Synthesis and Biological Activity of Novel Phenyltriazolinone Derivatives
Abstract
:1. Introduction
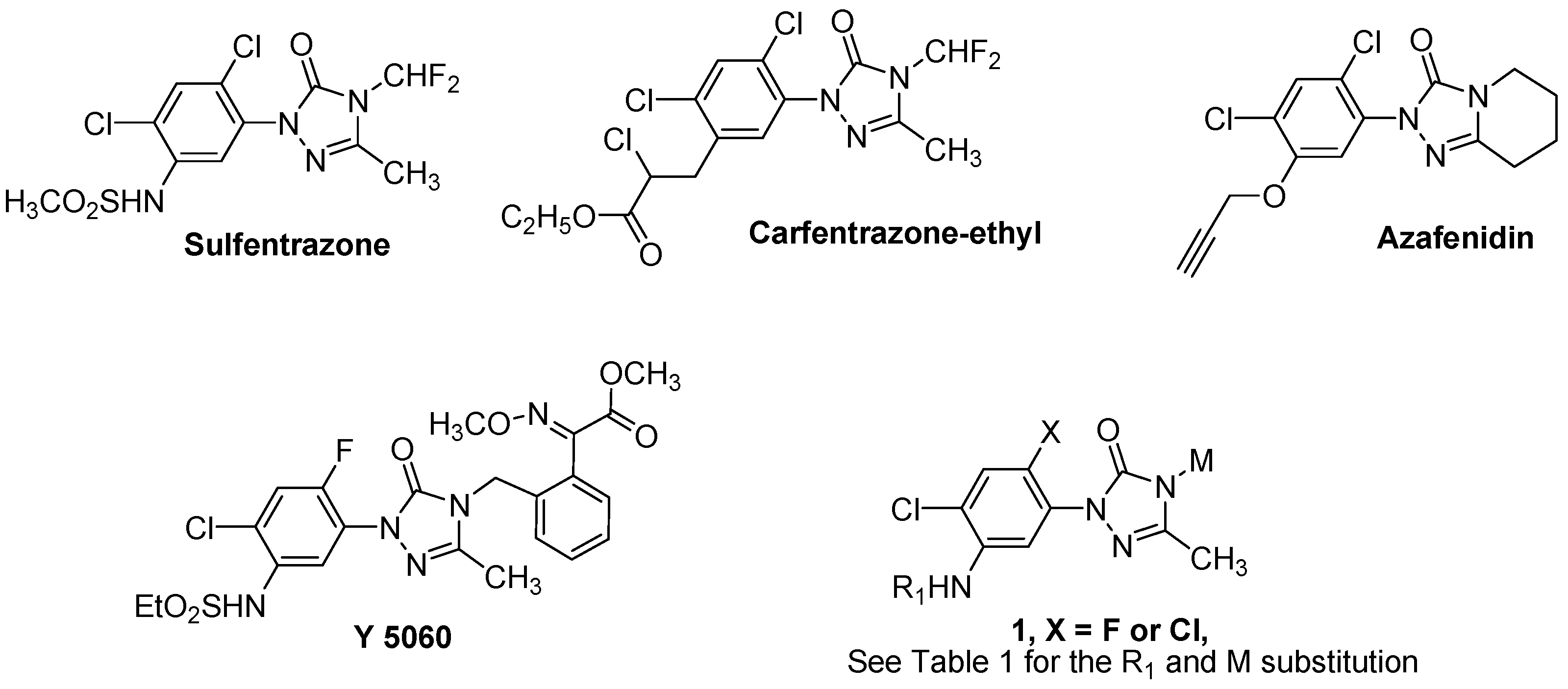
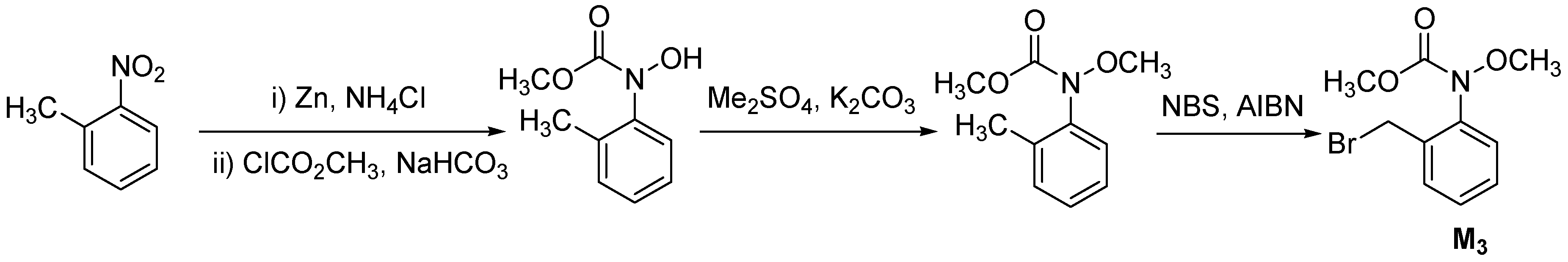
2. Results and Discussion
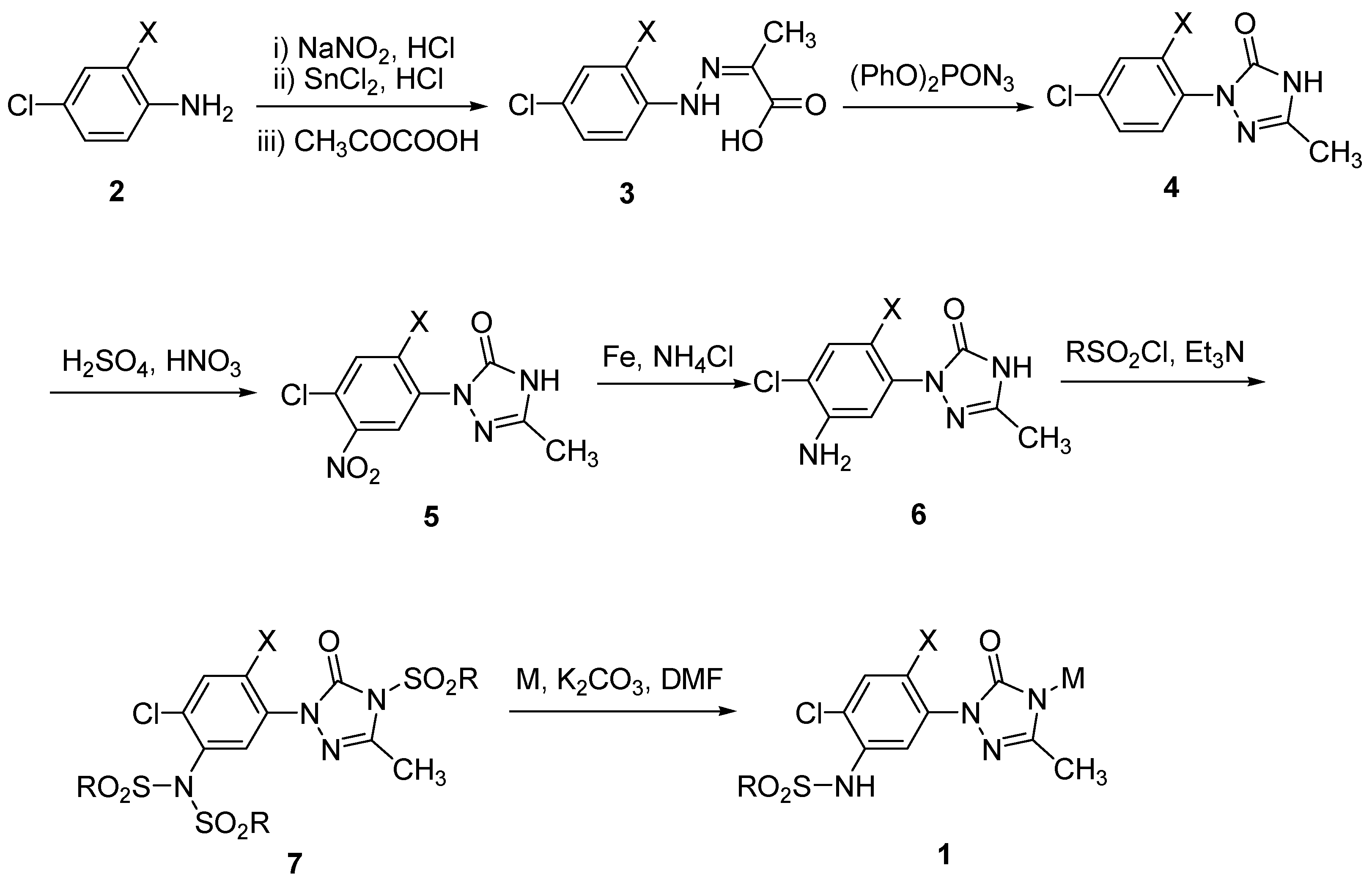
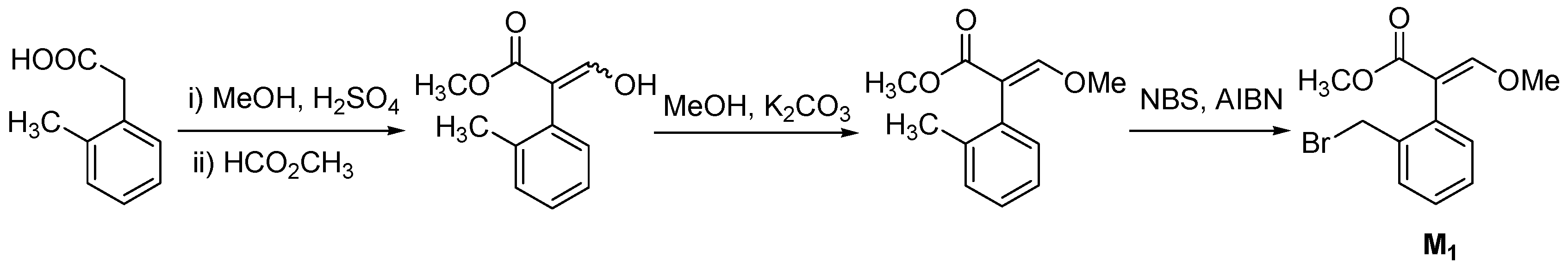
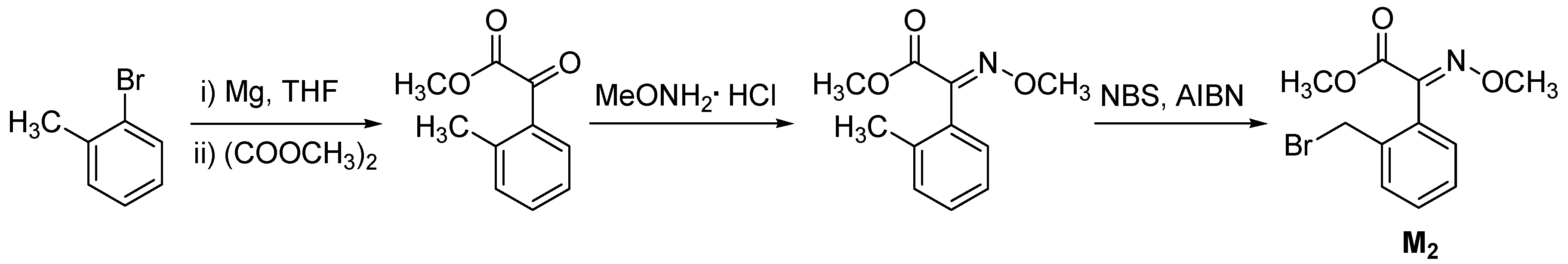
 | |||||
|---|---|---|---|---|---|
| No. | X | R | Ma | m.p. (°C) | Yield (%) |
| 1a | F | CH3- | M1 | 153-155 | 52 |
| 1b | F | CH3CH2- | M1 | 167-169 | 59 |
| 1c | F | Ph- | M1 | 187-190 | 39 |
| 1d | Cl | CH3- | M1 | 138-140 | 78 |
| 1e | Cl | CH3CH2- | M1 | 168-170 | 80 |
| 1f | Cl | Ph- | M1 | 194-196 | 48 |
| 1g | F | CH3- | M2 | 138-140 | 75 |
| 1h | F | Ph- | M2 | 164-166 | 46 |
| 1i | Cl | CH3- | M2 | 150-152 | 73 |
| 1j | Cl | CH3CH2- | M2 | 162-164 | 79 |
| 1k | Cl | Ph- | M2 | 190-192 | 44 |
| 1l | F | CH3- | M3 | 186-188 | 57 |
| 1m | F | CH3CH2- | M3 | 166-168 | 55 |
| 1n | F | Ph- | M3 | oil | 41 |
| 1o | Cl | CH3SO2- | M3 | 189-191 | 62 |
| 1p | Cl | CH3CH2SO2- | M3 | 174-176 | 70 |
| 1q | Cl | PhSO2- | M3 | oil | 46 |
 | |||||
3. Experimental
3.1. General
3.2. General Procedure for the Preparation of Phenylhydrazines 3
3.3. General Procedure for the Synthesis of Phenyltriazolinones 4
3.4. General Procedure for the Synthesis of Nitrophenyltriazolinones 5
3.5. General Procedure for the Synthesis of Aminophenyltriazolinones 6
3.6. General Procedure for the Synthesis of Compound 7
3.7. General Procedure for the Preparation of Target Molecules 1
Acknowledgements
References
