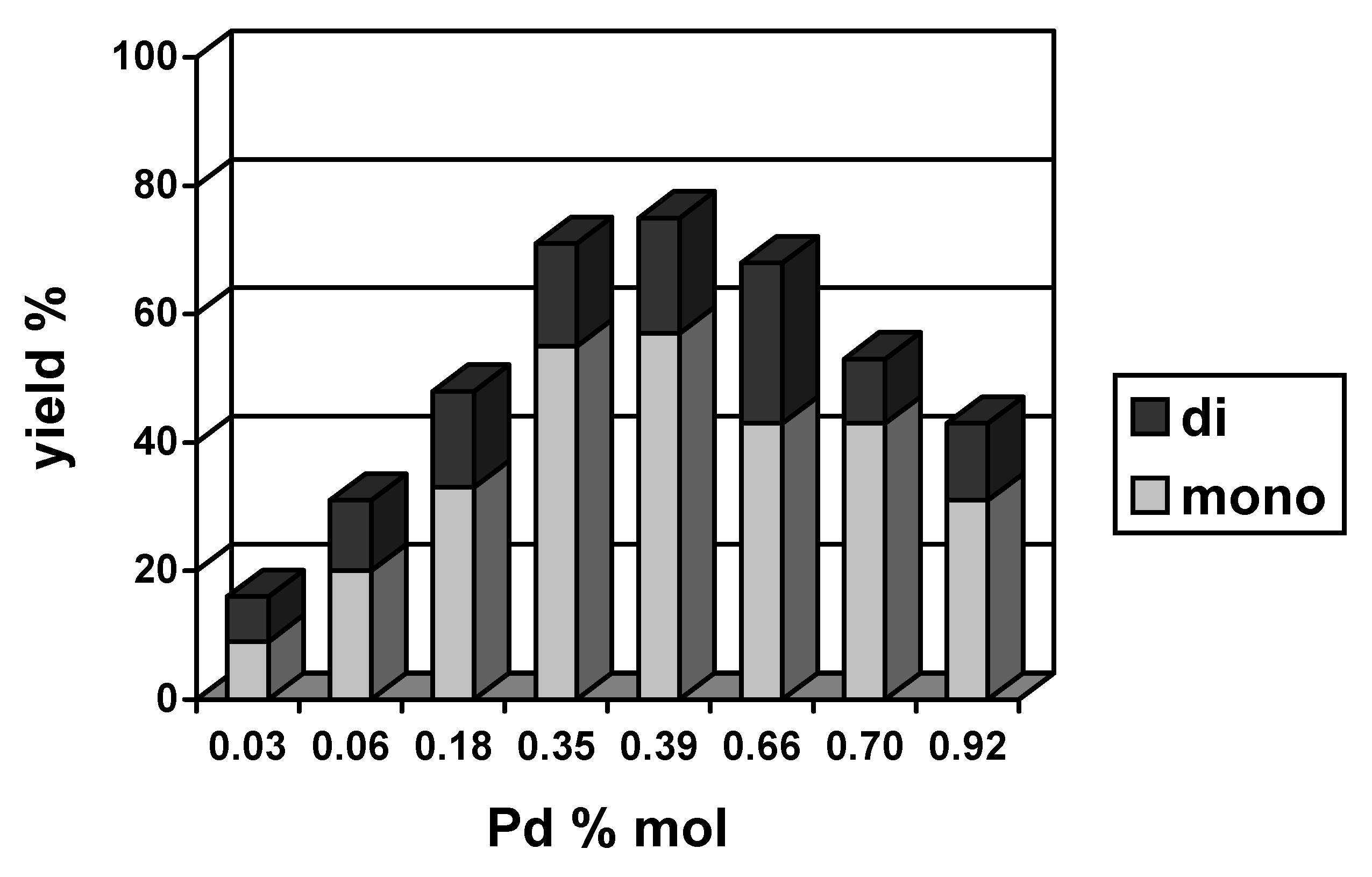
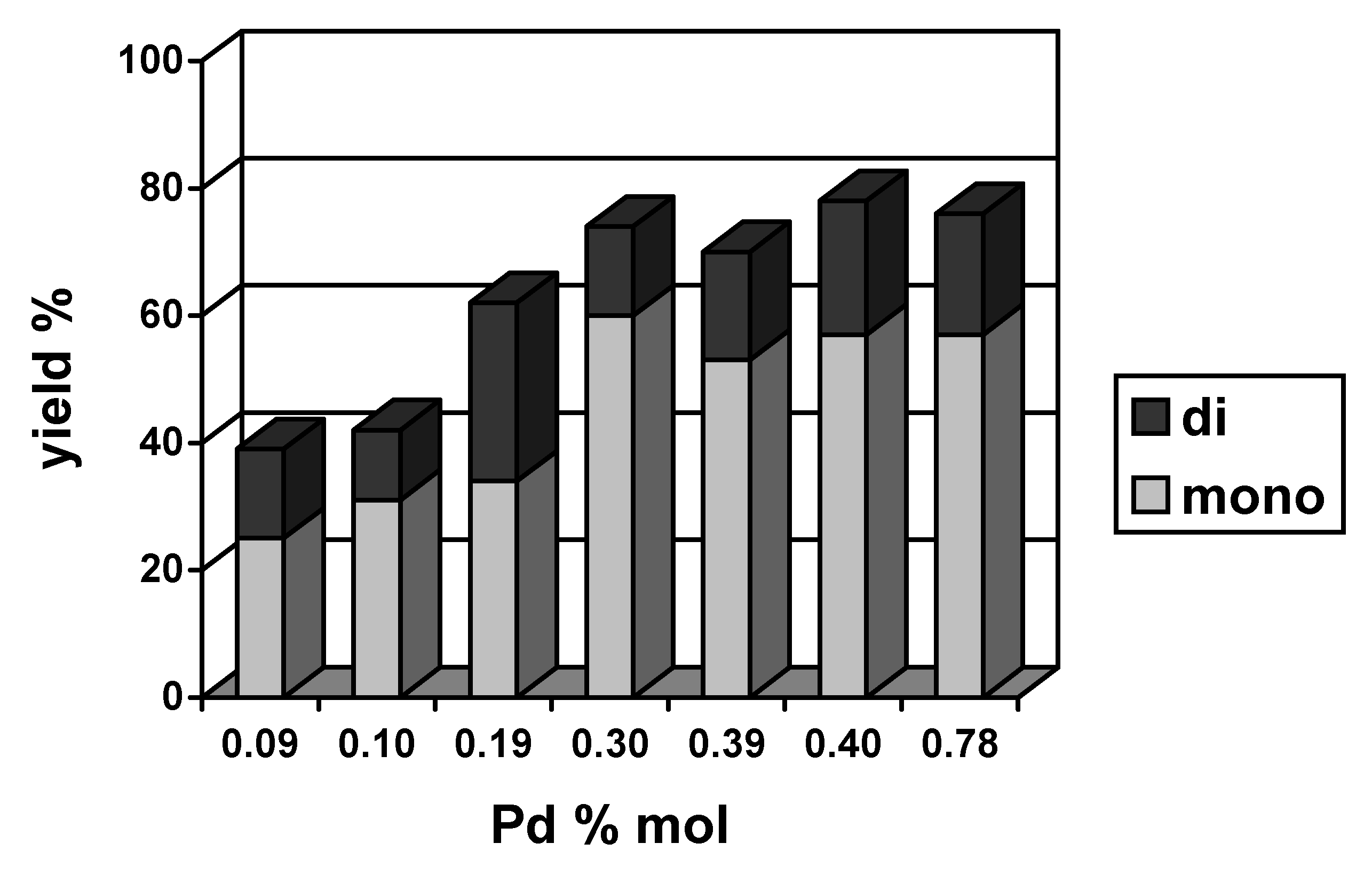
2.2. Heterogeneous catalysts
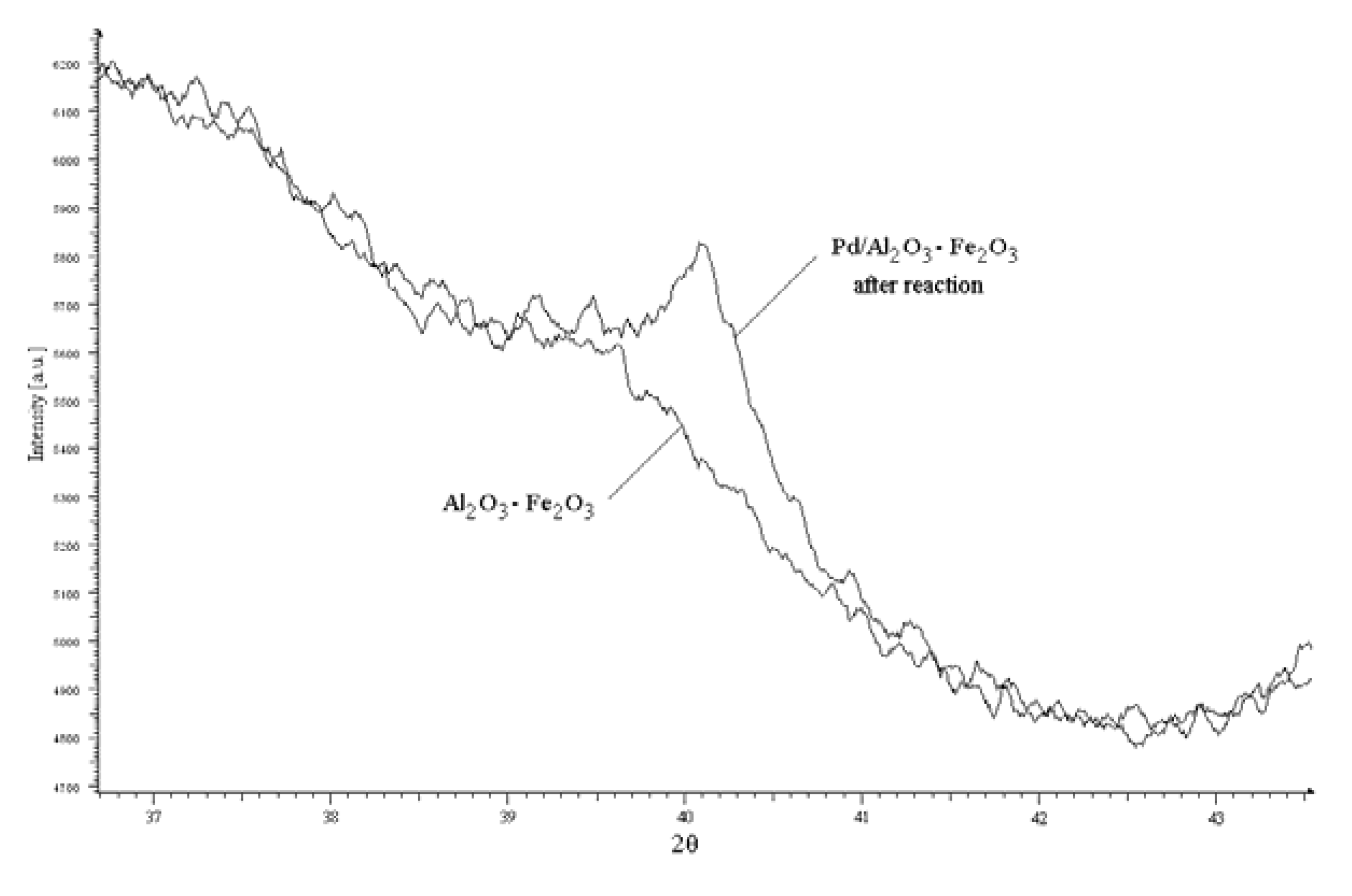
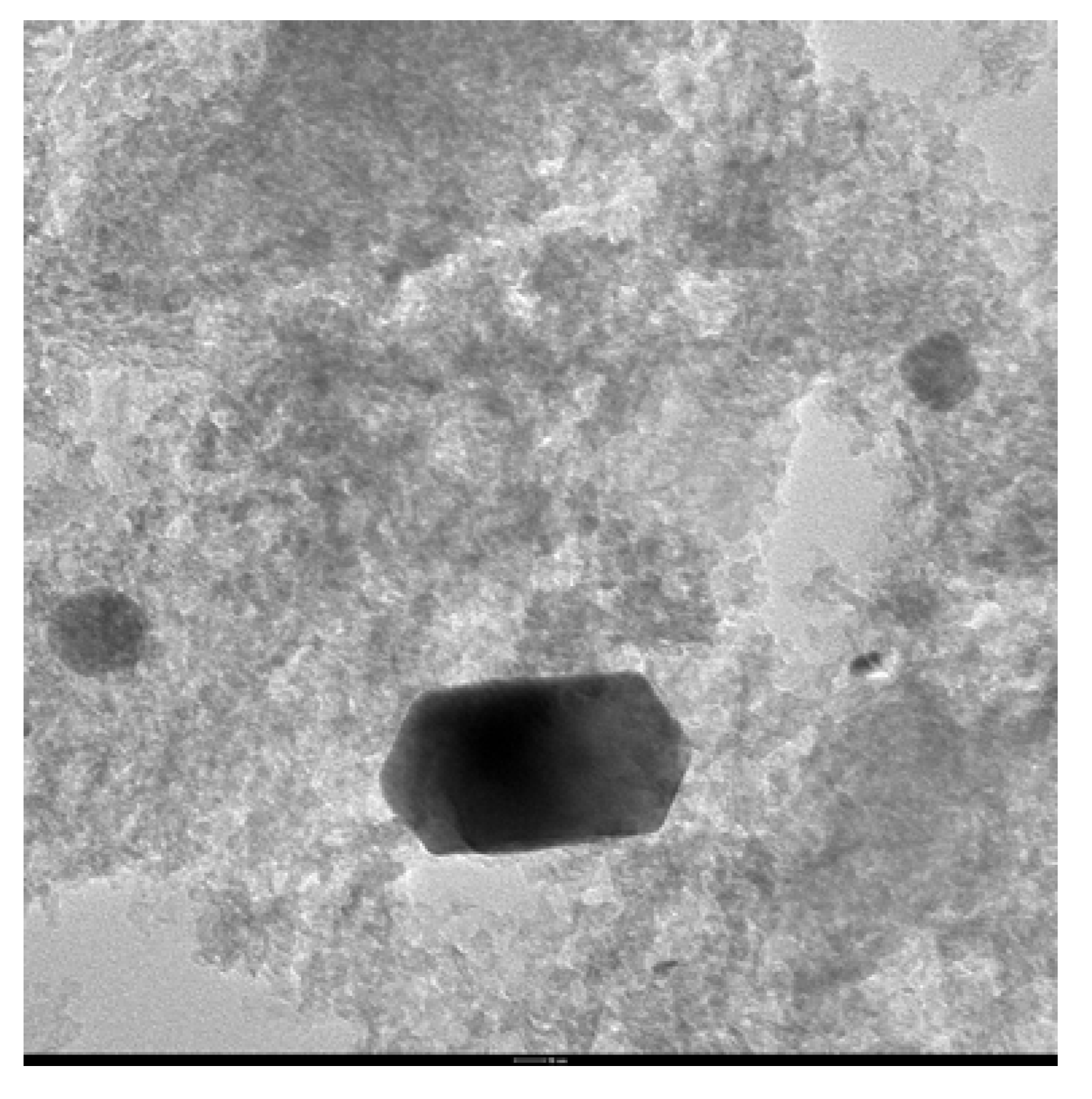
| Pd% mol | Solvent | [PPh3]:[Pd] | PhI conversion (%) | Yield
1+2+3 (%) | Selectivity 1:2:3 | Yield
4+5+6+7+8 (%) | Biphenyl
(%) |
|---|---|---|---|---|---|---|---|
| 0.26 | DMF | 39 | 31 | 16:13:71 | 4 | 5 | |
| 0.26 | [Bu4N]Br | 79 | 32 | 16:47:38 | 31 | 15 | |
| 0.35 | DMF | 43 | 33 | 15:12:73 | 5 | 6 | |
| 0.35 | [Bu4N]Br | 91 | 45 | 13:49:38 | 30 | 16 | |
| 0.53 | DMF | 64 | 42 | 14:10:76 | 10 | 12 | |
| 0.53 | [Bu4N]Br | 85 | 40 | 13:57:39 | 29 | 16 | |
| 0.35 | DMF | 5 | 27 | 17 | 18:12:70 | 4 | 5 |
| 0.35 | DMF | 10 | 32 | 22 | 18:5:77 | 4 | 5 |
| Catalyst | Base | PhI conversion (%) | Yield
1+2+3 (%) | Selectivity 1:2:3 | Yield
4+5+6+7+8 (%) | Biphenyl
(%) |
|---|---|---|---|---|---|---|
| Pd/Al2O3 + ZrO2 | NaHCO3 | 66 | 41 | 17:3:80 | 9 | 14 |
| KOH | 89 | 78 | 13:3:84 | 4 | 7 | |
| Pd/Al2O3 + CeO2 | NaHCO3 | 64 | 42 | 14:10:76 | 10 | 12 |
| KOH | 90 | 67 | 14:4:82 | 18 | 15 | |
| Pd/Al2O3 + Fe2O3 | NaHCO3 | 63 | 47 | 15:6:79 | 7 | 8 |
| KOH | 98 | 81 | 11:1:88 | 12 | 5 | |
| Pd/Al2O3 | NaHCO3 | 58 | 37 | 16:8:76 | 7 | 14 |
| NaOAc | 57 | 43 | 16:7:77 | 7 | 8 | |
| K2CO3 | 86 | 48 | 15:12:73 | 9 | 29 | |
| NaOH | 90 | 53 | 17:4:79 | 22 | 15 | |
| KOH | 88 | 65 | 12:0:88 | 12 | 11 |
3. Experimental
3.1. Preparation of alumina-based supports
3.2. Preparation of supported Pd(II) catalysts
3.3. Heck reaction procedure
