Abstract
Zingiber officinale Roscoe. (Family Zingiberaceae) is well known in Asia. The plant is widely cultivated in village gardens in the tropics for its medicinal properties and as a marketable spice in Malaysia. Ginger varieties are rich in physiologically active phenolics and flavonoids with a range of pharmacological activities. Experiments were conducted to determine the feasibility of increasing levels of flavonoids (quercetin, rutin, catechin, epicatechin, kaempferol, naringenin, fisetin and morin) and phenolic acid (gallic acid, vanillic acid, ferulic acid, tannic acid, cinnamic acid and salicylic acid), and antioxidant activities in different parts of Malaysian young ginger varieties (Halia Bentong and Halia Bara) with CO2 enrichment in a controlled environment system. Both varieties showed an increase in phenolic compounds and flavonoids in response to CO2 enrichment from 400 to 800 µmol mol-1 CO2. These increases were greater in rhizomes compared to leaves. High performance liquid chromatography (HPLC) results showed that quercetin and gallic acid were the most abundant flavonoid and phenolic acid in Malaysian young ginger varieties. Under elevated CO2 conditions, kaempferol and fisetin were among the flavonoid compounds, and gallic acid and vanillic acid were among the phenolic compounds whose levels increased in both varieties. As CO2 concentration was increased from 400 to 800 µmol mol-1, free radical scavenging power (DPPH) increased about 30% in Halia Bentong and 21.4% in Halia Bara; and the rhizomes exhibited more enhanced free radical scavenging power, with 44.9% in Halia Bentong and 46.2% in Halia Bara. Leaves of both varieties also displayed good levels of flavonoid compounds and antioxidant activities. These results indicate that the yield and pharmaceutical quality of Malaysian young ginger varieties can be enhanced by controlled environment production and CO2 enrichment.
1. Introduction
The increase of atmospheric CO2 due to global climate change and/or horticultural practices has direct and indirect effects on secondary metabolite synthesis in plants [1]. The responses observed in different plants show a wide range of patterns, either in the structure of primary and secondary metabolites or in the biomass production. These kinds of responses may occur in natural plant ecosystems, but may also be created deliberately by deliberate CO2 enrichment techniques in controlled environment systems to increase the production of some plants and some secondary compounds [2,3,4]. In the previous and current century, there has been an increase in the use of herbal products for traditional medicine and foods, which is attributable to a new way of life that is related to arising interest in the use of natural products. At the same time, there is also a keen interest in functional foods, which in some cases have shown little distinction between herbs and spices as a wide range of natural products are mostly produced from herbs or medicinal plants. They have been introduced to society as part of traditional cultural practices, and hence, there are only very soft rules existing about their commercial regulation [5].
Crops under enriched CO2 atmospheres acquire positive features with enhanced plant adaptation and growth. The greatest advantages of CO2 enrichment is in the enhancement of photosynthetic capacity, particularly under adverse climatic conditions and this would becomes most apparent in the vegetative growth of young plants [6,7]. Rising levels of atmospheric CO2 can alter plant growth and partitioning of secondary metabolites [8]. This can be proved by the results of the study of Wang et al. [9] showing that elevated CO2 concentrations in the atmosphere enhanced vegetative growth, carbohydrate accumulation, and fruit productivity in strawberry. It is well established that environmental factors can influence the production of secondary metabolites in plants [10]. For example, under nutrient deficient conditions, the levels of non-nitrogenous metabolites derived from the shikimic acid pathway such as phenolic acids, lignin, hydrolysable tannins, and proanthocyanidins usually increase in woody plants. The increase in C-based secondary metabolites frequently occurs when environmental conditions also promote an accumulation of non-structural carbohydrates (TNC) in plants. Elevated atmospheric CO2 concentrations often increase TNC concentrations in plants and possibly stimulate secondary metabolism [11].
Flavonoids belong to a large family of polyphenolic components synthesized by plants [12]. High contents of natural phenolic acids and flavonoids are found in green tea, fruits, and vegetables, while some amounts of phenolics exist in red wine and coffee [13,14]. Free radicals and single oxygen are recognized as major factors causing various chronic diseases such as cancer, diabetes, etc. Therefore, according to recent and previous studies, the health maintenance function of antioxidant components in various foods has received much attention in recent years [15,16]. Phenolic acids and flavonoids are antioxidants with health benefits such as anti-inflammatory and antitumor effect [17,18,19,20]. Sung-jin et al. [21] showed that some flavonoid components in green tea are effective in inhibiting cancer or induce mechanisms that may kill cancer cells and inhibit tumor invasion.
Zingiber officinale is one of the traditional folk medicinal plants that have been used by Polynesians for over 2,000 years for treating diabetes, high blood pressure, cancer, fitness and many other illnesses [22]. The use of ginger for food and cooking has a long history in Asia. Zingiber officinale contains a number of antioxidants such as ascorbic acid, and phenolic acids [23]. Easily cultivable, Zingiber officinale, with its wide range of antioxidants, can be a major source of natural or phytochemical antioxidants [24]. Although various extracts are obtained from ginger, it is the CO2 extracts that are richest in polyphenol compounds and have a composition that closely resembles that of the rhizomes [25,26].
Malikov et al. [30] reviewed the biosynthesis and properties of phenolic and flavonoid compounds detected from Scutellaria species. Increased concentration of flavonoids through CO2 enrichment has the potential to enhance the production and quality of medicinal plants such as Scutellaria. Increases in the levels of phenolic and flavonoid components of Populus tremuloides by CO2 enrichment method has been reported by Lindroth et al. [31].
The main objectives of this study were to evaluate the effects of carbon dioxide enrichment on concentration of phenolics and flavonoids compound in extracts of young ginger (Zingiber officinale) varieties (Halia Bentong and Halia Bara), and to determine the antioxidant activity.
