General Procedure for Solvent-Free Friedel-Crafts Acylation
A mixture of
1 (100 mg, 0.47 mmol), acylating agent (2.5 mL) and anhydrous AlCl
3 (400 mg, 3.00 mmol) was thoroughly ground in an agate mortar and pestle for 45 min in fume cupboard and then kept at room temperature for 1 h. The reaction mixture was then poured into crushed ice, basified with 30% NH
3 and extracted with EtOAc. The EtOAc layer was washed with water, dried (Na
2SO
4) and freed of solvent under reduced pressure. A solid mass thus obtained afforded compounds
2−
31 (
Table 1) through chromatographic procedures including column chromatography (silica gel; Merck 9385; CHCl
3-MeOH in increasing order of polarity from 9.95:0.05 to 8.25:1.25) and subsequent preparative TLC (Kieselgel 60 F
254 precoated aluminium cards; Merck, 9.9:0.1 to 9.5:0.5 CHCl
3-MeOH).
10-Acetyl-11-methoxy-3-methyl-5, 6-dihydro-β-carboline (2). Off white crystals (MeOH); mp 261–262 °C; IR (KBr) νmax: 3,418 (indole N-H), 1,668 (ketone C=O) cm-1; 1H-NMR (CDCl3): δ 7.89 (1H, s, H-9), 6.84 (1H, s, H-12), 3.88 (3H, s, OCH3), 3.80 (2H, t, J = 8.4 Hz, H-5), 2.84 (2H, t, J = 8.4 Hz, H-6), 2.70 (3H, s, H-2'), 2.36 (3H, s, H-14); 13C-NMR (CDCl3): δ 201.2 (C-1'), 157.9* (C-11), 157.7* (C-3), 140.1 (C-13), 129.4 (C-2), 124.8 (C-8), 123.5 (C-9), 119.9 (C-10), 119.1 (C-7), 93.7 (C-12), 55.8 (OCH3), 47.6 (C-5), 32.5 (C-2´), 21.6 (C-14), 19.3 (C-6); EIMS m/z (rel. int.%): 256 [M+] (30), 241 (100), 239 (47), 226 (6), 198 (6), 182 (22); HREIMS Calcd. for [C15H16N2O2]: 256.1212. Found: 256.1218.
10-Propionyl-11-methoxy-3-methyl-5, 6-dihydro-β-carboline (3). Off white crystals (MeOH); mp 263–264 °C; IR (KBr) νmax: 3,419 (indole N-H), 1,664 (ketone C=O) cm-1; 1H-NMR (CDCl3): δ 7.95 (1H, s, H-9), 6.85 (1H, s, H-12), 3.90 (3H, s, OCH3), 3.80 (2H, t, J = 8.4 Hz, H-5), 3.00 (2H, q, J = 7.2 Hz, H-2'), 2.82 (2H, t, J = 8.4 Hz, H-6), 2.35 (3H, s, H-14), 1.16 (3H, t, J = 7.2 Hz, H-3'); 13C-NMR (CDCl3): δ 203.4 (C-1'), 157.6 (C-3 and C-11), 140.0 (C-13), 129.2 (C-2), 124.8 (C-8), 123.3 (C-9), 119.7 (C-10), 119.0 (C-7), 93.6 (C-12), 55.7 (OCH3), 47.5 (C-5), 36.8 (C-2'), 21.5 (C-14), 19.3 (C-6), 8.8 (C-3'); EIMS m/z (rel. int.%): 270 [M+] (63), 241 (100), 226 (8), 198 (5), 182 (12); HREIMS Calcd. for [C16H18N2O2]: 270.1368. Found: 270.1370.
10-Butyryl-11-methoxy-3-methyl-5,6-dihydro-β-carboline (4). Off white crystals (MeOH); mp 266–267 °C; IR (KBr) νmax: 3,417 (indole N-H), 1,662 (ketone C=O) cm-1; 1H-NMR (CDCl3): δ 7.92 (1H, s, H-9), 6.84 (1H, s, H-12), 3.88 (3H, s, OCH3), 3.80 (2H, t, J = 8.4 Hz, H-5), 2.96 (2H, t, J = 7.4 Hz, H-2'), 2.81 (2H, t, J = 8.4 Hz, H-6), 2.33 (3H, s, H-14), 1.70 (2H, sextet, J = 7.4 Hz, H-3'), 0.95 (3H, t, J = 7.4 Hz, H-4'); 13C-NMR (CDCl3): δ 203.2 (C-1'), 157.7* (C-3), 157.5* (C-11), 140.0 (C-13), 129.2 (C-2), 124.9 (C-8), 123.2 (C-9), 119.6 (C-10), 118.9 (C-7), 93.6 (C-12), 55.7 (OCH3), 47.5 (C-5), 45.6 (C-2'), 21.6 (C-14), 19.3 (C-6), 18.2 (C-3'), 14.0 (C-4'); EIMS m/z (rel. int.%): 284 [M+] (79), 256 (2), 241 (100), 239 (25), 226 (6), 198 (4), 182 (10); HREIMS Calcd. for [C17H20N2O2]: 284.1525. Found: 284.1521.
10-Valeryl-11-methoxy-3-methyl-5,6-dihydro-β-carboline (5). Off white crystals (MeOH); mp 268–269 °C; IR (KBr) νmax: 3,421 (indole N-H), 1,662 (ketone C=O) cm-1; 1H-NMR (CDCl3): δ 7.87 (1H, s, H-9), 6.89 (1H, s, H-12), 3.87 (3H, s, OCH3), 3.76 (2H, t, J = 8.4 Hz, H-5), 2.95 (2H, t, J = 7.4 Hz, H-2'), 2.85 (2H, t, J = 8.4 Hz, H-6), 2.43 (3H, s, H-14), 1.68 (2H, quintet, J = 7.4 Hz, H-3'), 1.36 (2H, sextet, J = 7.4 Hz, H-4'), 0.91 (3H, t, J = 7.4 Hz, H-5'); 13C-NMR (CDCl3): δ 203.1 (C-1'), 158.1 (C-3), 157.7 (C-11), 140.2 (C-13), 129.6 (C-2), 125.2 (C-8), 123.4 (C-9), 119.5 (C-10), 118.6 (C-7), 93.8 (C-12), 55.7 (OCH3), 47.6 (C-5), 44.0 (C-2'), 26.4 (C-3'), 22.6 (C-4'), 21.8 (C-14), 19.6 (C-6), 14.1 (C-5'); EIMS m/z (rel. int.%): 298 [M+] (42), 256 (7), 241 (100), 239 (61), 226 (4), 198 (3), 182 (19), 57 (34); HREIMS Calcd. for [C18H22N2O2]: 298.1681. Found: 298.1694.