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- Heck, R.F. Palladium-catalyzed reactions of organic halides with olefins. Acc. Chem. Res. 1979, 12, 146–151. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Cherpakov, A.V. The Heck Reaction as a Sharpening Stone of Palladium Catalysis. Chem. Rev. 2000, 100, 3009–3066. [Google Scholar] [CrossRef]
- Whitcombe, N.J.; Hii, K.K.; Gibson, S.E. Advances in the Heck chemistry of aryl bromides and chlorides. Tetrahedron 2001, 57, 7449–7476. [Google Scholar] [CrossRef]
- Trzeciak, AM.; Ziółkowski, J.J. Structural and mechanistic studies of Pd-catalyzed C-C bond formation: The case of carbonylation and Heck reaction. Coord. Chem. Rev. 2005, 249, 2308–2322. [Google Scholar] [CrossRef]
- Tsuji, I. Palladium Reagents and Catalysts. In New Perspectives for the 21 st Century; John Wiley & Sons, Ltd.: Chichester, West Sussex, UK, 2004; pp. 105–176. [Google Scholar]
- Bedford, R.B.; Cazin, C.S.J.; Holder, D. The development of palladium catalysts for C-C and C-heteroatom bond forming reactions of aryl chloride substrates. Coord. Chem. Rev. 2004, 248, 2283–2321. [Google Scholar] [CrossRef]
- Peris, E.; Crabtree, R.H. Recent homogeneous catalytic applications of chelate and pincer N-heterocyclic carbenes. Coord. Chem. Rev. 2004, 248, 2239–2246. [Google Scholar] [CrossRef]
- Gniewek, A.; Trzeciak, A.M.; Ziółkowski, J.J.; Kępiński, L.; Wrzyszcz, J.; Tylus, W. Pd-PVP colloid as catalyst for Heck and carbonylation reactions: TEM and XPS studies. J. Catal. 2005, 229, 332–343. [Google Scholar] [CrossRef]
