Cherry Antioxidants: From Farm to Table
Abstract
:1. Introduction
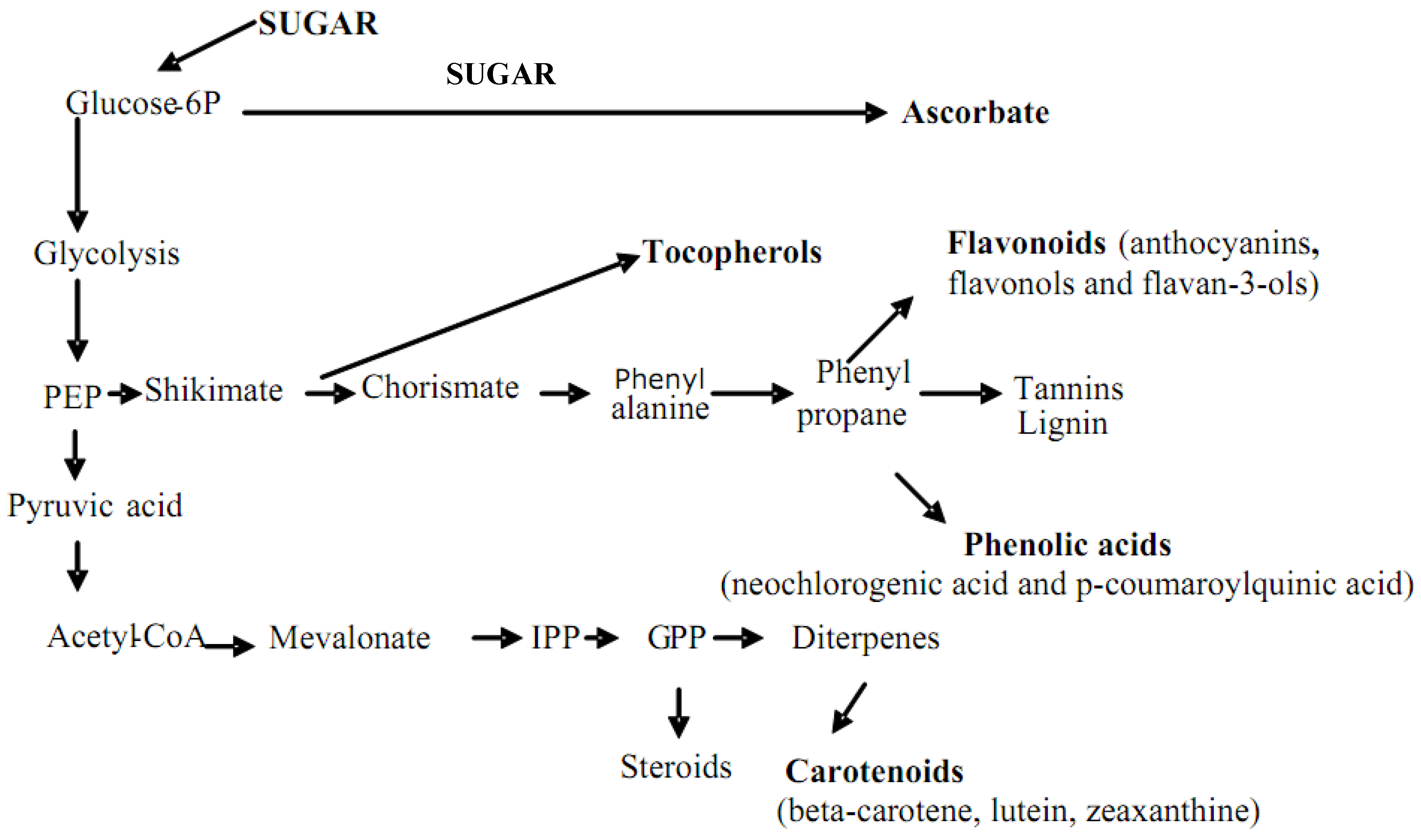
2. Nutritional Properties of Sweet and Sour Cherries
| Sweet cherries | Sour cherries | |
|---|---|---|
| Vitamin C | 7 mg | 10 mg |
| Niacin | 0.2 mg | 0.4 mg |
| Pantothenic Acid | 0.2mg | 0.1mg |
| Vitamin A | 64 IU | 1283 IU |
| Vitamin E (Alpha Tocopherol) | 0.1mg | 0.1mg |
| Vitamin K | 2 .1μg | 2.1 μg |
| Beta Carotene | 38 μg | 770 μg |
| Lutein+Zeaxanthin | 85 μg | 85 μg |
| Total Phenols | 109.8 mg | 228.9 mg |
2.1. Polyphenols in cherries
| Cultivar | Total phenols (mg GAE/100g fresh cherries ) | Total anthocyanins (CGE/100g fresh cherries) |
|---|---|---|
| sweet Brooksa | 60 ± 13 | 10 ± 2 |
| sweet Newstara | 75 ± 14 | 20 ± 5 |
| sweet Black Goldb | 92 ± 12 | 30 ± 9 |