10-Hexanoyl-11-methoxy-3-methyl-5,6-dihydro-β-carboline (6). Off white crystals (MeOH); mp 271–272 °C; IR (KBr) νmax: 3,420 (indole N-H), 1,665 (ketone C=O) cm-1; 1H-NMR (CDCl3, 400 MHz): δ 7.85 (1H, s, H-9), 6.88 (1H, s, H-12), 3.87 (3H, s, OCH3), 3.76 (2H, t, J = 8.3 Hz, H-5), 2.95 (2H, t, J = 7.4 Hz, H-2'), 2.82 (2H, t, J = 8.3 Hz, H-6), 2.36 (3H, s, H-14), 1.67 (2H, quintet, J = 7.4 Hz, H-3'), 1.31 (2H, m, H-4'), 1.23 (2H, m, H-5'), 0.88 (3H, t, J = 7.4 Hz, H-6'); 13C-NMR (CDCl3, 100 MHz): δ 203.2 (C-1'), 158.0* (C-3), 157.8* (C-11), 141.2 (C-13), 129.8 (C-2), 125.4 (C-8), 123.2 (C-9), 120.2 (C-10), 118.7 (C-7), 94.2 (C-12), 55.6 (OCH3), 47.5 (C-5), 44.1 (C-2'), 31.5 (C-4'), 25.1 (C-3'), 22.7 (C-5'), 21.8 (C-14), 19.6 (C-6), 14.0 (C-6'); EIMS m/z (rel. int.%): 312 [M+] (30), 256 (14), 241 (100), 239 (47), 226 (8), 198 (8), 182 (22); HREIMS Calcd. for [C19H24N2O2]: 312.1838. Found: 312.1831.
10-Heptanoyl-11-methoxy-3-methyl-5,6-dihydro-β-carboline (7). Off white crystals (MeOH); mp 274–275 °C; IR (KBr) νmax: 3,418 (indole N-H), 1,664 (ketone C=O) cm-1; 1H-NMR (CDCl3): δ 7.89 (1H, s, H-9), 6.88 (1H, s, H-12), 3.89 (3H, s, OCH3), 3.75 (2H, t, J = 8.3 Hz, H-5), 2.95 (2H, t, J = 7.4 Hz, H-2'), 2.86 (2H, t, J = 8.3 Hz, H-6), 2.45 (3H, s, H-14), 1.66 (2H, quintet, J = 7.4 Hz, H-3'), 1.28 (4H, m, H-4' and H-5'), 1.24 (2H, m, H-6'), 0.86 (3H, t, J = 7.4 Hz, H-7'); 13C-NMR (CDCl3): δ 203.2 (C-1'), 158.8 (C-3), 158.1 (C-11), 141.3 (C-13), 129.9 (C-2), 125.5 (C-8), 123.5 (C-9), 120.1 (C-10), 118.6 (C-7), 94.1 (C-12), 55.8 (OCH3), 47.5 (C-5), 43.7 (C-2'), 31.7 (C-5'), 29.7 (C-4'), 25.0 (C-3'), 22.5 (C-6'), 21.7 (C-14), 19.5 (C-6), 14.0 (C-7'); EIMS m/z (rel. int.%): 326 [M+] (68), 256 (16), 241 (100), 239 (20), 226 (4), 198 (4), 182 (5); HREIMS Calcd. for [C20H26N2O2]: 326.1994. Found: 326.1991.
10-Capryloyl-11-methoxy-3-methyl-5,6-dihydro-β-carboline (8). Off white crystals (MeOH); mp 275–276 °C; IR (KBr) νmax: 3,413 (indole N-H), 1,667 (ketone C=O) cm-1; 1H-NMR (C5D5N): δ 12.41 (1H, s, indole NH), 8.24 (1H, s, H-9), 7.27 (1H, s, H-12), 3.87 (2H, t, J = 8.8 Hz, H-5), 3.76 (3H, s, OCH3), 3.10 (2H, t, J = 7.2 Hz, H-2'), 2.92 (3H, s, H-14), 2.91 (2H, t, J = 8.8 Hz, H-6), 1.83 (2H, quintet, J = 7.2 Hz, H-3'), 1.36 (2H, quintet, J = 7.2 Hz, H-4'), 1.26 (6H, m, H-5' to H-7'), 0.81 (3H, t, J = 7.2 Hz, H-8'); 13C-NMR (C5D5N): δ 202.5 (C-1'), 157.0 (C-3 and C-11), 141.0 (C-13), 131.9 (C-2), 125.6 (C-8), 123.2 (C-9), 118.3* (C-10), 118.1* (C-7), 94.6 (C-12), 55.7 (OCH3), 47.5 (C-5), 43.9 (C-2'), 31.9 (C-6'), 29.6** (C-5'), 29.5** (C-4'), 25.1 (C-3'), 22.8 (C-7'), 21.9 (C-14), 19.4 (C-6), 14.2 (C-8'); EIMS m/z (rel. int.%): 340 [M+] (40), 256 (13), 241 (100), 149 (21), 57 (36); HREIMS Calcd. for [C21H28N2O2]: 340.2150. Found: 340.2170.
10-Nonanoyl-11-methoxy-3-methyl-5,6-dihydro-β-carboline (9). Off white crystals (MeOH); mp 276–277 °C; IR (KBr) νmax: 3,420 (indole N-H), 1,662 (ketone C=O) cm-1; 1H-NMR (CDCl3): δ 7.92 (1H, s, H-9), 6.88 (1H, s, H-12), 3.90 (3H, s, OCH3), 3.80 (2H, t, J = 8.2 Hz, H-5), 2.96 (2H, t, J = 7.5 Hz, H-2'), 2.79 (2H, t, J = 8.2 Hz, H-6), 2.39 (3H, s, H-14), 1.69 (2H, quintet, J = 7.5 Hz, H-3'), 1.34 (2H, quintet, J = 7.5 Hz, H-4'), 1.23 (8H, m, H-5' to H-8'), 0.85 (3H, t, J = 7.5 Hz, H-9'); 13C-NMR (CDCl3) δ: 202.5 (C-1'), 158.7 (C-3), 158.2 (C-11), 141.1 (C-13), 130.2 (C-2), 125.5 (C-8), 123.4 (C-9), 120.1 (C-10), 118.6 (C-7), 94.4 (C-12), 55.6 (OCH3), 47.3 (C-5), 44.1 (C-2'), 31.9 (C-7'), 29.8* (C-6'), 29.7* (C-5'), 29.5 (C-4'), 25.2 (C-3'), 22.9 (C-8'), 21.7 (C-14), 19.5 (C-6), 14.1 (C-9'); EIMS m/z (rel. int.%): 354 [M+] (46), 341 (6), 256 (17), 255 (13), 242 (17), 241 (100), 239 (54), 226 (4), 182 (12); HREIMS Calcd. for [C22H30N2O2]: 354.2307. Found: 354.2313.
