Remarkable Iodine-Catalyzed Synthesis of Novel Pyrrole- Bearing N-Polyaromatic β-Lactams
Abstract
:1. Introduction
2. Results and Discussion
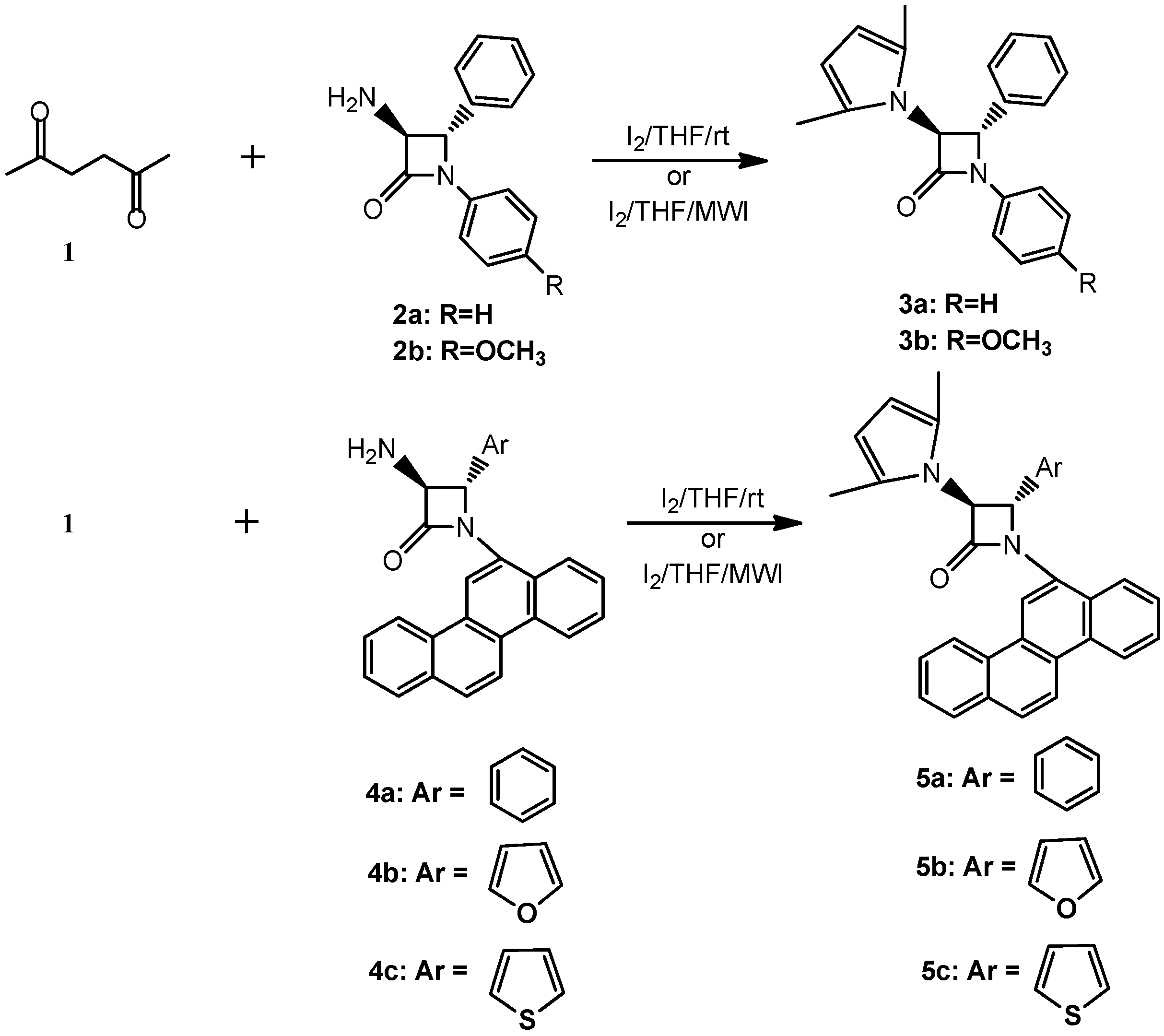
3. Experimental
3.1. General
3.1.1. General procedure for the synthesis of imines
3.1.2. General procedure for the synthesis of 3-phthalimido β-lactams
3.1.3. General procedure for the synthesis of 3-amino β-lactams
3.2. General procedure for the synthesis of pyrroles (±)-3 and (±)-5
4. Conclusion
Acknowledgements
References and Notes
- Lainton, J.A.H.; Hoffman, J.W.; Martin, D.R.; Compton, D.R. 1-Alkyl-3-(1-naphthoyl)pyrroles: A new class of cannabinoid. Tetrahedron Lett. 1995, 36, 1401–1404. [Google Scholar] [CrossRef]
- De Leon, C.Y.; Ganem, B. A new approach to porphobilinogen and its analogs. Tetrahedron 1997, 53, 7731–7752. [Google Scholar] [CrossRef]
- Gilchrist, T.L. Synthesis of aromatic heterocycles. J. Chem. Soc. Perkin Trans 1 1998, 615–628 and references cited therein. [Google Scholar] [CrossRef]
- Dieter, R.K.; Yu, H. A facile synthesis of polysubstituted pyrroles. Org. Lett. 2000, 2, 2283–2286. [Google Scholar] [CrossRef]
- Iwasawa, N.; Maeyama, K.; Saitou, M. Reactions of propargyl metallic species generated by the addition of alkynyllithiums to fischer-type carbene complexes. J. Am. Chem. Soc. 1997, 119, 1486–1487. [Google Scholar] [CrossRef]
- Furstner, A.; Weintritt, H.; Hupperts, A. A new, titanium-mediated approach to pyrroles: First synthesis of lukianol a and lamellarin-o-dimethyl ether. J. Org. Chem. 1995, 60, 6637–6641. [Google Scholar]
- Katritzky, A.; Jiang, J.; Steel, P.J. 1-Aza-1,3-bis(triphenylphosphoranylidene)propane: A novel :CHCH2N: Synthon. J. Org. Chem. 1994, 59, 4551–4555. [Google Scholar] [CrossRef]
- Arcadi, A.; Rossi, E. Synthesis of functionalized furans and pyrroles through annulation reactions of 4-pentynones. Tetrahedron 1998, 54, 15253–15272. [Google Scholar] [CrossRef]
- Periasamy, M.; Srinivas, G.; Bharathi, P. Conversion of Aryl methyl ketimines to 2,5-diarylpyrroles using TiCl4/Et3N. J. Org. Chem. 1999, 64, 4204–4205 and references cited therein. [Google Scholar] [CrossRef]
- Cooney, J.V.; McEwen, W.E. Synthesis of substituted pyrroles by intramolecular condensation of a Wittig reagent with the carbonyl group of a tertiary amide. J. Org. Chem. 1981, 46, 2570–2573. [Google Scholar]
- Ruault, P.; Pilard, J-F.; Touaux, B.; Boullet, F.T.; Hamelin, J. Rapid generation of amines by microwave irradiation of ureas dispersed on clay. Synlett 1994, 935–936. [Google Scholar]
- Danks, T.N. Microwave assisted synthesis of pyrroles. Tetrahedron Lett. 1999, 40, 3957–3960. [Google Scholar] [CrossRef]