- Reetz, M.T.; Westermann, E. Phosphane-Free Palladium-Catalyzed Coupling Reactions: The Decisive Role of Pd Nanoparticles. Angew. Chem. Int. Ed. 2000, 39, 165–168. [Google Scholar] [CrossRef]
- Calo, V.; Nacci, A.; Monopoli, A.; Laera, S.; Cioffi, N. Pd Nanoparticles Catalyzed Stereospecific Synthesis of β-Aryl Cinnamic Esters in Ionic Liquids. J. Org. Chem. 2003, 68, 2929–2933. [Google Scholar] [CrossRef]
- Pryjomska-Ray, I.; Trzeciak, A.M.; Ziółkowski, J.J. Base free efficient palladium catalysts of Heck reaction in molten tetrabutylammonium bromide. J. Mol. Catal. A: Chem. 2006, 257, 3–8. [Google Scholar]
- Glorius, F. N-heterocyclic carbenes in transition metal catalysis. In Top. Organomet. Chem.; Glorius, F., Ed.; Springer-Verlag: Berlin, Heidelberg, New York, NY, USA, 2007; Volume 3, pp. 1–218. [Google Scholar]
- Scott, M.N.; Nolan, S.P. Cross-coupling reactions catalyzed by palladium N-heterocyclic carbene complexes. In N-heterocyclic Carbenes in Synthesis; Nolan, S.P., Ed.; Wiley-VCH: Weinheim, Germany, 2006; pp. 55–70. [Google Scholar]
- Moreno-Manas, M.; Pleixats, R. Formation of carbon-carbon bonds under catalysis by transition-metal nanoparticles. Acc. Chem. Res. 2003, 36, 638–643. [Google Scholar] [CrossRef]
- Trzeciak, A.M.; Ziółkowski, J.J. Monomolecular, nanosized and heterogenized palladium catalysts for the Heck reaction. Coord. Chem. Rev. 2007, 251, 1281–1293. [Google Scholar]
- Evangelisti, C.; Panziera, N.; Petrici, P.; Vitulli, G.; Salvadori, P.; Battocchio, C.; Polzonetti, G. Palladium nanoparticles supported on polyvinylpyridine: Catalytic activity in Heck-type reactions and XPS structural studies. J. Catal. 2009, 262, 287–293. [Google Scholar]