10-Decanoyl-11-methoxy-3-methyl-5,6-dihydro-β-carboline (10). Off white crystals (MeOH); mp 279–280 °C; IR (KBr) νmax: 3,420 (indole N-H), 1,662 (ketone C=O) cm-1; 1H-NMR (CDCl3): δ 7.88 (1H, s, H-9), 6.86 (1H, s, H-12), 3.87 (3H, s, OCH3), 3.72 (2H, t, J = 8.2 Hz, H-5), 2.94 (2H, t, J = 7.2 Hz, H-2'), 2.83 (2H, t, J = 8.2 Hz, H-6), 2.40 (3H, s, H-14), 1.66 (2H, quintet, J = 7.2 Hz, H-3'), 1.34 (2H, quintet, J = 7.2 Hz, H-4'), 1.24 (10H, m, H-5' to H-9'), 0.85 (3H, t, J = 7.2 Hz, H-10'); 13C-NMR (CDCl3): δ 202.5 (C-1'), 159.0 (C-3), 158.0 (C-11), 141.5 (C-13), 130.5 (C-2), 125.5 (C-8), 123.5 (C-9), 120.2 (C-10), 118.7 (C-7), 94.4 (C-12), 55.6 (OCH3), 47.5 (C-5), 44.0 (C-2'), 32.0 (C-8'), 29.8 (C-5' to C-7'), 29.5 (C-4'), 25.3 (C-3'), 22.9 (C-9'), 21.8 (C-14), 19.7 (C-6), 14.2 (C-10'); EIMS m/z (rel. int.%): 368 [M+] (56), 256 (17), 241 (100), 239 (54), 226 (7), 198 (6), 182(12); HREIMS Calcd. for [C23H32N2O2]: 368.2464. Found: 368.2477.
12-Acetyl-11-methoxy-3-methyl-5,6-dihydro-β-carboline (11). Off white crystals (MeOH); mp 255–256 °C; IR (KBr) νmax: 3,414 (indole N-H), 1,637 (ketone C=O) cm-1; 1H-NMR (CDCl3): δ 10.60 (1H, br.s, indole NH), 7.70 (1H, d, J = 8.8 Hz, H-9), 6.85 (1H, d, J = 8.8 Hz, H-10), 4.00 (3H, s, OCH3), 3.82 (2H, t, J = 8.5 Hz, H-5), 2.80 (2H, t, J = 8.5 Hz, H-6), 2.71(3H, s , H-2'), 2.36 (3H, s, H-14); 13C-NMR (CDCl3,): δ 200.5 (C-1'), 159.9 (C-11), 157.6 (C-3), 137.4 (C-13), 129.6 (C-2), 127.0 (C-9), 121.1 (C-8), 116.1 (C-7), 110.4 (C-12), 105.7 (C-10), 56.3 (OCH3), 48.0 (C-5), 33.6 (C-2'), 21.8 (C-14), 19.1 (C-6); EIMS m/z (rel. int.%): 256 [M+] (100), 241 (39), 226 (4), 198 (7), 182 (5); HREIMS Calcd. for [C15H16N2O2]: 256.1212. Found: 256.1216.
12-Propionyl-11-methoxy-3-methyl-5,6-dihydro-β-carboline (12). Off white crystals (MeOH); mp 257–258 °C; IR (KBr) νmax: 3,414 (indole N-H), 1,637 (ketone C=O) cm-1; 1H-NMR (CDCl3): δ 10.67 (1H, br.s, indole NH), 7.70 (1H, d, J = 8.7 Hz, H-9), 6.86 (1H, d, J = 8.7 Hz, H-10), 4.00 (3H, s, OCH3), 3.82 (2H, t, J = 8.3 Hz, H-5), 3.14 (2H, q, J = 7.2 Hz, H-2'), 2.80 (2H, t, J = 8.3 Hz, H-6), 2.35 (3H, s, H-14), 1.20 (3H, t, J = 7.2 Hz, H-3'); 13C-NMR (CDCl3): δ 203.7 (C-1'), 159.7 (C-11), 157.5 (C-3), 137.6 (C-13), 129.6 (C-2), 126.7 (C-9), 121.2 (C-8), 116.0 (C-7), 110.1 (C-12), 105.8 (C-10), 56.3 (OCH3), 48.1 (C-5), 38.3 (C-2'), 21.9 (C-14), 19.2 (C-6), 8.4 (C-3'); EIMS m/z (rel. int.%): 270 [M+] (100), 255 (44), 241 (21), 226 (4), 213 (5), 198 (9) 182 (9); HREIMS Calcd. for [C16H18N2O2]: 270.1368. Found: 270.1357.
12-Butyryl-11-methoxy-3-methyl-5,6-dihydro-β-carboline (13). Off white crystals (MeOH); mp 260–261 °C; IR (KBr) νmax: 3,418 (indole N-H), 1,636 (ketone C=O) cm-1; 1H-NMR (CDCl3): δ 10.66 (1H, br.s, indole NH), 7.70 (1H, d, J = 8.7 Hz, H-9), 6.86 (1H, d, J = 8.7 Hz, H-10), 4.00 (3H, s, OCH3), 3.82 (2H, t, J =8.4 Hz, H-5), 3.08 (2H, t, J = 7.3 Hz, H-2'), 2.80 (2H, t, J = 8.4 Hz, H-6), 2.36 (3H, s, H-14), 1.75 (2H, sextet, J = 7.3 Hz, H-3'), 1.00 (3H, t, J = 7.3 Hz, H-4'); 13C-NMR (CDCl3): δ 203.4 (C-1'), 159.7 (C-11), 157.7 (C-3), 137.4 (C-13), 129.6 (C-2), 126.8 (C-9), 121.2 (C-8), 116.1 (C-7), 110.2 (C-12), 105.8 (C-10), 56.4 (OCH3), 47.8 (C-5), 45.9 (C-2'), 21.9 (C-14), 19.2 (C-6), 17.9 (C-3'), 14.0 (C-4'); EIMS m/z (rel. int.%): 284 [M+] (100), 269 (12), 255 (63), 241 (25), 226 (5), 213 (6), 198 (12), 182 (17); HREIMS Calcd. for [C17H20N2O2]: 284.1525. Found: 284.1533.