| sweet Hedelfingenb | 96 ± 20 | 40 ± 7 |
| sweet Reginab | 104 ± 6 | 41 ± 2 |
| sweet Hartlandb | 147 ± 19 | 76 ± 12 |
| sweet Cristalinaa | 155±20 | 79 ± 5 |
| sour Balatonb | 254.1 ± 6.0 | 45 ± 2.3 |
| sour Danubeb | 162 ± 1 | 65 ± 3 |
| sour Schattenmorelleb | 295 ± 34 | 72 ± 6 |
| sour Sumadinkab | 312 ± 8 | 109 ± 6 |
2.2. Effect of pre-harvest and post harvest conditions on cherry nutritional properties
2.2.1. Effect of pre-harvest factors
2.2.2. Post-harvest factors
2.2.3. Cherries processed products
3. Cherry Phenols Bioavailability and Physiological Roles
3.1. Bioactivity of phenolic compounds from sour and sweet cherry
3.1.1. Antioxidant activity
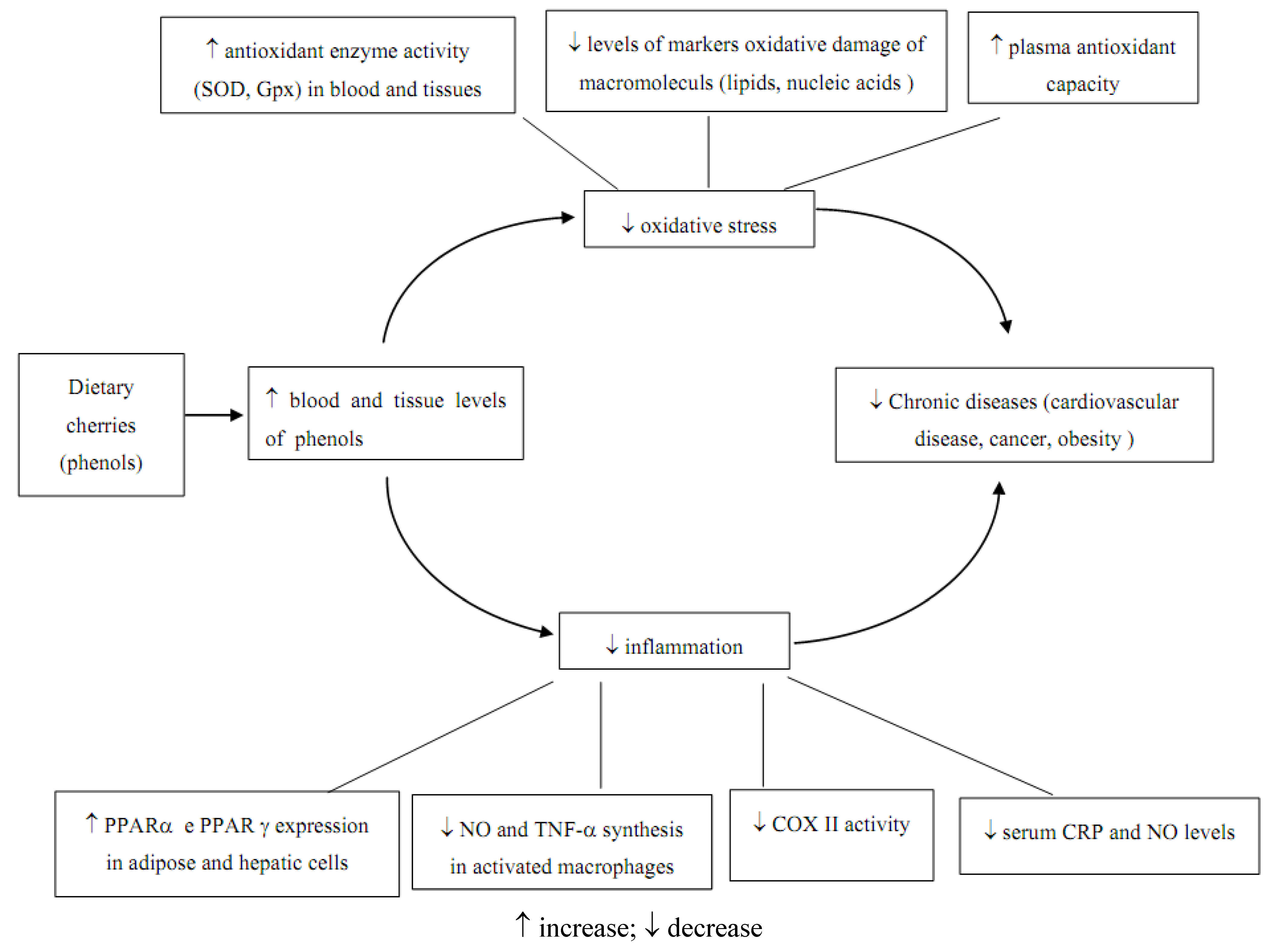
3.1.2. Anti inflammatory effect
3.2. Anticancer properties
4. Conclusions
- Sample Availability: Not Available.