2. Results and Discussion
2.1. HPLC Analysis of Flavonoids
The results obtained from the preliminary analysis of flavonoids are shown in Table 1. Increasing the CO2 concentration from 400 to 800 µmol mol-1 resulted in enhanced quercetin, catechin, kaempferol and fisetin levels in the leaves and rhizomes of both varieties, and increase in naringenin content in just the leaves. On the other hand, the contents of rutin, epicatechin and morin decreased in ginger parts with rising of CO2 concentration from ambient to 800 µmol mol-1. It can be seen from the data in this table that quercetin content in ginger, when compared with other plants, for example red chilli (0.799 mg g-1 DW), bird chilli (0.392 mg g-1 DW), bell pepper (0.448 mg g-1 DW), black tea (1.107 mg g-1 DW), onion (1.49 mg g-1 DW) and semambu (1.18 mg g-1 DW) [34] registered substantially high levels in both the leaves (1.33 mg g-1 DW) and rhizomes (1.27 mg g-1 DW) of Halia Bara exposed to elevated CO2 concentration at 800 µmol mol-1.

Table 1.
The concentrations of some flavonoids compounds in two varieties of Zingiber officinale, Halia Bentong (a) and Halia Bara (b) grown under different CO2 concentrations.
| Flavonoid compounds | (a) Halia Bentong | |||
|---|---|---|---|---|
| 400 | 800 | |||
| Leaves | Rhizomes | Leaves | Rhizomes | |
| Quercetin | 0.972 ± 0.013c | 0.895 ± 0.039c | 1.22 ± 0.07b | 1.138 ± 0.023b |
| Rutin | 0.171 ± 0.0028de | 0.452 ± 0.004a | 0.141 ± 0.031e | 0.388 ± 0.026b |
| Epicatechin | 0.122 ± 0.018a | 0.083 ± 0.007bc | 0.073 ± 0.008c | 0.048 ± 0.018d |
| Catechin | 0.409 ± 0.027d | 0.491 ± 0.019cd | 0.673 ± 0.044ab | 0.637 ± 0.034b |
| Kaempferol | 0.042 ± 0.002e | 0.053 ± 0.003de | 0.118 ± 0.014c | 0.148 ± 0.023b |
| Naringenin | 0.089 ± 0.0052c | 0.047 ± 0.003d | 0.127 ± 0.022b | 0.083 ± 0.004c |
| Fisetin | 0.986 ± 0.012e | 0.633 ± 0.033f | 2.05 ± 0.27c | 2.82 ± 0.19a |
| Morin | 0.514 ± 0.027e | 0.463 ± 0.014e | 0.49 ± 0.052e | 0.875 ± 0.036a |
| Flavonoid compounds | (b) Halia Bara | |||
| 400 | 800 | |||
| Leaves | Rhizomes | Leaves | Rhizomes | |
| Quercetin | 1.19 ± 0.122ab | 0.986 ± 0.032c | 1.33 ± 0.134a | 1.27 ± 0.01a |
| Rutin | 0.174 ± 0.007d | 0.334 ± 0.009c | 0.151 ± 0.025de | 0.404 ± 0.016b |
| Epicatechin | 0.12 ± 0.004a | 0.103 ± 0.0035ab | 0.096 ± 0.022bc | 0.037 ± 0.009d |
| Catechin | 0.668 ± 0.079ab | 0.533 ± 0.034c | 0.733 ± 0.014a | 0.682 ± 0.05ab |
| Kaempferol | 0.051 ± 0.002de | 0.068 ± 0.005d | 0.163 ± 0.011ab | 0.181 ± 0.009a |
| Naringenin | 0.061 ± 0.004d | 0.028 ± 0.003e | 0.155 ± 0.027a | 0.121 ± 0.011b |
| Fisetin | 1.53 ± 0.121d | 1.32 ± 0.12d | 2.38 ± 0.395b | 3.11 ± 0.185a |
| Morin | 0.765 ± 0.024b | 0.606 ± 0.006d | 0.661 ± 0.029c | 0.515 ± 0.025e |
All analyses are the mean of triplicate measurements ± standard deviation. Results expressed in mg g-1 of dry plant material. Means not sharing a common letter were significantly different at P ≤ 0.05.
Kaempferol is a rare flavonoid component in plants, but it was detected in the leaves (0.042–0.163 mg g-1 DW) and rhizomes (0.053–0.181 mg g-1 DW) of both Halia Bara and Halia Bentong. These contents were slightly higher than those recorded in green chilli (0.039 mg g-1 DW), sengkuang (0.037 mg g-1 DW), white radish (0.0383 mg g-1 DW), and pegaga (0.0205 mg g-1 DW). Nevertheless, both ginger varieties had lower kaempferol contents when compared to cekur manis (0.323 mg g-1 DW), pumpkin (0.371 mg g-1 DW), and carrot (0.140 mg g-1 DW) [35]. Tolonen et al. [36] reported very low kaempferol contents (9 mg/g DW) in white cabbages, and it was the only flavonoid found. Meanwhile, Kim [37] detected about 0.1–0.8 mg/g fm of quercetin and kaempferol aglycone contents in green cabbages. Exposing ginger plants to 800 µmol mol-1 of CO2 concentration saw the synthesis of kaempferol enhanced to 0.163 and 0.181 mg g-1 DW in Halia Bara leaves and rhizomes, respectively.
Fisetin is another rare yet well known flavonoid component in plants. Previous studies showed that fisetin had anti-inflammatory [35,38], anti-carcinogenic [39] and strong antioxidant [39] effects. Ginger leaves and rhizomes exhibited good potential levels of this flavonoid. It seemed that fisetin content could also be improved by increasing CO2 concentration in both of varieties, especially in Halia Bara (leaves: 1.53 increased to 2.38 mg g-1 DW; rhizome: 1.32 increased to 3.11 mg g-1 DW). Morin is another flavonoid belonging to the flavonols group. Morin acts as a chemopreventive agent in vitro and in vivo against oral carcinogenesis [40,41]. The importance of morin and related compounds as anti-tumour drugs has also been widely recognized [42]. In comparison with old fustic (Chlorophora tinctoria), osage orange (Maclura pomifera) [43], almonds (P. guajava L.) [44], mill (Prunus dulcis), fig (Chlorophora tinctoria) [43], onion and apple [44], both the leaves and rhizomes local ginger varieties showed good levels of morin when grown under both 400 and 800 µmol mol-1 CO2 conditions, indicating that the plant is naturally a good source of morin, although the content of the latter was variable. For example, the content of morin in the leaves decreased in both varieties with increasing of CO2 concentration, while a high content of morin (0.875 vs. 0.463 mg g-1 DW) was obtained from extract of Halia Bentong rhizomes grown under elevated CO2.