- Kulkarni, S.V.; Desai, V.C.; Prasad, V.A.; Rivadeneira, E.; Jelich, K.A. A progress for the manufacture substituted triazolinoes. Eur. Pat. 1,113,010, 2001. [Google Scholar]
- Schmitzer, P.R.; Graupner, P.R.; Chapin, E.L.; Fields, S.C.; Gilbert, J.R.; Gray, J.A.; Peacock, C.L.; Gerwick, B.C. Ribofuranosyl triazolone: a natural product herbicide with activity on adenylosuccinate synthetase following phosphorylation. J. Nat. Prod. 2000, 63, 777–781. [Google Scholar] [CrossRef]
- Brown, R.J.; Sun, K.M.; Frasier, D.A. Dihydrotriazole compounds and their use for controlling fungal plant diseases. US Pat. 5,977,149, 1999. [Google Scholar]
- Watanabe, Y.; Usui, H.; Kobayashi, S.; Yoshiwara, H.; Shibano, T.; Tanaka, T.; Morishima, Y.; Yasuoka, M.; Kanao, M. Syntheses and 5-HT2 antagonist activity of bicyclic 1,2,4-triazol-3(2H)-one and 1,3,5-triazine-2,4(3H)-dione derivatives. J. Med. Chem. 1992, 35, 189–194. [Google Scholar] [CrossRef]
- Xu, Y.; Mayhugh, D.; Saeed, A.; Wang, X.; Thompson, R.C.; Dominianni, S.J.; Kauffman, R.F.; Singh, J.; Bean, J.S.; Bensch, W.R.; Barr, R.J.; Osborne, J.; Montrose-Rafizadeh, C.; Zink, R.W.; Yumibe, N.P.; Huang, N.; Luffer-Atlas, D.; Rungta, D.; Maise, D.E.; Mantlo, N.B. Design and synthesis of a potent and selective triazolone-based peroxisome proliferator-activated receptor alpha agonist. J. Med. Chem. 2003, 46, 5121–5124. [Google Scholar]