12-Valeryl-11-methoxy-3-methyl-5,6-dihydro-β-carboline (14). Off white crystals (MeOH); mp 263–264 °C; IR (KBr) νmax: 3,416 (indole N-H), 1,638 (ketone C=O) cm-1; 1H-NMR (CDCl3): δ 10.65 (1H, br.s, indole NH), 7.70 (1H, d, J = 8.8 Hz, H-9), 6.86 (1H, d, J = 8.8 Hz, H-10), 4.00 (3H, s, OCH3), 3.82 (2H, t, J = 8.1 Hz, H-5), 3.11 (2H, t, J = 7.3 Hz, H-2'), 2.80 (2H, t, J = 8.1 Hz, H-6), 2.36 (3H, s, H-14), 1.70 (2H, quintet, J = 7.3 Hz, H-3'), 1.42 (2H, sextet, J = 7.3, H-4'), 0.96 (3H, t, J = 7.3 Hz, H-5'); 13C-NMR (CDCl3): δ 203.4 (C-1'), 159.6 (C-11), 157.7 (C-3), 137.6 (C-13), 129.5 (C-2), 126.8 (C-9), 121.2 (C-8), 116.0 (C-7), 110.2 (C-12), 105.8 (C-10), 56.3 (OCH3), 47.9 (C-5), 44.8 (C-2'), 26.7 (C-3'), 22.6 (C-4'), 21.8 (C-14), 19.1 (C-6), 14.1 (C-5'); EIMS m/z (rel. int.%): 298 [M+] (100), 283 (9), 255 (52), 241 (28), 226 (4), 213 (6), 198 (8), 182 (12); HREIMS Calcd. for [C18H22N2O2]: 298.1681. Found: 298.1692.
12-Hexanoyl-11-methoxy-3-methyl-5,6-dihydro-β-carboline (15). Off white crystals (MeOH); mp 265–266 °C; IR (KBr) νmax: 3,412 (indole N-H), 1,637 (ketone C=O) cm-1; 1H-NMR (CDCl3): δ 10.66 (1H, br.s, indole NH), 7.70 (1H, d, J = 8.6 Hz, H-9), 6.87 (1H, d, J = 8.6 Hz, H-10), 4.00 (3H, s, OCH3), 3.83 (2H, t, J = 8.0 Hz, H-5), 3.10 (2H, t, J = 7.1 Hz, H-2'), 2.81 (2H, t, J = 8.0 Hz, H-6), 2.37 (3H, s, H-14), 1.70 (2H, quintet, J = 7.1 Hz, H-3'), 1.37 (2H, m, H-4'), 1.29 (2H, m, H-5'), 0.91 (3H, t, J = 7.1 Hz, H-6'); 13C-NMR (CDCl3): δ 203.4 (C-1'), 159.7 (C-11), 157.9 (C-3), 137.7 (C-13), 129.5 (C-2), 126.8 (C-9), 121.1 (C-8), 116.4 (C-7), 110.2 (C-12), 105.9 (C-10), 56.3 (OCH3), 47.6 (C-5), 45.1 (C-2'), 31.7 (C-4'), 24.2 (C-3'), 22.6 (C-5'), 21.5 (C-14), 19.1 (C-6), 14.0 (C-6'); EIMS m/z (rel. int.%): 312 [M+] (100), 297 (12), 255 (62) , 241 (39), 226 (7), 213 (8), 198 (9), 182 (9); HREIMS Calcd. for [C19H24N2O2]: 312.1838. Found: 312.1826.
12-Heptanoyl-11-methoxy-3-methyl-5,6-dihydro-β-carboline (16). Off white crystals (MeOH); mp 268–269 °C; IR (KBr) νmax: 3,417 (indole N-H), 1,637 (ketone C=O) cm-1; 1H-NMR (CDCl3): δ 10.73 (1H, br.s, indole NH), 7.72 (1H, d, J = 8.8 Hz, H-9), 6.88 (1H, d, J = 8.8 Hz, H-10), 4.01 (3H, s, OCH3), 3.85 (2H, t, J = 8.4 Hz, H-5), 3.10 (2H, t, J = 7.4 Hz, H-2'), 2.85 (2H, t, J = 8.4 Hz, H-6), 2.42 (3H, s, H-14), 1.71 (2H, quintet, J = 7.4 Hz, H-3'), 1.39 (2H, quintet, J = 7.4, H-4'), 1.27 (4H, m, H-5' and H-6'), 0.88 (3H, t, J = 7.4 Hz, H-7'); 13C-NMR (CDCl3): δ 203.2 (C-1'), 159.5 (C-11), 157.8 (C-3), 137.7 (C-13), 129.6 (C-2), 127.0 (C-9), 121.3 (C-8), 116.2 (C-7), 110.2 (C-12), 106.1 (C-10), 56.4 (OCH3), 46.8 (C-5), 45.2 (C-2'), 31.5 (C-5'), 28.9 (C-4'), 25.0 (C-3'), 22.5 (C-6'), 21.6 (C-14), 19.1 (C-6), 14.0 (C-7'); EIMS m/z (rel. int.%): 326 [M+] (100), 269 (6), 255 (43), 241 (20), 226 (4), 213 (6), 198 (5), 182 (8), 57 (29); HREIMS Calcd. for [C20H26N2O2]: 326.1994. Found: 326.1999.