Similar trends of increasing concentration of flavonoid components with increasing CO2 concentration was observed in Betula pendula and strawberry [2,9]. Wang et al. [9], reported growing strawberry plants under CO2 enrichment conditions (950 µmol mol-1) significantly enhanced fruit p-coumaroylglucose, dihydroflavonol, quercetin 3-glucoside, quercetin 3-glucuronide, and kaempferol 3-glucoside contents, as well as cyanidin 3-glucoside, pelargonidin 3-glucoside, and pelargonidin 3-glucoside succinate content. This finding is in agreement with Sttute et al. [32] who showed the ability of elevated CO2 concentrations to enhance flavonoid components (apygenin, baicalin, scutellarein) in Scutellaria species. The percentages of increase or decrease in flavonoid contents of ginger when exposed to 800 µmol mol-1 concentrations of CO2 are tabulated in Table 2.

Table 2.
Percent of increase or decrease of flavonoid compounds in two varieties of Zingiber officinale grown under elevated CO2 concentration (800 µmol mol-1).
| Flavonoid compounds | Halia Bentong | Halia Bara | ||
|---|---|---|---|---|
| Leaves | Rhizomes | Leaves | Rhizomes | |
| Quercetin | +25.5 | +27.2 | +9.2 | +28.8 |
| Rutin | -17.5 | -14.2 | -13.2 | +21.0 |
| Epicatechin | -40.2 | -42.2 | -20.0 | -64.1 |
| Catechin | +64.5 | +29.7 | +9.7 | +28.0 |
| Kaempferol | +181.0 | +179.2 | +219.6 | +166.2 |
| Naringenin | +42.7 | +76.6 | +154.1 | +332.1 |
| Fisetin | +107.9 | +345.5 | +55.6 | +135.6 |
| Morin | -4.7 | +89.0 | -13.6 | -15.0 |
Results expressed in percent; + and – indicate respectively increases and decreases of component concentrations when exposed to CO2.
According to data from this table, kaempferol levels were more enhanced in varieties grown under elevated carbon dioxide conditions and after that, fisetin and naringenin were more enhanced. On average flavonoid compounds increased 44.9% in leaves and 86.3% in rhizomes of Halia Bentong and 50.1 % in leaves and 79% in rhizomes of Halia Bara when exposed to elevated carbon dioxide conditions. To the best of our knowledge the current study is the first report of fisetin, morin and naringenin in young ginger varieties. Figure 1 shows the HPLC chromatogram of flavonoids analysis in Halia Bentong extract (leaves).
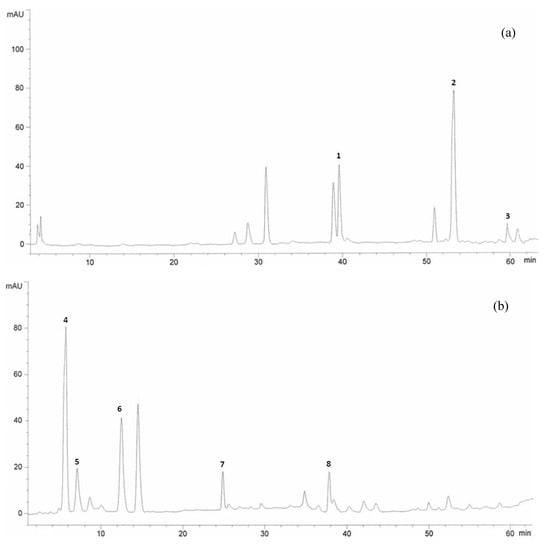
Figure 1.
HPLC chromatogram of Halia Bentong ginger (Zingiber officinale) leaves extracts at wavelengths of 360 nm (a), and 280 nm (b). Identification of compounds: quercetin (1), rutin (2), kaempferol (3), fisetin (4), morin (5), catechin (6), epicatechin (7), naringenin (8).
2.2. HPLC Analysis of Phenolic Compounds
Like the alteration of flavonoid accumulation in both varieties of ginger when they were exposed to 400 to 800 µmol mol-1 CO2 the phenolic contents also increased in the leaves more than in the rhizomes. Phenolic contents are influenced by the interaction between varieties and parts of the plants. Partitioning and accumulation of phenolics in different parts of ginger grown under ambient CO2 followed the trend of leaves > rhizomes. Rhizomes in both of varieties had more phenolic content when exposed to elevated CO2. Among the phenolic acid compounds, gallic acid had a higher content in both ginger varieties (Table 3). Elevated CO2 had significant effects (p ≤ 0.001) on the synthesis of phenolics. What is interesting in this data is that vanillic acid, cinnamic acid and salicylic acid were not detected in ginger grown under ambient (400 µmol mol-1) conditions. Conversely, tannic acid was not detected in gingers grown under elevated CO2 (800 µmol mol-1). Results imply that different CO2 concentrations have variable effects on each of the phenolic components. Among the studied phenolic compounds vanillic acid, cinnamic acid and salicylic acid were not detected in Halia Bentong grown under ambient CO2. Also cinnamic acid and salicylic acid were not detected in Halia Bara grown under ambient CO2. Tannic acid was not detected in those varieties grown under elevated (800 µmol mol-1) CO2.

Table 3.