- Patil, B.S.; Krishnamurthy, G.; BhojyaNaik, H.S.; Latthe, P.R.; Ghate, M. Synthesis, characterization and antimicrobial studies of 2-(4-methoxy-phenyl)-5-methyl-4-(2-arylsulfanyl-ethyl)-2,4-dihydro-[1,2,4]triazolo-3-ones and their corresponding sulfones. Eur. J. Med. Chem. 2010, 45, 3329–3334. [Google Scholar] [CrossRef]
- Cowden, C.J.; Wilson, R.D.; Bishop, B.C.; Cottrell, I.F.; Davies, A.J.; Dolling, U.H. A new synthesis of 1,2,4-triazolin-5-ones: application to the convergent synthesis of an NK1 antagonist. Tetrahedron Lett. 2000, 41, 8661–8864. [Google Scholar] [CrossRef]
- Chang, L.L.; Ashton, W.T.; Flanagan, K.L.; Chen, T.B.; O'Malley, S.S.; Zingaro, G.J.; Siegl, P.K.S.; Kivlighn, S.D.; Lotti, V.J. Triazolinone biphenylsulfonamides as angiotensin II receptor antagonists with high affinity for both the AT1 and AT2 subtypes. J. Med. Chem. 1994, 37, 4464–4478. [Google Scholar] [CrossRef]
- Wolf, A.D. Substituierte bicyclesche triazole und diese enthaltende zusammensetzungen. DE Pat. 1978. 2,801,429. [Google Scholar]
- Theodoridis, G. Herbicidal aryl triazolinones. US Pat. 4,818,275, 1989. [Google Scholar]
- Meazza, G.; Bettarini, F.; La Porta, P.; Piccardi, P.; Signorini, E.; Portoso, D.; Fornara, L. Synthesis and herbicidal activity of novel heterocyclic protoporphyrinogen oxidase inhibitors. Pest Manag. Sci. 2004, 60, 1178–1188. [Google Scholar] [CrossRef]
- Hiraki, M.; Ohki, S.; Sato, Y.; Jablonkai, I.; Boger, P.; Wakaba-yashi, K. Protoporphyrinogen-IX Oxidase Inhibitors: Bioactivation of Thiadiazolidines. Pestic. Biochem. Phys. 2001, 70, 159–167. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, J.; Yang, G.F. A DFT-based QSARs study of protoporphyrinogen oxidase inhibitors: phenyl triazolinones. Bioorg. Med. Chem. 2004, 12, 6183–6191. [Google Scholar] [CrossRef]