References
- Hu, F.B. Plant-based foods and prevention of cardiovascular disease: an overview. Am. J. Clin. Nutr. 2003, 78, 544S–551S. [Google Scholar]
- He, F.J.; Nowson, C.A.; Lucas, M.; MacGregor, G.A. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: Meta-analysis of cohort studies. J. Hum. Hypertens. 2007, 21, 717–728. [Google Scholar] [CrossRef]
- Riboli, E.; Norat, T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am. J. Clin. Nutr. 2003, 78 (3 Suppl.), 559S–569S. [Google Scholar]
- Steinmetz, K.A.; Potter, J.D. Vegetables, fruit, and cancer prevention: A review. J. Am. Diet. Assoc. 1996, 96, 1027–1039. [Google Scholar] [CrossRef]
- Prior, R.L. Fruits and vegetables in the prevention of cellular oxidative damage. Am. J. Clin. Nutr. 2003, 78, 570–578. [Google Scholar]
- Szajdek, A.; Borowska, E.J. Bioactive compounds and health-promoting properties of berry fruits: A review. Plant Foods Hum. Nutr. 2008, 63, 147–156. [Google Scholar] [CrossRef]
- Benzie, I.F. Lipid peroxidation: A review of causes, consequences, measurement and dietary influences. Int. J. Food Sci. Nutr. 1996, 47, 233–261. [Google Scholar] [CrossRef]
- Machlin, L.J.; Bendich, A. Free radical tissue damage: Protective role of antioxidant nutrients. FASEB J. 1987, 1, 441–445. [Google Scholar]
- Bernalte, M.J.; Sabio, E.; Herna´ndez, M.T.; Gervasini, C. Influence of storage delay on quality of “Van” sweet cherry. PostharVest. Biol. Technol. 2003, 28, 303–312. [Google Scholar] [CrossRef]
- Esti, M.; Cinquante, L.; Sinesio, F.; Moneta, E.; Matteo, M. Physicochemical and sensory fruit characteristics of two sweet cherry cultivars after cool storage. Food Chem. 2002, 76, 399–405. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M.; Metheney, P. Consumer acceptance of “Brooks” and “Bing” cherries is mainly dependent on fruit SSC and visual skin color. Postharvest Biol. Technol. 2003, 28, 159–167. [Google Scholar] [CrossRef]
- Burkhardt, S.; Tan, D.X.; Manchester, L.C.; Hardeland, R.; Reiter, R.J. Detection and quantification of the antioxidant melatonin in Montmorency and Balaton tart cherries (Prunus cerasus). J. Agric. Food Chem. 2001, 49, 4898–4902. [Google Scholar] [CrossRef]
- Serrano, M.; Guille´n, F.; Martı´nez-Romero, D.; Castillo, S.; Valero, D. Chemical constituents and antioxidant activity of sweet cherry at different ripening stages. J. Agric. Food Chem. 2005, 53, 2741–2745. [Google Scholar] [CrossRef]
- Mozeticˇ, B.; Trebsˇe, P.; Hribar, J. Determination and quantitation of anthocyanins and hydroxycinnamic acids in different cultivars of sweet cherries (Prunus avium L.) from Nova Gorica Region (Slovenia). Food Technol. Biotechnol. 2002, 40, 207–212. [Google Scholar]
- Chaovanalikit, A.; Wrolstad, R.E. Total anthocyanins and total phenolics of fresh and processed cherries and their antioxidant properties. J. Food Sci. 2004, 69, FST67–FST72. [Google Scholar]
- Gao, L.; Mazza, G. Characterization, quantitation, and distribution of anthocyanins and colorless phenolics in sweet cherries. J. Agric. Food Chem. 1995, 43, 343–346. [Google Scholar] [CrossRef]
- Chandra, A.; Nair, M.G.; Iezzoni, A. Evaluation and characterization of the anthocyanin pigments in tart cherries (Prunus cerasus L.). J. Agric. Food Chem. 1992, 40, 967–969. [Google Scholar] [CrossRef]