The concentrations of some phenolics compounds in two varieties of Zingiber officinale, Halia Bentong (a) and Halia Bara (b) grown under different CO2 concentrations.
| Phenolic compounds | (a) Halia Bentong | |||
|---|---|---|---|---|
| 400 | 800 | |||
| Leaves | Rhizomes | Leaves | Rhizomes | |
| Gallic acid | 0.173 ± 0.0091d | 0.141 ± 0.031d | 0.576 ± 0.049b | 0.489 ± 0.043c |
| Vanillic acid | nd | nd | 0.229 ± 0.058b | 0.335 ± 0.028a |
| Ferulic acid | 0.081 ± 0.022f | 0.116 ± 0.016ef | 0.117 ± 0.026de | 0.21 ± 0.022b |
| Tannic acid | 0.388 ± 0.072a | nd | nd | nd |
| Cinnamic acid | nd | nd | 0.134 ± 0.027a | 0.0336 ± 0.255b |
| Salicylic acid | nd | nd | 0.22 ±0.021b | 0.037 ± 0.0125c |
| Phenolic compounds | (b) Halia Bara | |||
| 400 | 800 | |||
| Leaves | Rhizomes | Leaves | Rhizomes | |
| Gallic acid | 0.191±0.008d | 0.152+0.0081d | 0.645±0.066a | 0.537±0.034bc |
| Vanillic acid | 0.082±0.016c | nd | 0.24±0.052b | 0.357±0.038a |
| Ferulic acid | 0.071±0.017f | 0.148+0.017cd | 0.162±0.014c | 0.285±0.038a |
| Tannic acid | 0.224±0.041b | nd | nd | nd |
| Cinnamic acid | nd | nd | 0.125±0.027a | 0.0457±0.01b |
| Salicylic acid | nd | nd | 0.269±0.027a | 0.0417±0.044c |
All analyses are the mean ± standard deviation (N = 2). Results expressed in mg g-1 of dry plant material. nd: non detected. Means not sharing a common letter in each row (a:H.Bentong and b: H. Bara) were significantly different at P ≤ 0.05.
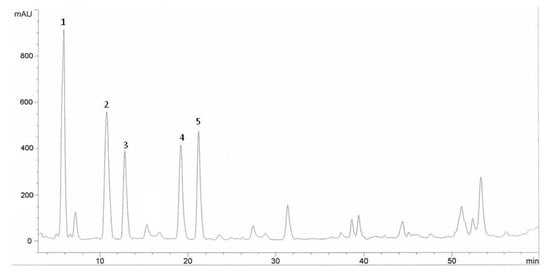
Figure 2.
HPLC chromatogram of Halia Bentong ginger (Zingiber officinale) leaves extracts. Identification of compounds: gallic acid (1), vanillic acid (2), ferulic acid (3), cinnamic acid (4), salicylic acid (5).
Salicylic acid belongs to plant phenolics group and is found in some plant species, with the highest levels being observed in the inflorescences of thermogenic plants and in spice herbs [45]. A high content of salicylic acid (0.269 mg g-1 DW) was detected in extract of Halia Bara leaves grown under 800 µmol mol-1 CO2. The results of previous studies showed that salicylic acid could enhance plant growth and yield. Jeyakumar et al. [46] reported that salicylic acid was able to enhance the dry matter production in black gram, while Nagasubramaniam et al. [45] stated that salicylic acid increased plant height, leaf area, crop growth rate, and total dry matter production in baby corn. Salicylic acid was able to enhance plant growth by improving nutrition uptake. According to previous studies we could say that increasing of cinnamic acid in ginger might be one of the reasons for increased ginger growth under elevated carbon dioxide.

Table 4.
Percent of increase or decrease of phenolic compounds in two varieties of Zingiber officinale grown under elevated CO2 concentration (800 µmol mol-1).
| Phenolic compounds | Halia Bentong | Halia Bara | ||
|---|---|---|---|---|
| Leaves | Rhizomes | Leaves | Rhizomes | |
| Gallic acid | +232.4 | +246.8 | +252.4 | +262.8 |
| Vanillic acid | +100 | +100 | +192.6 | +100 |
| Ferulic acid | +44.4 | +81 | +128.2 | +92.5 |
| Tannic acid | -100 | 0 | -100 | 0 |
| Cinnamic acid | +100 | +100 | +100 | +100 |
| Salicylic acid | +100 | +100 | +100 | +100 |
Results expressed in percent; + and – indicate respectively increase and decrease of component concentrations when exposed to CO2.
According to data from Table 4, gallic acid more enhanced in those varieties grown under elevated carbon dioxide conditions and after that vanillic acid and ferulic acid were more enhanced. On average phenolic compounds increased 79.4% in leaves and 107.6% in rhizomes of Halia Bentong and 112.2% in leaves and 109.2% in rhizomes of Halia Bara when exposed to elevated carbon dioxide conditions.
2.3. Radical Scavenging Activity (DPPH)
Under ambient conditions (400 µmol mol-1 CO2), ginger leaves exhibited higher radical scavenging activity than rhizomes (Figure 3). At a concentration of 30 µg mL-1 leaves of Halia Bara showed a 50.0% inhibition of free radicals, and at 45 µg mL-1, the scavenging activity of the methanolic extract of Halia Bara leaves grown under ambient CO2 concentration (400 µmol mol-1) reached 62.1%, while at the same extract concentration, that of the rhizomes was 42.0% (Figure 4), while 50% free radical scavenging activity was observed for Halia Bentong leaves at 45 µg mL-1 extract concentration, implying that Halia Bara, under natural environmental conditions, had higher antioxidant properties than Halia Bentong, and that was found in the leaves.
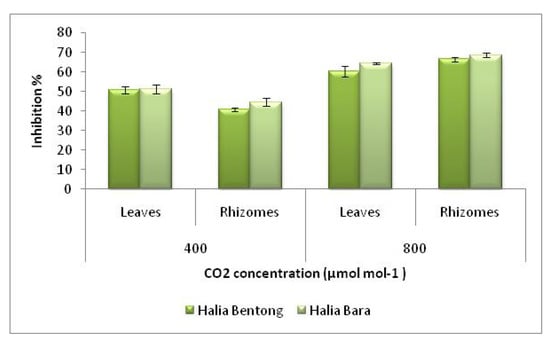
Figure 3.