12-Capryloyl-11-methoxy-3-methyl-5,6-dihydro-β-carboline (17). Off white crystals (MeOH); mp 271–272 °C; IR (KBr) νmax: 3,413 (indole N-H), 1,637 (ketone C=O) cm-1; 1H-NMR (CDCl3): δ 10.70 (1H, br.s, indole NH), 7.72 (1H, d, J = 8.8 Hz, H-9), 6.88 (1H, d, J = 8.8 Hz, H-10), 4.00 (3H, s, OCH3), 3.85 (2H, t, J = 8.1 Hz, H-5), 3.10 (2H, t, J = 7.2 Hz, H-2'), 2.84 (2H, t, J = 8.1 Hz, H-6), 2.42 (3H, s, H-14), 1.70 (2H, quintet, J = 7.2 Hz, H-3'), 1.34 (2H, quintet, J = 7.2, H-4'), 1.26 (6H, m, H-5' to H-7'), 0.85 (3H, t, J = 7.2 Hz, H-8'); 13C-NMR (CDCl3): δ 203.4 (C-1'), 159.6 (C-11), 157.7 (C-3), 137.9 (C-13), 129.3 (C-2), 126.6 (C-9), 121.3 (C-8), 116.6 (C-7), 110.1 (C-12), 106.1 (C-10), 56.1 (OCH3), 47.1 (C-5), 45.1 (C-2'), 31.9 (C-6'), 29.5* (C-5'), 29.4* (C-4'), 24.7 (C-3'), 22.7 (C-7'), 21.4 (C-14), 19.4 (C-6), 14.0 (C-8'); EIMS m/z (rel. int.%): 340[M+] (100), 255 (43), 241 (20), 239 (28), 198 (8), 182 (15); HREIMS Calcd. for [C21H28N2O2]: 340.2150. Found: 340.2170.
12-Nonanoyl-11-methoxy-3-methyl-5,6-dihydro-β-carboline (18). Off white crystals (MeOH); mp 273–274 °C; IR (KBr) νmax: 3,414 (indole N-H), 1,638 (ketone C=O) cm-1; 1H-NMR (CDCl3): δ 10.77 (1H, br.s, indole NH), 7.72 (1H, d, J = 8.8 Hz, H-9), 6.88 (1H, d, J = 8.8 Hz, H-10), 4.00 (3H, s, OCH3), 3.87 (2H, t, J = 8.1 Hz, H-5), 3.10 (2H, t, J = 7.3 Hz, H-2'), 2.88 (2H, t, J = 8.1 Hz, H-6), 2.46 (3H, s, H-14), 1.71 (2H, quintet, J = 7.3 Hz, H-3'), 1.40 (2H, quintet, J = 7.3, H-4'), 1.26 (8H, m, H-5' to C-8'), 0.86 (3H, t, J = 7.3 Hz, H-9'); 13C-NMR (CDCl3): δ 203.5 (C-1'), 159.7 (C-11), 157.9 (C-3), 137.8 (C-13), 129.4 (C-2), 126.7 (C-9), 121.3 (C-8), 116.5 (C-7), 110.1 (C-12), 106.1 (C-10), 56.1 (OCH3), 47.3 (C-5), 45.1 (C-2'), 31.9 (C-7'), 29.7 (C-6'), 29.5* (C-5'), 29.4* (C-4'), 24.6 (C-3'), 22.9 (C-8'), 21.6 (C-14), 19.3 (C-6), 14.1 (C-9'); EIMS m/z (rel. int.%): 354 [M+] (100), 339 (5), 269 (5), 255 (31), 241 (18), 226 (4), 213 (5), 198 (3), 182 (5); HREIMS Calcd. for [C22H30N2O2]: 354.2307. Found: 354.2313.
12-Decanoyl-11-methoxy-3-methyl-5,6-dihydro-β-carboline (19). Off white crystals (MeOH); mp 275–276 °C; IR (KBr) νmax: 3,415 (indole N-H), 1,638 (ketone C=O) cm-1; 1H-NMR (CDCl3): δ 10.70 (1H, br.s, indole NH), 7.70 (1H, d, J = 8.8 Hz, H-9), 6.87 (1H, d, J = 8.8 Hz, H-10), 4.00 (3H, s, OCH3), 3.84 (2H, t, J = 8.2 Hz, H-5), 3.10 (2H, t, J = 7.3 Hz, H-2'), 2.83 (2H, t, J = 8.2 Hz, H-6), 2.40 (3H, s, H-14), 1.71 (2H, quintet, J = 7.3 Hz, H-3'), 1.34 (2H, m, H-4'), 1.25 (10H, m, H-5' to H-9'), 0.86 (3H, t, J = 7.3 Hz, H-10'); 13C-NMR (CDCl3): δ 203.4 (C-1'), 159.8 (C-11), 158.3 (C-3), 137.9 (C-13), 129.4 (C-2), 126.9 (C-9), 121.1 (C-8), 116.8 (C-7), 110.2 (C-12), 106.0 (C-10), 56.3 (OCH3), 47.1 (C-5), 45.2 (C-2'), 31.9 (C-8'), 29.7* (C-7'), 29.6* (C-6'), 29.5* (C-5'), 29.3 (C-4'), 24.5 (C-3'), 22.7 (C-9'), 21.2 (C-14), 19.1 (C-6), 14.1 (C-10'); EIMS m/z (rel. int.%): 368 [M+] (100), 353 (12) 269 (4) 255 (27), 241 (22), 226 (4), 213 (8), 198 (9), 182 (22), 57 (28); HREIMS Calcd. for [C23H32N2O2]: 368.2464. Found: 368.2452.
2-Acetyl-3-(2-acetamidoethyl)-7-methoxyindole (20). Colorless crystalline solid (CHCl3-MeOH, 1:1); mp 152–153 °C; IR (KBr) νmax: 3,436, 3,314, 3,270 (indolic and amide N-H), 1,690, 1,640 (ketone and amide C=O) cm-1; 1H-NMR (CDCl3): δ 8.86 (1H, br.s, indole NH) 7.54 (1H, d, J = 8.8 Hz, H-5), 6.79 (1H, dd, J = 8.8 and 1.8 Hz, H-6), 6.75 (1H, br.s, H-8), 5.85 (1H, br.t, CONH), 3.83 (3H, s, OCH3), 3.54 (2H, q, J = 6.8 Hz, H-2'), 3.28 (2H, t, J = 6.8 Hz, H-1'), 2.60 (3H, s, 2-COCH3), 1.90 (3H, s, H-2''); 13C-NMR (CDCl3): δ 189.8 (2-COCH3), 170.3 (C-1''), 160.0 (C-7), 137.3 (C-9), 131.8 (C-2), 122.8 (C-4), 122.0 (C-5), 120.9 (C-3), 112.4 (C-6), 93.4 (C-8), 55.5 (OCH3), 40.8 (C-2'), 28.1 (2-COCH3), 25.3 (C-1'), 23.3 (C-2''); EIMS m/z (rel. int.%): 274 [M+] (14), 215 (100), 203 (42), 202 (57), 188 (46), 160 (37), 145 (22); HREIMS Calcd. for [C15H18N2O3]: 274.1317. Found: 274.1322.