- Theodoridis, G.; Bahr, J.T.; Hotzman, F.W.; Sehgel, S.; Suarez, D.P. New generation of protox-inhibiting herbicides. Crop Protect. 2000, 19, 533–535. [Google Scholar] [CrossRef]
- Dayan, F.E.; Green, H.M.; Weete, J.D.; Hancock, H.G. Postemergence activity of sulfentrazone: Effects of surfactants and leaf surfaces. Weed Sci. 1996, 44, 797–803. [Google Scholar]
- Dayan, F.E.; Duke, S.O.; Weete, J.D.; Hancock, H.G. Selectivity and mode of action of carfentrazone-ethyl, a novel phenyl triazolinone herbicide. Pestic. Sci. 1997, 51, 65–73. [Google Scholar] [CrossRef]
- Dayan, F.E.; Duke, S.O. Phytotoxicity of protoporphyrinogen oxidase inhibitors: Phenomenology, mode of action and mechanisms of resistance. In Herbicide Activity: Toxicology, Biochemistry and Molecular Biology; Roe, R.M., Burton, J.D., Kuhr, R.J., Eds.; IOS Press: Washington, DC, USA, 1997; pp. 11–35. [Google Scholar]
- Dayan, F.E.; Duke, S.O. Protoporphyrinogen oxidase-inhibiting herbicides. In Haye's Handbook of Pesticide Toxicology, 3rd; Krieger, R., Doull, J., Hodgson, E., Maibach, H., Reiter, L., Ritter, L., Ross, J., Slikker, W.J., Van Hemmen, J., Eds.; Academic Press-Elsevier: San Diego, CA, USA, 2010; pp. 1733–1751. [Google Scholar]
- Patzoldt, W.L.; Hager, A.G.; McCormick, J.S.; Tranel, P.J. A codon deletion confers resistance to herbicides inhibiting protoporphyrinogen oxidase. Proc. Nat. Acad. Sci. USA 2006, 103, 12329–12334. [Google Scholar]