DPPH scavenging activities of the methanolic extracts in different parts of two varieties of Zingiber officinale (error bar represents standard deviation).
The effect of antioxidants on DPPH scavenging is due to their hydrogen donating ability. The results of the current study showed that DPPH radical scavenging abilities of the extracts of ginger parts were less than those of butylated hydroxytoluene (BHT) (83.7%) and α-tocopherol (92.3%) at 45 µg mL-1. Antioxidant activity in the leaves and rhizomes were enhanced by increasing the CO2 concentration (Figure 3). When the CO2 concentration was increased from 400 to 800 µmol mol-1, the free radical scavenging power increased about 30.0% in Halia Bentong and 21.4% in Halia Bara. When comparing the ginger parts, it was found that the free radical scavenging power was more enhanced in the rhizomes than in the leaves (44.9% in Halia Bentong; 46.2% in Halia Bara). The above results also suggested that Halia Bentong seemed to be more responsive to increased CO2 concentration than Halia Bara, although the rhizomes of the latter were more receptive to elevated CO2 in the improvement of the antioxidative power. At low concentrations (10 and 15 µg mL-1) the DPPH activities of Halia Bara leaves was higher (35% and 38%, respectively) than BHT (20% and 32%, respectively). The DPPH scavenging activities of rhizomes in both varieties also increased after the concentration was increased to 35 µg mL-1, and these activities were higher than those recorded from the leaves using the same concentration. Our finding is in agreement with that of Wang et al. [9], who reported the increase in free radical scavenging power of strawberry by elevated CO2 concentrations (950 µmol mol-1). This study demonstrated that ginger has good free radical scavenging ability and, hence, can be used as a radical inhibitor or scavenger, possibly acting as a primary antioxidant. At the same time, with the anticipated rise in CO2 concentrations in the future with the current climate change scenario, it is anticipated that the antioxidant properties of ginger extracts could be enhanced, as the results indicate that an increased atmospheric carbon dioxide concentration could have a major effect on the antioxidant capacities of young ginger varieties.
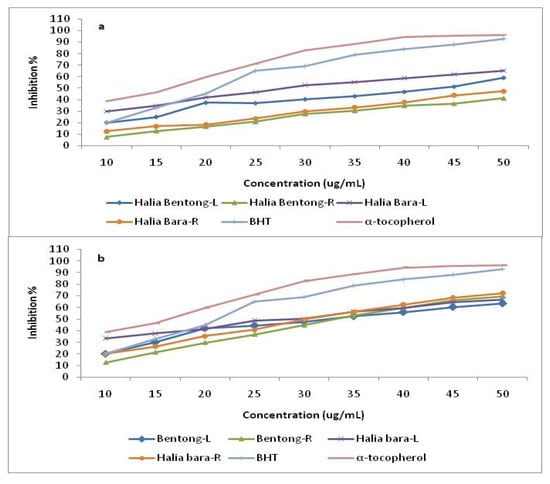
Figure 4.
DPPH radical scavenging activity of the methanolic extracts in different parts of two varieties of Zingiber officinale compared with positive controls butylated hydroxytoluene (BHT) and α-tocopherol. L and R represent the leaves and rhizomes at 400 µmol mol-1 CO2 (a), and 800 µmol mol-1 CO2 (b).
3. Experimental
3.1. Plant Materials
Rhizomes of two varieties of Zingiber officinale Roscoe. (Halia Bentong and Halia Bara) were germinated for two weeks in small pots and then transferred to 15 cm by 18 cm polyethylene bags filled with a soiless mixture of burnt rice husk and coco peat in a ratio of 1:1. After 2 weeks, the plants were transferred to a CO2 growth chamber (Conviron EF7, Winnipeg, Canada) with two different CO2 concentrations.
3.2. Growth Chamber Microclimate
First 400 µmol mol-1 was used as ambient CO2 condition, and 800 µmol mol-1 as the elevated CO2 concentration. Pure carbon dioxide (99.8% purity) was supplied from a high concentration carbon dioxide cylinder and injected through a pressure regulator into the closed fumigation chamber. The flow and concentration of carbon dioxide to the chamber was monitored and controlled with a CO2 PPM3 Controller. Water being supplied throughout the experiment using a drip system to ensure normal plant growth as water was supplied directly to the root zone of seedlings by pen drippers. Photoperiod, relative humidity, and air temperature of the chamber were controlled using an integrated control, monitoring, and data management system software package (Dynamac Corp., Rockville, MD). Plants were harvested after 16 weeks of CO2 exposure, and leaves and rhizomes were separated, freeze dried and finally kept at -80 ºC for future analysis. The experiments were carried out at the Biosystem Laboratory, Engineering Faculty, Universiti Putra Malaysia (UPM).
3.3. High Performance Liquid Chromatography (HPLC)
3.3.1. Flavonoid Extract Preparation
Aliquots of leaves and rhizomes (0.25 g) were extracted with 60% aqueous methanol (20 mL). Six M HC1 (5 mL) was added to each extract to give a 25 mL solution of 1.2 M HC1 in 50% aqueous methanol. Extracts were refluxed at 90 ºC for 2 h. Extract aliquots of 500 μL, taken both before and after hydrolysis, were filtered through a 0.45 μm filter [47].
3.3.2. Analysis of Flavonoids Composition by HPLC
Reversed-phase HPLC was used to assay flavonoid compositions. The Agilent HPLC system (Tokyo, Japan) used consisted of a Model 1100 pump equipped with a multi-solvent delivery system and an L-7400 ultraviolet (UV) detector. The column was an Agilent C18 (5 µm, 4.0 mm internal diameter 250 mm). The mobile phase composed of: (A) 2% acetic acid (CH3COOH) and (B) 0.5% acetic acid-acetonitrile (CH3CN),(50:50 v/v), and gradient elution was performed as follows: 0 min, 95:5; 10 min, 90:10; 40 min, 60:40, 55 min, 45:55; 60 min, 20:80; and 65 min, 0:100. The mobile phase was filtered under vacuum through a 0.45 um membrane filter before use. The flow rate was 1 mL min-1 and UV absorbance was measured at 280-365 nm. The operating temperature was maintained at room temperature [48]. Identification of the flavonoids was achieved by comparison with retention times of standards, UV spectra and calculation of UV absorbance ratios after co-injection of samples and standards. Commercial standards were purchased from Sigma–Aldrich (St Louis, MO, USA).