- Calo, V.; Nacci, A.; Lopez, L.; Napoli, A. Arylation of α-substituted acrylates in ionic liquids catalyzed by a Pd-benzothiazole carbene complex. Tetrahedron Lett. 2001, 42, 4701–4703. [Google Scholar] [CrossRef]
- Calo, V.; Nacci, A.; Lopez, L.; Mannarini, N. Heck reaction in ionic liquids catalyzed by a Pd-benzothiazole carbene complex. Tetrahedron Lett. 2000, 41, 8973–8976. [Google Scholar] [CrossRef]
- Jeffery, T.; Galland, J.-C. Tetraalkylammonium salts in Heck-type reactions using an alkali-metal hydrogen carbonate or an alkali-metal acetate as the base. Tetrahedron Lett. 1994, 35, 4101–4106. [Google Scholar]
- Jeffery, T.; David, M. [Pd/Base/QX] catalyst systems for directing Heck-type reactions. Tetrahedron Lett. 1998, 39, 5751–5754. [Google Scholar] [CrossRef]
- Biffis, A.; Zecca, M.; Basato, M. Metallic palladium in the Heck reaction: active catalyst or convenient precursor? Eur. J. Chem. 2001, 5, 1131–1133. [Google Scholar]
- Pryjomska-Ray, I.; Gniewek, A.; Trzeciak, A.M.; Ziółkowski, J.J.; Tylus, W. Homogeneous/heterogenous palladium based catalytic system for Heck reaction. The reversible transfer of palladium between solution and support. Topics Catal. 2006, 40, 173–184. [Google Scholar]
- Pröckl, S.S.; Kleist, W.; Gruber, M.A.; Köhler, K. In situ generation of highly active dissolved palladium species from solid catalysts - a concept for the activation of aryl chlorides in the Heck reaction. Angew. Chem. Int. Ed. 2004, 43, 1881–1882. [Google Scholar] [CrossRef]