- Chandra, A.; Rana, J.; Li, Y. Separation, identification, quantification, and method validation of anthocyanins in botanical supplement raw materials by HPLC and HPLC-MS. J. Agric. Food Chem. 2001, 49, 3515–3521. [Google Scholar] [CrossRef]
- Kim, D.; Heo, H.J.; Kim, Y.J.; Yang, H.S.; Lee, C.Y. Sweet and Sour Cherry Phenolics and Their Protective Effects on Neuronal Cells. J. Agric. Food Chem. 2005, 53, 9921–9927. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Kim, D.O.; Padilla-Zakour, O.I. Jam processing effect on phenolics and antioxidant capacity in anthocyanin-rich fruits: Cherry, plum, and raspberry. J. Food Sci. 2004, 69, S395–S400. [Google Scholar]
- Wang, S.Y. Effect of Pre-harvest Conditions on Antioxidant Capacity in Fruits. Acta Hort. 2006, 712. [Google Scholar]
- Jakobek, L.; Seruga, M.; Voca, S.; Sindrak, Z.; Dobricevic, N. Flavonol and phenolic acid composition of sweet cherries (cv. Lapins) produced on six different vegetative rootstocks. Sci. Hort-Amsterdam 2009, 123, 23–28. [Google Scholar] [CrossRef]
- Usenik, V.; Štampar, F. Seasonal changes in polyphenols of ‘Lapins’ sweet cherry grafted on different rootstocks. Acta Hortic. 2005, 667, 239–246. [Google Scholar]
- Karlidag, H.; Ercisli, S.; Sengul, M.; Tosun, M. Physico-chemical diversity in fruits of wild-growing sweet cherries (Prunus avium L.). Biotechnol. Biotechnol. Eq. 2009, 23, 3. [Google Scholar]
- Kevers, C.; Falkowski, M.; Tabart, J.; Defraigne, J.O.; Dommes, J.; Pincemail, J. Evolution of Antioxidant Capacity during Storage of Selected Fruits and Vegetables. J. Agric. Food Chem. 2007, 55, 8596–8603. [Google Scholar]
- Serrano, M.; Diaz-Mula, H.M.; Zapata, P.J.; Castillo, S.; Guillén, F.; Martinez-Romero, D.; Valverde Juan, M.; Valero, D. Maturity Stage at Harvest Determines the Fruit Quality and Antioxidant Potential after Storage of Sweet Cherry Cultivars. J. Agric. Food Chem. 2009, 57, 3240–3246. [Google Scholar] [CrossRef]
- Gonçalves, B.; Landbo, A.K.; Knudsen, D.; Silva, A.P.; Moutinho-Pereira, J.; Rosa, E.; Meyer, A.S. Effect of Ripeness and Postharvest Storage on the Phenolic Profiles of Cherries (Prunus avium L.). J. Agric. Food Chem. 2004, 52, 523–530. [Google Scholar]
- Kirakosyan, A.; Seymour, E.M.; Urcuyo Llanes, D.E.; Kaufman, P.B.; Bolling, S.F. Chemical profile and antioxidant capacities of tart cherry products. Food Chem. 2009, 115, 20–25. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar]
- Fazzari, M.; Fukumoto, L.; Mazza, G.; Livrea, M.A; Tesoriere, L.; Di Marco, L. In vitro Bioavailability of Phenolic Compounds from Five Cultivars of Frozen Sweet Cherries (Prunus avium L.). J. Agric. Food Chem. 2008, 56, 3561–3568. [Google Scholar] [CrossRef]
- Kalt, W.; Blumberg, J.B.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.; Graf, B.A.; O'Leary, J.M.; Milbury, P.E. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J. Agric. Food Chem. 2008, 56, 705–712. [Google Scholar]
- Milbury, P.E.; Vita, J.A.; Blumberg, J.B. Anthocyanins are Bioavailable in Humans following an Acute Dose of Cranberry Juice. J. Nutr. 2010, 140, 1099–1104. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar]
- Blando, F.; G.erardi, C.; Nicoletti, I. Sour Cherry (Prunus cerasus L.) Anthocyanins as Ingredients for Functional Foods. J. Biomed. Biotechnol. 2004, 5, 253–258. [Google Scholar]