- Dayan, F.E.; Daga, P.R.; Duke, S.O.; Lee, R.M.; Tranel, P.J.; Doerksen, R.J. Biochemical and structural consequences of a glycine deletion in the α-8 helix of protoporphyrinogen oxidase. Biochim. Biophys. Acta 2010, 1804, 1548–1556. [Google Scholar] [CrossRef]
- Shapiro, R.; DiCosimo, R.; Hennessey, S.M.; Stieglitz, B.; Campopiano, O.; Chiang, G.C. Discovery and development of a commercial synthesis of azafenidin. Org. Process Res. Dev. 2001, 5, 593–598. [Google Scholar] [CrossRef]
- Sauter, H.; Steglich, W.; Anke, T. Strobilurins: Evolution of a new class of active substances. Angew. Chem. Int. Ed. 1999, 38, 1328–1349. [Google Scholar] [CrossRef]
- Li, M.; Liu, C.L.; Yang, J.C.; Zhang, J.B.; Li, Z.N.; Z, H.; Li, Z.M. Synthesis and biological activity of new (E)-alpha-(Methoxyimino)benzeneacetate derivatives containing a substituted pyrazole ring. J. Agric. Food Chem. 2010, 58, 2664–2667. [Google Scholar]
- Zhao, P.L.; Liu, C.L.; H, W.; Wang, Y.Z.; Yang, G.F. Synthesis and fungicidal evaluation of novel chalcone-based strobilurin analogues. J. Agric. Food Chem. 2007, 55, 5697–5700. [Google Scholar] [CrossRef]
- Luo, Y.P.; Jiang, L.L.; Wang, G.D.; Chen, Q.; Yang, G.F. Syntheses and herbicidal activities of novel triazolinone derivatives. J. Agric. Food Chem. 2008, 56, 2118–2124. [Google Scholar]
- Li, Y.; Zhang, H.Q.; Liu, J.; Yang, X.P.; Liu, Z.J. Stereoselective synthesis and antifungal activities of (E)-alpha-(methoxyimino)benzeneacetate derivatives containing 1,3,5-substituted pyrazole ring. J. Agric. Food Chem. 2006, 54, 3636–3640. [Google Scholar] [CrossRef]