3.3.3. Preparation of Phenolics Extracts
Phenolics extracts were prepared by first carefully pipetting phosphoric acid (H3PO4, 1.2 mL) into about 950 mL water in a 1-L volumetric flask, mixing and bringing to volume with water. Then leaves and rhizomes (0.25 g) were extracted with 20 mL, of this phosphoric acid solution. Five mL of 6 M HC1 was added to each extract to give a 25 mL solution of 1.2 M HC1 in 50% MeOH. Extracts were refluxed at 90 ºC for 2 h and solution were filtered through a 0.45 μm filter [49]
3.3.4. Analysis of Phenolics Acids Composition by HPLC
An Agilent HPLC system (Tokyo, Japan) consisting of a Model 1100 pump equipped with a multi-solvent delivery system and a L-7400 ultraviolet (UV) detector was used. The column was an Agilent C18 (5 µm, 4.6 mm internal diameter 250 mm). The mobile phase was composed of phosphoric acid (aqueous) and (B) acetonitrile and gradient elution was performed as follows: 0 min, 85:15; 12 min, 75:25; 20 min, 75:25; 22 min, 85:15 and 30 min, 85:15. The mobile phase was filtered under vacuum through a 0.45 lm membrane filter before use. The flow rate and injection volume were 1 mL min-1 and 20 μL. UV absorbance was measured at 220-365 nm. The operating temperature was maintained at room temperature [49]. Identification of the phenolic acids were achieved by comparison with retention times of standards, UV spectra and calculation of UV absorbance ratios after co-injection of samples and standards. Commercial standards were purchased from Sigma–Aldrich.
3.4. Determination of Antioxidant Activities
Radical Scavenging Assay (DPPH)
1,1-Diphenyl-2-picrylhydrazyl (DPPH) was purchased from Sigma–Aldrich. Butylated hydroxytoluene (BHT) and α-tocopherol were purchased from Merck (India). In order to determine the radical scavenging ability, the method reported by Mensor et al. [50], was used. Briefly, an alcohol solution of DPPH (1 mL, 3mg/mL) was added to 2.5 mL samples containing different concentrations of extracts originating from different parts of the ginger varieties. The samples were first kept in a dark place at room temperature and their absorbance was read at 518 nm after 30 min. The antiradical activity (AA) was determined using the following formula:
AA% = 100- ((Abs: sample - Abs: empty sample) × 100)/ Abs: control
Blank samples contained 1 mL ethanol + 2.5 mL of various concentrations of ginger extract; control sample contained 1 mL of 0.3 mM DPPH + 2.5 mL ethanol. The concentration of the samples, the control, and the empty samples were measured in comparison with ethanol. The synthetic antioxidants BHT (butylated hydroxytoluene) and α-tocopherol were used as positive controls.
3.5. Statistical Analysis
The experiment was split-split plot and results were expressed as mean ± standard deviation (SD). Where applicable, the data were subjected to one way analysis of variance (ANOVA) and the differences between samples were determined by Duncan’s Multiple Range test using the Statistical Analysis System (SAS, 1999) and MSTATC program. P values ≤ 0.05 were regarded as significant.
4. Conclusions
The results of current study indicate that the synthesis of phenolics and flavonoids in ginger can be increased and affected by using CO2 enrichment in a controlled environment (CE). Following that, the antioxidant activity in young ginger extracts could also be improved. The HPLC analyses of flavonoids and phenolic compounds of CO2-enriched ginger plants also exhibited the ability of such treated plants to synthesize new compounds such as vanillic acid, cinnamic acid and salicylic acid. According to previous studies salicylic acid can enhance plant growth. Among the flavonoid compounds synthesized, fisetin and kaempferol, together with the phenolic compounds gallic acid and vanillic acid were dramatically enhanced in ginger parts by the elevated CO2 enrichment. This is a first report of fisetin, morin and naringenin in young ginger. Increases of these components in the rhizomes were more than in the leaves, and this was attributed to simultaneous improvement of antioxidant activities in rhizomes of those varieties when grown under elevated carbon dioxide conditions. This is a significant finding because the practice of CO2 enrichment has the potential to increase the value of the chemical product per unit area, and increase bio-activity per gram dry matter, emphasizing the implication on the phytochemistry of valuable medicinal and food plants such as in Zingiber officinale.
Natural antioxidants, especially phenolics and flavonoids from tea, wine, fruits, vegetables, and spices are already exploited commercially, either as antioxidant additives or as nutritional supplements. In recent years many plant species have been investigated in the search for novel antioxidants, but generally there is still a demand to find more information concerning the antioxidant potential of plant species as they are safe and also bioactive. The results on antioxidant activity of ginger indicate a good potential in this area for both the rhizomes as well as the leaves, especially when grown under elevated carbon dioxide. On the other hand, the impacts of cultural conditions and CO2 concentration on biopharmaceutical production in herbs have not been widely investigated and it needs to be understood, especially when the objective is the optimization of the herb chemistry. From this work, it can also be suggested that the composition of the phenolics and flavonoids will have to be considered in the development of any CE production system for medicinal plants. Further work is required to confirm this suggestion.
Acknowledgments
The authors are grateful to the Ministry of Higher Education Malaysia and the Research Management Centre of UPM for financing this work under the Fundamental Research Grant Scheme FRGS/PHASE1-2009/FUNDAMENTAL SCIENCE/UPM/ (01-11-08-646FR).