2-Acetyl-3-(2-propionylamidoethyl)-7-methoxyindole (21). Colorless crystalline solid (CHCl3-MeOH, 1:1); mp 156–157 °C; IR (KBr) νmax: 3,432, 3,315, 3,274 (indolic and amide N-H), 1,691, 1,637 (ketone and amide C=O) cm-1; 1H-NMR (CDCl3): δ 8.80 (1H, br.s, indole NH), 7.55 (1H, d, J = 8.8 Hz, H-5), 6.79 (1H, dd, J = 8.8 and 2.0 Hz, H-6), 6.75 (1H, d, J = 2.0 Hz, H-8), 5.73 (1H, br.t, CONH), 3.84 (3H, s, OCH3), 3.55 (2H, q, J = 6.9 Hz, H-2'), 3.28 (2H, t, J = 6.9 Hz, H-1'), 2.61 (3H, s, 2-COCH3), 2.12 (2H, q, J = 7.6 Hz, H-2''), 1.08 (3H, t, J = 7.6 Hz, H-3''); 13C-NMR (CDCl3): δ 189.7 (2-COCH3), 174.0 (C-1''), 160.0 (C-7), 137.2 (C-9), 131.8 (C-2), 122.8 (C-4), 122.1 (C-5), 120.9 (C-3), 112.4 (C-6), 93.4 (C-8), 55.5 (OCH3), 40.7 (C-2'), 29.7 (C-2''), 28.1 (2-COCH3), 25.4 (C-1'), 9.7 (C-3''); EIMS m/z (rel. int.%): 288 [M+] (13), 215 (100), 203 (32), 202 (51) 188 (19), 57 (53); HREIMS Calcd. for [C16H20N2O3]: 288.1474. Found: 288.1477.
2-Acetyl-3-(2-butyrylamidoethyl)-7-methoxyindole (22). Colorless crystalline solid (CHCl3-MeOH, 1:1); mp 161–162 °C; IR (KBr) νmax: 3,430, 3,315, 3,258 (indolic and amide N-H), 1,695, 1,631(ketone and amide C=O) cm-1; 1H-NMR (CDCl3): δ 8.80 (1H, br.s, indole NH), 7.56 (1H, d, J = 8.8 Hz, H-5), 6.79 (1H, dd, J = 8.8 and 2.1 Hz, H-6), 6.75 (1H, d, J = 2.1 Hz, H-8), 5.74 (1H, br.t, CONH), 3.84 (3H, s, OCH3), 3.55 (2H, q, J = 6.7 Hz, H-2'), 3.28 (2H, t, J = 6.7 Hz, H-1'), 2.62 (3H, s, 2-COCH3), 2.07 (2H, t, J = 7.4 Hz, H-2''), 1.59 (2H, sextet, J = 7.4 Hz, H-3''´), 0.89 (3H, t, J = 7.4 Hz, H-4''); 13C-NMR (CDCl3): δ 189.8 (2-COCH3), 173.2 (C-1''), 160.0 (C-7), 137.3 (C-9), 131.9 (C-2), 122.8 (C-4), 122.1 (C-5), 120.9 (C-3), 112.4 (C-6), 93.4 (C-8), 55.5 (OCH3), 40.6 (C-2'), 38.7 (C-2''), 28.1 (2-COCH3), 25.4 (C-1'), 19.0 (C-3''), 13.8 (C-4''); EIMS m/z (rel. int.%): 302 [M+] (6), 259 (3) 215 (100), 203 (33), 202 (39), 188 (20), 160 (20); HREIMS Calcd. for [C17H22N2O3]: 302.1630. Found: 302.1642.
2-Acetyl-3-(2-valerylamidoethyl)-7-methoxyindole (23). Colorless crystalline solid (CHCl3-MeOH, 1:1); mp 163–164 °C; IR (KBr) νmax: 3,420, 3,314, 3,260 (indolic and amide N-H), 1,692, 1,633 (ketone and amide C=O) cm-1; 1H-NMR (CDCl3): δ 8.81 (1H, br.s, indole NH), 7.55 (1H, d, J = 8.8 Hz, H-5), 6.79 (1H, dd, J = 8.8 and 2.0 Hz, H-6), 6.75 (1H, d, J = 2.0 Hz, H-8), 5.74 (1H, br.t, CONH), 3.84 (3H, s, OCH3), 3.54 (2H, q, J = 6.9 Hz, H-2'), 3.28 (2H, t, J = 6.9 Hz, H-1'), 2.62 (3H, s, 2-COCH3), 2.09 (2H, t, J = 7.3 Hz, H-2''), 1.54 (2H, quintet, J = 7.3 Hz, H-3''´), 1.26 (2H, sextet, J = 7.3 Hz, H-4''), 0.87 (3H, t, J = 7.3 Hz, H-5''); 13C-NMR (CDCl3): δ 189.7 (2-COCH3), 173.3 (C-1''), 160.0 (C-7), 137.2 (C-9), 131.9 (C-2), 122.9 (C-4), 122.1 (C-5), 120.9 (C-3), 112.4 (C-6), 93.4 (C-8), 55.5 (OCH3), 40.6 (C-2'), 36.5 (C-2''), 28.1 (2-COCH3), 27.7 (C-3''), 25.5 (C-1'), 22.4 (C-4''), 13.8 (C-5''); EIMS m/z (rel. int.%): 316 [M+] (9), 273 (2), 215 (100), 203 (32), 202 (38), 188 (17), 160 (18), 85 (33), 57 (66); HREIMS Calcd. for [C18H24N2O3]: 316.1787. Found: 316.1802.