- Lister, C.E.; Wilson, P.E.; Sutton, K.H.; Morrison, S.C. Understanding the health benefits of blackcurrants. Acta Hort. 2002, 585, 443–449. [Google Scholar]
- Tsuda, T.; Watanabe, M.; Ohshima, K.; Norinobu, S.; Choi, S.W.; Kawakishi, S.; Osawa, T. Antioxidative activity of the anthocyanin pigments cyanidin 3-O-beta-D-glucoside and cyanidin. J. Agric. Food Chem. 1994, 42, 2407–2410. [Google Scholar] [CrossRef]
- Tsuda, T.; Horio, F.; Kitoh, J.; Osawa, T. Protective effects of dietary cyanidin 3-O-beta-D-glucoside on liver ischemia-reperfusion injury in rats. Arch. Biochem. Biophys. 1999, 368, 361–366. [Google Scholar] [CrossRef]
- Heinonen, M.; Meyer, A.S.; Frankel, E.N. Antioxidant Activity of Berry Phenolics on Human Low-Density Lipoprotein and Liposome Oxidation. J. Agric. Food Chem. 1998, 46, 4107–4111. [Google Scholar] [CrossRef]
- Kim, D.O.; Heo, H.J.; Kim, Y.J.; Yang, H.S.; Lee, C.Y. Sweet and sour cherries phenolics and their protective effects on neuronal cells. J. Agric. Food Chem. 2005, 53, 9921–9927. [Google Scholar] [CrossRef]
- Sarić, A.; Sobocanec, S.; Balog, T.; Kusić, B.; Sverko, V.; Dragović-Uzelac, V.; Levaj, B.; Cosić, Z.; Macak Safranko, Z.; Marotti, T. Improved antioxidant and anti-inflammatory potential in mice consuming sour cherry juice (Prunus cerasus cv. Maraska). Plant Foods Hum. Nutr. 2009, 64, 231–237. [Google Scholar] [CrossRef]
- Prior, R.L.; Gu, L.; Wu, X.; Jacob, R.A.; Sotoudeh, G.; Kader, A.A.; Cook, R.A. Plasma Antioxidant Capacity Changes Following a Meal as a Measure of the Ability of a Food to Alter In Vivo Antioxidant Status. J. Am. Coll. Nutr. 2007, 26, 170–181. [Google Scholar]
- Jacob, R.A.; Spinozzi, G.M.; Simon, V.A.; Kelley, D.S.; Prior, R.L.; Hess-Pierce, B.; Kader, A.A. Consumption of cherries lowers plasma urate in healthy women. J. Nutr. 2003, 133, 1826–1829. [Google Scholar]
- Traustadóttir, T.; Davies, S.S.; Stock, A.A.; Su, Y.; Heward, C.B.; Roberts, L.J., 2nd; Harman, S.M. Tart cherry juice decreases oxidative stress in healthy older men and women. J. Nutr. 2009, 139, 1896–1900. [Google Scholar] [CrossRef]
- Kelley, D.S.; Rasooly, R.; Jacob, R.A.; Kader, A.A.; Mackey, B.E. Consumption of Bing sweet cherries lowers circulating concentrations of inflammation markers in healthy men and women. J. Nutr. 2006, 136, 981–986. [Google Scholar]
- Ridker, P.M. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in primary prevention of cardiovascular disease. Circulation 2001, 103, 1813–1818. [Google Scholar] [CrossRef]
- Abramson, S.B.; Amin, A.R.; Clancy, R.M.; Attur, M. The role of nitric oxide in tissue destruction. Best Pract. Res. Clin. Rheumatol. 2001, 15, 831–845. [Google Scholar] [CrossRef]
- Onur, O.; Akinci, A.S.; Akbiyik, F.; Unsal, I. Elevated levels of nitrate in rheumatoid arthritis. Rheumatol. Int. 2001, 20, 154–158. [Google Scholar] [CrossRef]
- Wang, J.; Mazza, G. Inhibitory effects of anthocyanins and other phenolic compounds on nitric oxide production in LPS/IFN-gamma-activated RAW 264.7 macrophages. J. Agric. Food Chem. 2002, 50, 850–857. [Google Scholar] [CrossRef]
- Wang, J.; Mazza, G. Effects of anthocyanins and other phenolic compounds on the production of tumor necrosis factor alpha in LPS/IFN-gamma-activated RAW 264.7 macrophages. J. Agric. Food Chem. 2002, 50, 4183–4189. [Google Scholar] [CrossRef]