- Becker, F.F.; Banik, B.K. Polycyclic aromatic compounds as anticancer agents: Synthesis and biological evaluation of some chrysene derivatives. Bioorg. Med. Chem. Lett. 1998, 8, 2877–2880. [Google Scholar] [CrossRef]
- Becker, F.F.; Mukhopadhyay, C.; Hackfeld, L.; Banik, I.; Banik, B.K. Polycyclic aromatic compounds as anticancer agents: Synthesis and biological evaluation of dibenzofluorene derivatives. Bioorg. Med. Chem. 2000, 8, 2693–2699. [Google Scholar] [CrossRef]
- Banik, B.K.; Becker, F.F. Polycyclic aromatic compounds as anticancer agents. 4. Structure-activity relationships of chrysene and pyrene derivatives. Bioorg. Med. Chem. 2001, 9, 593–605. [Google Scholar] [CrossRef]
- Banik, B.K.; Becker, F.F. Synthesis, electrophilic substitution and structure-activity relationship studies of polycyclic aromatic compounds towards the development of anticancer agents. Curr. Med. Chem. 2001, 8, 1513–1533. [Google Scholar] [CrossRef]
- Banik, I.; Becker, F.F.; Banik, B.K. Stereoselective synthesis of β-lactams with polyaromatic imines: Entry to new and novel anticancer agents. J. Med. Chem. 2003, 46, 12–15. [Google Scholar] [CrossRef]
- Banik, B.K.; Suhendra, M.; Banik, I.; Becker, F.F. Indium/ammonium chloride mediated selective reduction of aromatic nitro compounds: Practical synthesis of 6-aminochrysene. Synth. Commun. 2000, 30, 3745–3754. [Google Scholar] [CrossRef]
- Banik, B.K.; Banik, I.; Hackfeld, L.; Becker, F.F. Indium-mediated reductive cyclizations in aqueous ethanol: highly efficient synthesis of heterocyclic compounds of biological interest. Heterocycles 2002, 56, 467–470. [Google Scholar] [CrossRef]
- Banik, B.K.; Hackfeld, L.; Becker, F.F. Studies on the indium-mediated reduction of imines. Synth. Commun. 2001, 31, 1581–1586. [Google Scholar] [CrossRef]
- Samajdar, S.; Becker, F.F.; Banik, B.K. Surface-mediated highly efficient oxidation of alcohols by bismuth nitrate. Synth. Commun. 2001, 31, 2691–2695. [Google Scholar]
- Banik, B.K.; Mukhopadhyay, C.; Becker, F.F. An expeditious one pot synthesis of dibenzo[a,g]fluorene. Synth. Commun. 2001, 31, 2399–2403. [Google Scholar] [CrossRef]
- Dasgupta, S.K.; Banik, B.K. A new entry to N-unsubstituted β-lactams through a solid-phase approach. Tetrahedron Lett. 2002, 43, 9445–9447. [Google Scholar]
- Banik, B.K.; Samajdar, S.; Banik, I. A facile synthesis of oxazines by indium-induced reduction-rearrangement of the nitro β-lactams. Tetrahedron Lett. 2003, 44, 1699–1701. [Google Scholar] [CrossRef]
- Srivastava, N.; Banik, B.K. Bismuth nitrate-catalyzed versatile Michael reactions. J. Org. Chem. 2003, 68, 2109–2114. [Google Scholar] [CrossRef]