2-Acetyl-3-(2-hexanoylamidoethyl)-7-methoxyindole (24). Colorless crystalline solid (CHCl3-MeOH, 1:1); mp 167–168 °C; IR (KBr) νmax: 3,428, 3,317, 3,270 (indolic and amide N-H), 1,692, 1,633 (ketone and amide C=O) cm-1; 1H-NMR (CDCl3): δ 8.80 (1H, br.s, indole NH) 7.55 (1H, d, J = 8.8 Hz, H-5), 6.79 (1H, dd, J = 8.8 and 2.0 Hz, H-6), 6.75 (1H, d, J = 2.0 Hz, H-8), 5.73 (1H, br.t, CONH), 3.84 (3H, s, OCH3), 3.55 (2H, q, J = 6.9 Hz, H-2'), 3.28 (2H, t, J = 6.9 Hz, H-1'), 2.61 (3H, s, 2-COCH3), 2.08 (2H, t, J = 6.7 Hz, H-2''), 1.56 (2H, quintet, J = 6.7 Hz, H-3''´), 1.25 (4H, m, H-4'' and H-5''), 0.86 (3H, t, J = 6.7 Hz, H-6''); 13C-NMR (CDCl3): δ 189.7 (2-COCH3), 173.3 (C-1''), 160.0 (C-7), 137.3 (C-9), 131.9 (C-2), 122.9 (C-4), 122.1 (C-5), 120.9 (C-3), 112.4 (C-6), 93.5 (C-8), 55.5 (OCH3), 40.7 (C-2'), 36.8 (C-2''), 31.5 (C-4''), 28.1 (2-COCH3), 25.5 (C-1'), 25.3 (C-3''), 22.4 (C-5''), 13.9 (C-6''); EIMS m/z (rel. int.%): 330 [M+] (8), 287 (2), 215 (100), 203 (30), 202 (22), 188 (12), 160 (7), 145 (5), 99 (6); HREIMS Calcd. for [C19H26N2O3]: 330.1943. Found: 330.1956.
2-Acetyl-3-(2-heptanoylamidoethyl)-7-methoxyindole (25). Colorless crystalline solid (CHCl3-MeOH, 1:1); mp 171–172 °C; IR (KBr) νmax: 3,430, 3,314, 3,274 (indolic and amide N-H), 1,691, 1,637 (ketone and amide C=O) cm-1; 1H-NMR (CDCl3): δ 8.79 (1H, br.s, indole NH), 7.56 (1H, d, J = 8.8 Hz, H-5), 6.79 (1H, dd, J = 8.8 and 2.1 Hz, H-6), 6.75 (1H, d, J = 2.1 Hz, H-8), 5.72 (1H, br.t, CONH), 3.84 (3H, s, OCH3), 3.54 (2H, q, J = 7.0 Hz, H-2'), 3.28 (2H, t, J = 7.0 Hz, H-1'), 2.61 (3H, s, 2-COCH3), 2.08 (2H, t, J = 7.0 Hz, H-2''), 1.55 (2H, quintet, J = 7.0 Hz, H-3''´), 1.25 (6H, m, H-4'' to H-6''), 0.85 (3H, t, J = 7.0 Hz, H-7''); 13C-NMR (CDCl3): δ 189.7 (2-COCH3), 173.3 (C-1''), 160.0 (C-7), 137.3 (C-9), 131.9 (C-2), 122.9 (C-4), 122.1 (C-5), 120.9 (C-3), 112.4 (C-6), 93.5 (C-8), 55.5 (OCH3), 40.7 (C-2'), 36.8 (C-2''), 31.5 (C-5''), 28.9 (C-4''), 28.1 (2-COCH3), 25.6* (C-3''), 25.5* (C-1'), 22.4 (C-6''), 14.0 (C-7''); EIMS m/z (rel. int.%): 344 [M+] (8), 301 (3), 215 (100), 203 (27), 202 (18), 188 (15), 160 (12), 145 (7), 113 (4); HREIMS Calcd. for [C20H28N2O3]: 344.2100. Found: 344.2110.
2-Acetyl-3-(2-capryloylamidoethyl)-7-methoxyindole (26). Colorless crystalline solid (CHCl3-MeOH, 1:1); mp 172–173 °C; IR (KBr) νmax: 3,436, 3,315, 3,270 (indolic and amide N-H), 1,695, 1,637 (ketone and amide C=O) cm-1; 1H-NMR (CDCl3): δ 8.85 (1H, br.s, indole NH), 7.55 (1H, d, J = 8.8 Hz, H-5), 6.79 (1H, dd, J = 8.8 and 2.2 Hz, H-6), 6.74 (1H, d, J = 2.2 Hz, H-8), 5.77 (1H, br.t, CONH), 3.83 (3H, s, OCH3), 3.54 (2H, q, J = 6.9 Hz, H-2'), 3.28 (2H, t, J = 6.9 Hz, H-1'), 2.61 (3H, s, 2-COCH3), 2.08 (2H, t, J = 7.3 Hz, H-2''), 1.54 (2H, quintet, J = 7.3 Hz, H-3''´), 1.24 (8H, m, H-4'' to H-7''), 0.85 (3H, t, J = 7.3 Hz, H-8''); 13C-NMR (CDCl3): δ 189.7 (2-COCH3), 173.3 (C-1''), 160.0 (C-7), 137.3 (C-9), 131.9 (C-2), 122.9 (C-4), 122.1 (C-5), 120.9 (C-3), 112.4 (C-6), 93.5 (C-8), 55.5 (OCH3), 40.7 (C-2'), 36.8 (C-2''), 31.7 (C-6''), 29.3 (C-5''), 29.0 (C-4''), 28.1 (2-COCH3), 25.6* (C-3''), 25.5* (C-1'), 22.6 (C-7''), 14.0 (C-8''); EIMS m/z (rel. int.%): 358 [M+] (7), 315 (2), 215 (100), 203 (27), 202 (21), 160 (10), 145 (4), 127 (4), 57 (24); HREIMS Calcd. for [C21H30N2O3]: 358.2256. Found: 358.2261.