- Kleist, W.; Lee, J.-K.; Köhler, K. Pd/MOx materials synthesized by sol-gel coprecipitation as catalysts for carbon-carbon coupling reactions of aryl bromides and chlorides. Eur. J. Inorg. Chem. 2009, 2, 261–266. [Google Scholar]
- Köhler, K.; Wagner, M.; Djakovitch, L. Supported palladium as catalyst for carbon-carbon bond construction (Heck reaction) in organic synthesis. Catal. Today 2001, 66, 105–114. [Google Scholar]
- Pröckl, S.S.; Kleist, W.; Köhler, K. Design of highly active heterogeneous palladium catalysts for the activation of aryl chlorides in Heck reactions. Tetrahedron 2005, 61, 9855–9859. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Len, C.; Fihri, A. Silica-supported palladium: sustainable catalysts for cross-coupling reactions. Coord. Chem. Rev. 2009, 253, 2599–2626. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Molnar, A. Silica-supported Pd catalysts for Heck coupling reactions. Tetrahedron 2007, 63, 6949–6976. [Google Scholar] [CrossRef]
- Djakovitch, L.; Heise, H.; Köhler, K. Heck reactions between aryl halides and olefins catalysed by Pd-complexes entrapped into zeolites NaY. J. Organomet. Chem. 1999, 584, 16–26. [Google Scholar]
- Dams, M.; Drijkoningen, L.; Pauwels, B.; Van Tendeloo, G.; De Vos, D.E.; Jakobs, P.A. Pd-Zeolites as heterogeneous catalysts in Heck chemistry. J. Catal. 2002, 209, 225–236. [Google Scholar]
- Yin, Y.; Liebscher, J. Carbon-carbon coupling reactions catalyzed by heterogeneous palladium catalysts. Chem. Rev. 2007, 107, 133–173. [Google Scholar]