References and Notes
- Bazzaz, F.A. The response of natural ecosystems to the rising global CO2 levels. Annu. Rev. Ecol. Syst. 1990, 21, 167–196. [Google Scholar]
- Lavola, A.; Julkunen, T.R. The effect of elevated carbon dioxide and fertilization on primary and secondary metabolites in Befula pendula (Roth). Oecologia 1994, 99, 315–321. [Google Scholar] [CrossRef]
- Mark, S.J.; Jackson, S.B. Growth responses of Quercus petraea, Fraxinus excelsior and Pinus sylvestris to elevated carbon dioxide, ozone and water supply. New Phytol. 2000, 146, 437–451. [Google Scholar] [CrossRef]
- Tisserat, B.; Herman, C.; Silman, R.; Bothast, R.J. Using ultrahigh carbon dioxide levels enhances plantlet growth in vitro. Horttechnology 1997, 7, 282–289. [Google Scholar]
- Brevoort, P. The blooming United State botanical market: a new overview. Herbalgram 1998, 44, 33–46. [Google Scholar]
- Jaafar, H.Z.E. Carbon dioxide enrichment technology for improved productivity under controlled environment system in the tropics. Acta Hort. 2006, 742, 353–363. [Google Scholar]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Harun, M.H.; Yusop, R. Changes in the growth and photosynthetic patterns of oil palm (Elaeis guineensis Jacq.) seedlings exposed to short term CO2 enrichment in a closed top chamber. Acta Physiol. Plant. 2010, 32, 305–313. [Google Scholar] [CrossRef]
- Mattson, W.J.; Julkunen-Tiitto, R.; Herms, D.A. CO2 enrichment and carbon partitioning to phenolics: do plant responses accord better with the protein competition or the growth-differentiation balance models? Oikos 2005, 111, 337–347. [Google Scholar] [CrossRef]
- Wang, Y.S.H.; Bunce, A.J.; Maas, L.J. Elevated carbon dioxide increases contents of antioxidant compounds in field-grown strawberries. J. Agr. Food Chem. 2003, 51, 4315–4320. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A.; Wahab, P.E.M.; Halim, M.R.A. Effect of Different Light Intensities on Total Phenolics and Flavonoids Synthesis and Anti-oxidant Activities in Young Ginger Varieties (Zingiber officinale Roscoe). Int. J. Mol. Sci. 2010, 11, 3885–3897. [Google Scholar]
- Booker, F.L. Influence of carbon dioxide enrichment, ozone and nitrogen fertilization on cotton (Gossypium hirsutum L.) leaf and root composition. Plant Cell Environ. 2000, 23, 573–583. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A. Identification and Concentration of Some Flavonoid Components in Malaysian Young Ginger (Zingiber officinale Roscoe) Varieties by a High Performance Liquid Chromatography Method. Molecules 2010, 15, 6231–6243. [Google Scholar] [CrossRef]
- Yao, L.H. Flavonoids in food and their health benefits. Plant Food. Hum. Nutr. 2004, 59, 113–122. [Google Scholar]
- Ho, C.T.; Lee, C.Y.; Hungan, M.T. Phenolic compounds in food and their effects on health (analysis, occurrence, and chemistry). Amer. Chem. Soc. 1992, 2–19. [Google Scholar]
- Byers, T.; Guerrero, N. Epidemilogic evidence for vitamin C and vitamin E in cancer prevention. Amer. J. Clin. Nutr. 1995, 62, 1385–1392. [Google Scholar]
- Namiki, M. Antioxidant/antimutagens in food, critical reviews of food science and nutrition. Food Sci. Nutr. 1990, 29, 273–300. [Google Scholar]
- Heijnen, C.G.; Haenen, G.R.; Vanacker, F.A.; Vijgh, W.J.; Bast, A. Flavonoids as peroxynitrite scavengers:the role of the hydroxyl groups. Toxicol. Vitro 2001, 15, 3–6. [Google Scholar] [CrossRef]
- Chun, O.K.; Kim, D.O.; Lee, C.Y. Superoxide radical scavenging activity of the major polyphenols in fresh plums. J. Agr. Food Chem. 2003, 51, 8067–8072. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research science. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Chen, G. Effect of low fat and/or high fruit and vegetable diets on plasma level of 8-isoprostane-F2alpha in nutrition and breast health study. Nutr. Cancer 2004, 50, 155–160. [Google Scholar] [CrossRef]
- Sung-Jin, P.; Hoon, M.; Young-Youn, K.; Jun-Young, P.; Jun-Woo, P.; Myung-Jin, K.; Soon-Min, H. Anticancer effects of genistein, green tea catechins, and cordycepin on oral squamous cell carcinoma. J. Korean Oral Maxillofac. Surg. 2008, 34, 1–10. [Google Scholar]
- Tepe, B.; Sokmen, M.; Akpulat, H.A.; Sokmen, A. Screening of the antioxidant potentials of six Salvia species from Turkey. Food Chem. 2006, 95, 200–204. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Lim, Y.Y.; Wong, L.F.; Lianto, F.S.; Wong, S.K.; Lim, K.K.; Joe, C.E.; Lim, T.Y. Antioxidant and tyrosinase inhibition properties of leaves and rhizomes of ginger species. Food Chem. 2008, 109, 477–483. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Nakatani, N. Antioxidant effect of some ginger constituents. J. Food Sci. 1993, 578, 1407–1410. [Google Scholar] [CrossRef]
- Chen, C.H.; Kuo, M.; Wu, C.H; Ho, C.H. Pungent compounds of ginger (Zingiber officinale .L) Rosc.) extracted by liquid carbon dioxide. J. Agr. Food Chem. 1986, 34, 477–480. [Google Scholar] [CrossRef]
