Liquid-Phase Synthesis of Cyanuric Acid from Urea
Abstract
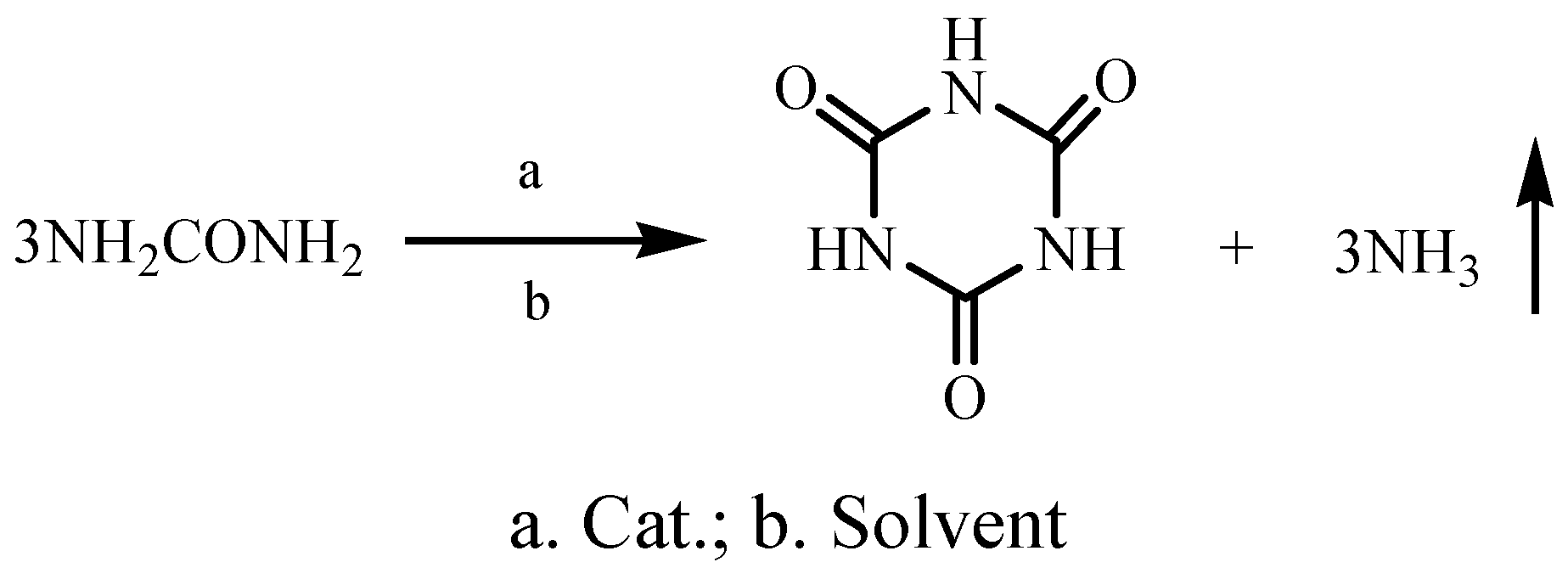
:Introduction
Results and Discussion
Experimental
General
Synthesis of cyanuric acid
Conclusions
Acknowledgements
References and Notes
- Patil, S.P.; Padmanabhan, D. A facile preparation of (2,4,6-14C)-cyanuric acid under solvent-free conditions. J. Label Compd. Radiophar. 2002, 45, 539–542. [Google Scholar] [CrossRef]
- Zeng, Y.F. The application of cyanuric acid in chemical industry. Fine Chem. Ind. 1987, 2, 42–47. [Google Scholar]
- Onoda, H.; Takenaka, A. Influence of addition of urea and its related compounds on formation of various neodymium and cerium phosphates. Mater. Chem. Phys. 2003, 82, 194–198. [Google Scholar] [CrossRef]
- Qiu, Y.; Gao, L. Blue-emitting cyanuric acid–melamine complexes from urea thermolysis. Mater. Res. Bull. 2005, 40, 794–799. [Google Scholar] [CrossRef]
- Gerd, F.; Janna, G.; Klapotke, Thomas M.K.; Burkhard, K. Synthesis, properties and dimerization study of isocyanic acid. Z. Naturforsch. B Chem. Sci. 2002, 57, 19–24. [Google Scholar]
- Chun, H.; Min, S.S. Methods and devices for preparing biuret and cyanuric acid. CN Pat. 10117296, 2008. [Google Scholar]
- Shinichi, K.; Takahiro, T. Preparation of isocyanuric acid from urea. JP Pat. 04364173, 2008. [Google Scholar]
- Arakelyan, A.A.; Meliksetyan, A.A. Improvement of the production of cyanuric acid. Khim. Prom. 1988, 7, 442. [Google Scholar]
- Jakub, D.; Lubomir, H. Method of manufacturing cyanuric acid. PL Pat. 166711, 1995. [Google Scholar]
- Ohata, Y.; Aihara, M. Method for producing cyanuric acid. US Pat. 39, 53443, 1976. [Google Scholar]
- Sidner, B. Preparation of cyanuric acid. US Pat. 35, 63987, 1971. [Google Scholar]
- Jie, Y.; Gongying, W. Preparation of biuret in solvent. Fine Chem. Ind. 1987, 2, 42–47. [Google Scholar]
Sample Availability: Available from the authors. |
| Entry | Solvent | Yield (%) |
|---|---|---|
| 1 | kerosene | 88.7 |
| 2 | diesel | 80.5 |
| 3 | sulfolane | 54.8 |
| 4 | sulfolane: cyclohexanol = 3:1 | 64.6 |
| Entry | Temperature (°C) | Yield (%) |
|---|---|---|
| 1 | 180 | 85.7 |
| 2 | 190 | 88.9 |
| 3 | 200 | 87.4 |
| 5 | 210 | 80.4 |
| 5 | 220 | 67.5 |
| Entry | Catalyst | Yield (%) |
|---|---|---|
| 1 | (NH4)2SO4 | 82.4 |
| 2 | CaCl2 | 77.6 |
| 3 | NH4Cl | 80.9 |
| 4 | ZnCl2 | 79.4 |
| 5 | KNO3 | 82.4 |
| 6 | NaCl | 76.3 |
| 7 | NH4NO3 | 80.6 |
| 8 | CaSO4 | 75.3 |
| 9 | Ca(NO3)2 | 77.3 |
| 10 | ZnSO4 | 81.2 |
| 11 | ZnNO3 | 82.0 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
She, D.-M.; Yu, H.-L.; Huang, Q.-L.; Li, F.-M.; Li, C.-J. Liquid-Phase Synthesis of Cyanuric Acid from Urea. Molecules 2010, 15, 1898-1902. https://doi.org/10.3390/molecules15031898
She D-M, Yu H-L, Huang Q-L, Li F-M, Li C-J. Liquid-Phase Synthesis of Cyanuric Acid from Urea. Molecules. 2010; 15(3):1898-1902. https://doi.org/10.3390/molecules15031898
Chicago/Turabian StyleShe, Dong-Mei, Hai-Lin Yu, Qi-Liang Huang, Fen-Ming Li, and Chun-Jiu Li. 2010. "Liquid-Phase Synthesis of Cyanuric Acid from Urea" Molecules 15, no. 3: 1898-1902. https://doi.org/10.3390/molecules15031898
APA StyleShe, D.-M., Yu, H.-L., Huang, Q.-L., Li, F.-M., & Li, C.-J. (2010). Liquid-Phase Synthesis of Cyanuric Acid from Urea. Molecules, 15(3), 1898-1902. https://doi.org/10.3390/molecules15031898