- Zhao, F.; Shirai, M.; Ikushima, Y.; Arai, M. The leaching and re-deposition of metal species from and onto conventional supported palladium catalysts in the Heck reaction of iodobenzene and methyl acrylate in N-methylpyrrolidone. J. Mol. Catal. A: Chem. 2002, 180, 211–219. [Google Scholar]
- Wagner, M.; Hartung, G.G.; Beller, M.; Köhler, K. Pd-catalyzed Heck arylation of cycloalkenes–studies on selectivity comparing homogeneous and heterogeneous catalysts. J. Mol. Catal. A: Chem. 2004, 219, 121–130. [Google Scholar]
- Hartung, G.; Koehler, K.; Beller, M. Highly selective palladium – catalyzed Heck reaction of aryl bromides with cycloalkenes. Org. Lett. 1999, 1, 709–711. [Google Scholar] [CrossRef]
- Kaganovsky, L.; Cho, K.-B.; Gelman, D. New trans-chelating ligands and their complexes and catalytic properties in the Mizoroki – Heck arylation of cyclohexene. Organometallics 2008, 27, 5139–5145. [Google Scholar] [CrossRef]
- Dodd, D.W.; Toews, H.E.; Carneiro, F.D.S.; Jennings, M.C.; Jones, N.D. Model intermolecular, asymmetric Heck reactions catalyzed by chiral pyridyloxazoline palladium(II) complexes. Inorg. Chim. Acta 2006, 329, 2850–2858. [Google Scholar]
- Berthiol, F.; Doucet, H.; Santelli, M. Heck reaction of aryl halides with linear or cyclic alkenes catalysed by tetraphosphine/palladium catalyst. Tetrahedron Lett. 2003, 44, 1221–1225. [Google Scholar] [CrossRef]
- Rosol, M.; Moyano, A. 1,-carbopalladated-4-ferrocenyl-1,3-oxazolines as catalysts for Heck reactions: Further evidence in support of the Pd(0)/Pd(II) mechanism. J. Organomet. Chem. 2005, 690, 2291–2296. [Google Scholar] [CrossRef]