- Banik, B.K.; Manhas, M.S.; Bose, A.K. Stereospecific glycosylation via ferrier rearrangement for optical resolution. J. Org. Chem. 1994, 59, 4714–4716. [Google Scholar] [CrossRef]
- Banik, B.K.; Manhas, M.S.; Bose, A.K. Studies on lactams. 103. Enantiopure α-hydroxy-β-lactams via stereoselective glycosylation. Tetrahedron Lett. 1997, 38, 5077–5080. [Google Scholar]
- Banik, B.K.; Zegrocka, O.; Manhas, M.S.; Bose, A.K. Studies on lactams. 104. Enantiomerically pure β-lactams with the thienamycin side chain via glycosylation. Heterocycles 1997, 27, 173–176. [Google Scholar]
- Banik, B.K.; Fernandez, M.; Alvarez, C. Iodine-catalyzed highly efficient Michael reaction of indoles under solvent-free condition. Tetrahedron Lett. 2005, 46, 2479–2482. [Google Scholar]
- Banik, B.K.; Garcia, I.; Moarles, F.; Aguilar, C. Novel synthesis of substituted pyrrole bound to indolinone via molecular iodine-catalyzed reaction. Heterocycl. Commun. 2007, 13, 109–112. [Google Scholar]
- Banik, B.K.; Garza, R. Molecular iodine-catalyzed deprotection of acetals and ketals in acetone. Chem. Edu. 2007, 12, 75–76. [Google Scholar]
- Bandyopadhyay, D.; Xavier, M.; Banik, B.K. Highly stereoselective β-lactam synthesis via the Staudinger reaction using polyaromatic imines. Heterocycl. Commun. 2009, 15, 229–231. [Google Scholar]
- Rivera, G.; Bandyopadhyay, D.; Jaggi, S.; Gonzales, R.C.; Banik, B.K. An expeditious synthesis of 3-amino β-lactams derived from polyaromatic compounds. Heterocycl. Commun. 2009, 15, 323–325. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2010 by the authors;
Share and Cite
Bandyopadhyay, D.; Rivera, G.; Salinas, I.; Aguilar, H.; Banik, B.K. Remarkable Iodine-Catalyzed Synthesis of Novel Pyrrole- Bearing N-Polyaromatic β-Lactams. Molecules 2010, 15, 1082-1088. https://doi.org/10.3390/molecules15021082
Bandyopadhyay D, Rivera G, Salinas I, Aguilar H, Banik BK. Remarkable Iodine-Catalyzed Synthesis of Novel Pyrrole- Bearing N-Polyaromatic β-Lactams. Molecules. 2010; 15(2):1082-1088. https://doi.org/10.3390/molecules15021082
Chicago/Turabian StyleBandyopadhyay, Debasish, Gildardo Rivera, Isabel Salinas, Hector Aguilar, and Bimal K. Banik. 2010. "Remarkable Iodine-Catalyzed Synthesis of Novel Pyrrole- Bearing N-Polyaromatic β-Lactams" Molecules 15, no. 2: 1082-1088. https://doi.org/10.3390/molecules15021082
APA StyleBandyopadhyay, D., Rivera, G., Salinas, I., Aguilar, H., & Banik, B. K. (2010). Remarkable Iodine-Catalyzed Synthesis of Novel Pyrrole- Bearing N-Polyaromatic β-Lactams. Molecules, 15(2), 1082-1088. https://doi.org/10.3390/molecules15021082