- Beautement, K.; Clough, J.M.; de Fraine, P.J.; Godfrey, C.R.A. Fungicidal β-methoxyacrylates: From natural products to novel synthetic agricultural fungicides. Pestic. Sci. 1991, 31, 499–519. [Google Scholar] [CrossRef]
- Li, Y.X.; Luo, Y.P.; Xi, Z.; Niu, C.W.; He, Y.Z.; Yang, G.F. Design and syntheses of novel phthalazin-1(2H)-one derivatives as acetohydroxyacid synthase inhibitors. J. Agric. Food Chem. 2006, 54, 9135–9139. [Google Scholar] [CrossRef]
- Weckbecker, C.; Drauz, K. Process for the preparation of triazolinone herbicides. US Pat. 5,856,495, 1999. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, Q.; Wang, G.; Huang, S.; Lin, L.; Yang, G. Synthesis and Biological Activity of Novel Phenyltriazolinone Derivatives. Molecules 2010, 15, 9024-9034. https://doi.org/10.3390/molecules15129024
Wu Q, Wang G, Huang S, Lin L, Yang G. Synthesis and Biological Activity of Novel Phenyltriazolinone Derivatives. Molecules. 2010; 15(12):9024-9034. https://doi.org/10.3390/molecules15129024
Chicago/Turabian StyleWu, Qiongyou, Guodong Wang, Shaowei Huang, Long Lin, and Guangfu Yang. 2010. "Synthesis and Biological Activity of Novel Phenyltriazolinone Derivatives" Molecules 15, no. 12: 9024-9034. https://doi.org/10.3390/molecules15129024
APA StyleWu, Q., Wang, G., Huang, S., Lin, L., & Yang, G. (2010). Synthesis and Biological Activity of Novel Phenyltriazolinone Derivatives. Molecules, 15(12), 9024-9034. https://doi.org/10.3390/molecules15129024