- Wheatley, B.M.M.; Keay, B.A. Use of deuterium labelling studies to determine the stereochemical outcome of palladium migration during an asymmetric intermolecular Heck reaction. J. Org. Chem. 2007, 72, 7253–7259. [Google Scholar] [CrossRef]
- Gron, L.U.; Tinsley, A.S. Tailoring aqueous solvents for organic reactions: Heck coupling reactions in high temperature water. Tetrahedron Lett. 1999, 40, 227–230. [Google Scholar] [CrossRef]
- Gniewek, A.; Ziółkowski, J.J.; Trzeciak, A.M.; Zawadzki, M.; Grabowska, H.; Wrzyszcz, J. Palladium nanoparticles supported on alumina-based oxides as heterogeneous catalysts of the Suzuki-Miyaura reaction. J. Catal. 2008, 254, 121–130. [Google Scholar]
- Mieczyńska, E.; Pryjomska-Ray, I.; Trzeciak, A.M.; Grabowska, H.; Zawadzki, M. The Heck arylation of mono- and disubstituted olefins catalyzed by palladium supported on alumina-based oxides. New J. Chem. 2010, submitted. [Google Scholar]
- Wojtków, W.; Trzeciak, A.M.; Choukroun, R.; Pellegatta, J.L. Pd-colloid catalyzed methoxycarbonylation of iodobenzene in ionic liquids. J. Mol. Catal. A: Chem. 2004, 224, 81–86. [Google Scholar]
- De Vries, J.G. A unifying mechanism for all high-temperature Heck reactions. The role of palladium colloids and anionic species. Dalton Trans. 2006, 421–429. [Google Scholar] [CrossRef]
- Calo, V.; Nacci, A.; Monopoli, A.; Fornaro, A.; Sabbatini, L.; Cioffi, N.; Ditaranto, N. Heck reaction catalyzed by nanosized palladium on chitosan in ionic liquids. Organometallics 2004, 23, 5154–5158. [Google Scholar] [CrossRef]