- Ramanthan, L.; Das, N.P. Effect of natural copper chelating compounds on the pro-oxidant activity of ascorbic acid in steam-cooked ground fish. Int. J. Food Sci. Technol. 1993, 28, 279–288. [Google Scholar]
- Kaufman, P.B.; Cseke, L.J.; Warber, S.; Duke, J.A.; Brielmann, H.L. Natural Products from Plant; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Wink, M. Introduction: Biochemistry, Role and Biotechnology of Secondary Products; CRC Press: Boca Raton, FL, USA, 1999; pp. 1–16. [Google Scholar]
- Liu, L.; King, J.S.; Giardina, P.C.H. Effects of elevated concentrations of atmospheric CO2 and tropospheric O3 on leaf litter production and chemistry in trembling aspen and paper birch communities. Tree Physiol. 2005, 25, 1511–1522. [Google Scholar] [CrossRef]
- Malikov, V.M.; Yuledashev, M.P. Phenolic compounds of plants of the Scutellaria L. genus: distribution, structure, and properties. Chem. Nat. Compd. 2002, 28, 358–406. [Google Scholar]
- Lindroth, R.L.; Kinney, K.K.; Platz, C.L. Responses of deciduous trees to elevated atmospheric CO2: Productivity, phytochemistry, and insect performance. Ecology 1993, 74, 763–777. [Google Scholar] [CrossRef]
- Stutte, G.W.; Eraso, I. Carbon dioxide enrichment enhances growth and flavonoid content of two Scutellaria species. J. Amer. Soc. Hort. Sci. 2008, 133, 631–638. [Google Scholar]
- Caldwell, C.R.; Britz, S.J.; Mirecki, R.M. Effect of temperature, elevated carbon dioxide, and drought during seed development on the isoflavone content of dwarf soybean (Glycine max (L.) Merrill] grown in controlled environments. J. Agr. Food Chem. 2005, 53, 1125–1129. [Google Scholar] [CrossRef]
- Khoo, H.M.; Suhaila, M. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agr. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef]
- Geraets, L.; Haegens, A.; Brauers, K.; Haydock, J.A.; Vernooy, J.H.; Wouters, E.F.; Bast, A.; Hageman, G.J. Inhibition of LPS-induced pulmonary inflammation by specific flavonoids. Biochem. Biophys. Res. Commun. 2009, 382, 598–603. [Google Scholar] [CrossRef]
- Tolonen, M.; Taipale, M.; Viander, B.; Pihlava, J.M.; Korhonen, H.; Ryhänen, E.L. Plant-derived biomolecules in fermented cabbage. J. Agr. Food. Chem. 2002, 50, 6798–6803. [Google Scholar]
- Kim, D.O.; Padilla-Zakour, O.I.; Griffiths, P.D. Flavonoids and antioxidant capacity of various cabbage genotypes at juvenile stage. J. Food Sci. 2004, 69, 685–689. [Google Scholar]
- Park, H.H.; Lee, S.; Son, H.Y.; Park, S.B.; Kim, M.S.; Choi, E.J.; Singh, T.S.; Ha, J.H.; Lee, M.G.; Kim, J.E.; Hyun, M.C.; Kwon, T.K.; Kim, Y.H.; Kim, S.H. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch. Pharm. Res. 2008, 31, 1303–1311. [Google Scholar] [CrossRef]
- Lim, D.O.Y.; Park, J.H. Induction of P53 contributes to apoptosis of HCT-116 human colon cancer cells induced by the dietary compound fisetin. Amer. J. Phys. Gastrointest. Liver Physiol. 2009, 296, 1060–1068. [Google Scholar] [CrossRef]
- Brown, J.; Prey, J.; Harrison, P.R. Enhanced sensitivity of human oral tumours to the flavonol, morin, during cancer progression: involvement of the Akt and stress kinase pathways. Carcinogenesis 2003, 24, 171–177. [Google Scholar] [CrossRef]
- Kawabata, K.; Tanaka, T.; Honjo, S.; Kakumoto, M.; Hara, A.; Makita, H.; Tatematsu, N.; Ushida, J; Tsuda, H.; Mori, H. Chemopreventive effect of dietary flavonoid morin on chemically induced rat tongue carcinogenesis. Int. J. Cancer 1999, 83, 381–386. [Google Scholar] [CrossRef]
- Song, Y.M.; Kang, J.W.; Zhou, J.; Wang, Z.H.; Lua, X.Q.; Wang, L.F.; Gao, J.Z. Study on the fluorescence spectra and electrochemical behavior of ZnL2 and morin with DNA. Spectrochim. Acta PT A: Mol. Bio. 2000, 56, 2491–2497. [Google Scholar] [CrossRef]
- Wijeratne, S.S.K.; Abou-Zaid, M.M.; Shahidi, F. Antioxidant polyphenols in almond and its coproducts. J. Agr. Food Chem. 2006, 54, 312–318. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar] [CrossRef]
- Nagasubramaniam, A.; Pathmanabhan, G.; Mallika, V. Studies on improving production potential of baby corn with foliar spray of plant growth regulators. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2007, 21, 154–157. [Google Scholar]
- Jeyakumar, P.; Velu, G.; Rajendran, C.; Amutha, R.; Savery, M.A.J.R.; Chidambaram, S. Varied responses of blackgram (Vigna munga) to certain foliar applied chemicals and plant growth regulators. Legume Res. Int. J. 2008, 31, 110–113. [Google Scholar]
- Crozier, A; Jensen, E.; Lean, M.E.J; Mc Donald, M.S. Quantitative analysis of flavonoids by reversed-phase high performance liquid chromatography. J. Chromatogr. 1997, 761, 315–321. [Google Scholar] [CrossRef]
- Wang, T.C.; Chuang, Y.C.; Ku, Y.H. Quantification of bioactive compounds in citrus fruits cultivated in Taiwan. Food Chem. 2007, 102, 1163–1171. [Google Scholar] [CrossRef]
- Standard Operating Protocol, HPLC Analysis of Phenolic acids (SOP), SOP No.: CB0103; Botanical Center for Age-Related Diseases: West Lafayette, IN, USA, 2001; p. 9.
- Mensor, L.L.; Menezes, F.S.; Leitao, G.G.; Reis, A.S.; Santos, T.S.; Coube, C.S. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).