- Wang, H.; Nair, M.G.; Strasburg, G.M. Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J. Nat. Prod. 1999, 62, 294–296. [Google Scholar] [CrossRef]
- Mulabagal, V.; Lang, G.A.; DeWitt, D.L.; Dalavoy, S.S.; Nair, M.G. Anthocyanin content, lipid peroxidation and cyclooxygenase enzyme inhibitory activities of sweet and sour cherries. J. Agric. Food Chem. 2009, 57, 1239–1246. [Google Scholar] [CrossRef]
- Tall, J.M.; Seeram, N.P.; Zhao, C.; Nair, M.G.; Meyer, R.A.; Raja, S.N. Tart cherry anthocyanins suppress inflammation-induced pain behavior in rat. Behav. Brain Res. 2004, 153, 181–188. [Google Scholar] [CrossRef]
- Blau, L.W. Cherry diet control for gout and arthritis. Tex. Rep. Biol. Med. 1950, 8, 309–311. [Google Scholar]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of tart cherry juice on indices of recovery following marathon running. Scand. J. Med. Sci. Sports 2009. [Google Scholar] [CrossRef]
- Connolly, D.A.; McHugh, M.P.; Padilla-Zakour, O.I.; Carlson, L.; Sayers, S.P. Efficacy of a tart cherry juice blend in preventing the symptoms of muscle damage. Br. J. Sports Med. 2006, 40, 679–683. [Google Scholar] [CrossRef]
- Kuehl, K.S.; Perrier, E.T.; Elliot, D.L.; Chesnutt, J.C. Efficacy of tart cherry juice in reducing muscle pain during running: A randomized controlled trial. JISSN 2010, 7, 17. [Google Scholar]
- Seymour, E.M.; Singer, A.A.; Kirakosyan, A.; Urcuyo-Llanes, D.E.; Kaufman, P.B.; Bolling, S.F. Altered hyperlipidemia, hepatic steatosis, and hepatic peroxisome proliferator-activated receptors in rats with intake of tart cherry. J. Med. Food 2008, 11, 252–259. [Google Scholar] [CrossRef]
- Seymour, E.M.; Lewis, S.K.; Urcuyo-Llanes, D.E.; Tanone, I.I.; Kirakosyan, A.; Kaufman, P.B.; Bolling, S.F. Regular tart cherry intake alters abdominal adiposity, adipose gene transcription, and inflammation in obesity-prone rats fed a high fat diet. J. Med. Food 2009, 12, 935–942. [Google Scholar] [CrossRef]
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef]
- Kang, S.Y.; Seeram, N.P.; Nair, M.G.; Bourquin, L.D. Tart cherry anthocyanins inhibit tumor development in Apc(Min) mice and reduce proliferation of human colon cancer cells. Cancer Lett. 2003, 194, 13–19. [Google Scholar]
- Bobe, G.; Wang, B.; Seeram, N.P.; Nair, M.G.; Bourquin, L.D. Dietary anthocyanin-rich tart cherry extract inhibits intestinal tumorigenesis in APC(Min) mice fed suboptimal levels of sulindac. J. Agric. Food Chem. 2006, 54, 9322–9328. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ferretti, G.; Bacchetti, T.; Belleggia, A.; Neri, D. Cherry Antioxidants: From Farm to Table. Molecules 2010, 15, 6993-7005. https://doi.org/10.3390/molecules15106993
Ferretti G, Bacchetti T, Belleggia A, Neri D. Cherry Antioxidants: From Farm to Table. Molecules. 2010; 15(10):6993-7005. https://doi.org/10.3390/molecules15106993
Chicago/Turabian StyleFerretti, Gianna, Tiziana Bacchetti, Alberto Belleggia, and Davide Neri. 2010. "Cherry Antioxidants: From Farm to Table" Molecules 15, no. 10: 6993-7005. https://doi.org/10.3390/molecules15106993
APA StyleFerretti, G., Bacchetti, T., Belleggia, A., & Neri, D. (2010). Cherry Antioxidants: From Farm to Table. Molecules, 15(10), 6993-7005. https://doi.org/10.3390/molecules15106993