- Colussi, S.; Trovarelli, A.; Groppi, G.; Llorca, J. The effect of CeO2 on the dynamics of Pd-PdO transformation over Pd/Al2O3 combustion catalysts. Catal. Comm. 2007, 8, 1263–1266. [Google Scholar] [CrossRef]
- Romanova, R.G.; Petrova, E.V. Acid-base surface propertis of binary systems based on aluminum and zirconium oxides. Kinet. Catal. 2006, 47, 138–147. [Google Scholar] [CrossRef]
- Enache, D.; Roy-Auberger, M.; Esterle, K.; Revel, R. Preparation of Al2O3-ZrO2 mixed supports; thair characteristics and hydrothermal stability. Colloids Surf. A: Physicochem. Eng. Aspects 2003, 220, 223–233. [Google Scholar]
- McLafferty, F.W.; Staffer, D.B. The Wiley/NBS Registry of Mass Spectra Data; Wiley-Interscience Publication, John Wiley&Sons: New York, NY, USA, 1989; p. 453. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mieczyńska, E.; Trzeciak, A.M. Selective Heck Arylation of Cyclohexene with Homogeneous and Heterogeneous Palladium Catalysts. Molecules 2010, 15, 2166-2177. https://doi.org/10.3390/molecules15042166
Mieczyńska E, Trzeciak AM. Selective Heck Arylation of Cyclohexene with Homogeneous and Heterogeneous Palladium Catalysts. Molecules. 2010; 15(4):2166-2177. https://doi.org/10.3390/molecules15042166
Chicago/Turabian StyleMieczyńska, Ewa, and Anna M. Trzeciak. 2010. "Selective Heck Arylation of Cyclohexene with Homogeneous and Heterogeneous Palladium Catalysts" Molecules 15, no. 4: 2166-2177. https://doi.org/10.3390/molecules15042166
APA StyleMieczyńska, E., & Trzeciak, A. M. (2010). Selective Heck Arylation of Cyclohexene with Homogeneous and Heterogeneous Palladium Catalysts. Molecules, 15(4), 2166-2177. https://doi.org/10.3390/molecules15042166