2-Acetyl-3-(2-nonanoylamidoethyl)-7-methoxyindole (27). Colorless crystalline solid (CHCl3-MeOH, 1:1); mp 178–179 °C ; IR (KBr) νmax: 3,440, 3,320, 3,275 (indolic and amide N-H), 1,690, 1,634 (ketone and amide C=O) cm-1; 1H-NMR (CDCl3): δ 8.80 (1H, br.s, indole NH), 7.55 (1H, d, J = 8.8 Hz, H-5), 6.79 (1H, dd, J = 8.8 and 1.9 Hz, H-6), 6.75 (1H, d, J = 1.9 Hz, H-8), 5.75 (1H, br.t, CONH), 3.84 (3H, s, OCH3), 3.54 (2H, q, J = 6.9 Hz, H-2'), 3.28 (2H, t, J = 6.9 Hz, H-1'), 2.62 (3H, s, 2-COCH3), 2.08 (2H, t, J = 7.1 Hz, H-2''), 1.55 (2H, quintet, J = 7.1 Hz, H-3''´), 1.24 (10H, m, H-4'' to H-8''), 0.85 (3H, t, J = 7.1 Hz, H-9''); 13C-NMR (CDCl3): δ 189.7 (2-COCH3), 173.4 (C-1''), 160.0 (C-7), 137.2 (C-9), 131.9 (C-2), 122.8 (C-4), 122.1 (C-5), 120.9 (C-3), 112.4 (C-6), 93.4 (C-8), 55.5 (OCH3), 40.6 (C-2'), 36.8 (C-2''), 31.8 (C-7''), 29.3 (C-5''and C-6''), 29.1 (C-4''), 28.1 (2-COCH3), 25.6* (C-3''), 25.5* (C-1'), 22.6 (C-8''), 14.1 (C-9''); EIMS m/z (rel. int.%): 372 [M+] (3), 215 (100), 203 (29), 202 (35), 188 (17), 174 (19), 160 (18), 145 (12), 57 (35), 55 (28); HREIMS Calcd. for [C22H32N2O3]: 372.2413. Found 372.2426.
2-Acetyl-3-(2-decanoylamidoethyl)-7-methoxyindole (28). Colorless crystalline solid (CHCl3-MeOH, 1:1); mp 183–184 °C; IR (KBr) νmax: 3,423, 3,317, 3,274 (indolic and amide N-H), 1,690, 1,637 (ketone and amide C=O) cm-1; 1H-NMR (CDCl3): δ 8.90 (1H, br.s, indole NH), 7.55 (1H, d, J = 8.9 Hz, H-5), 6.78 (1H, dd, J = 8.9 and 1.7 Hz, H-6), 6.74 (1H, d, J = 1.7 Hz, H-8), 5.79 (1H, br.t, CONH), 3.83 (3H, s, OCH3), 3.54 (2H, q, J = 6.9 Hz, H-2'), 3.28 (2H, t, J = 6.9 Hz, H-1'), 2.61 (3H, s, 2-COCH3), 2.08 (2H, t, J = 6.9 Hz, H-2''), 1.54 (2H, quintet, J = 6.9 Hz, H-3''´), 1.25 (12H, m, H-4'' to H-9''), 0.85 (3H, t, J = 6.9 Hz, H-10''); 13C-NMR (CDCl3): δ 189.7 (2-COCH3), 173.4 (C-1''), 160.0 (C-7), 137.3 (C-9), 131.9 (C-2), 122.8 (C-4), 122.1 (C-5), 120.9 (C-3), 112.4 (C-6), 93.4 (C-8), 55.6 (OCH3), 40.7 (C-2'), 36.8 (C-2''), 31.8 (C-8''), 29.4* (C-7''), 29.3 (C-5'' and C-6''), 29.2* (C-4''), 28.1 (2-COCH3), 25.6** (C-3''), 25.5** (C-1'), 22.7 (C-9''), 14.1 (C-10''); EIMS m/z (rel. int.%): 386 [M+] (10), 243 (3), 215 (100), 203 (30), 202 (14), 188 (12), 174 (9), 145 (4), 160 (7); HREIMS Calcd. for [C23H34N2O3]: 386.2569. Found: 386.2580.
2-Acetyl-3-(2-capryloylamidoethyl)-8-capryloyl-7-methoxyindole (29). Colorless crystalline solid (CHCl3-MeOH, 1:1); mp 196–198 °C; IR (KBr) νmax: 3,436, 3,290 (indolic and amide N-H), 1,692, 1,637 (ketone and amide C=O) cm-1; 1H-NMR (CDCl3): δ 11.02 (1H, br.s, indole NH), 7.87 (1H, d, J = 9.0 Hz, H-5), 6.89 (1H, d, J = 9.0 Hz, H-6), 6.17 (1H, br.t, CONH), 4.02 (3H, s, OCH3), 3.52 (2H, q, J = 6.4 Hz, H-2'), 3.28 (2H, t, J = 6.4 Hz, H-1'), 3.08 (2H, t, J = 7.2 Hz, H-2'''), 2.62 (3H, s, 2-COCH3), 2.05 (2H, t, J = 6.5 Hz, H-2''), 1.71 (2H, quintet, J = 7.2 Hz, H-3'''´), 1.56 (2H, quintet, J = 6.5 Hz, H-3''´), 1.34 (2H, m, H-4'''), 1.28 (6H, m, H-5''' to H-7'''), 1.24 (8H, m, H-4'' to H-7''), 0.86 (3H, t, J = 7.2 Hz, H-8'''), 0.84 (3H, t, J = 6.5 Hz, H-8''); 13C-NMR (CDCl3): δ 203.0 (C-1'''), 190.7 (2-COCH3), 173.5 (C-1''), 161.0 (C-7), 137.2 (C-9), 132.4 (C-2), 128.1 (C-5), 123.7 (C-4), 121.4 (C-3), 109.7 (C-8), 106.8 (C-6), 56.4 (OCH3), 45.1 (C-2'''), 41.0 (C-2'), 36.8 (C-2''), 31.8 (C-6'''), 31.6 (C-6''), 29.5 (C-5'''), 29.3* (C-5''), 29.2* (C-4'''), 29.0 (C-4''), 28.1 (2-COCH3), 25.6 (C-1'), 24.5 (C-3''') 24.3 (C-3''), 22.6 (C-7'''), 22.5 (C-7''), 14.1 (C-8'''), 14.0 (C-8''); EIMS m/z (rel. int.%): 484 [M+] (5), 441 (2), 341 (100), 329 (10), 328 (9), 270 (8), 257 (15), 242 (20), 230 (15), (63); HREIMS Calcd. for [C29H44N2O4 ]: 484.3301. Found: 484.3303.
2-Acetyl-3-(2-nonanoylamidoethyl)-8-nonanoyl-7-methoxyindole (30). Colorless crystalline solid (CHCl3-MeOH, 1:1); mp 199–200 °C; IR (KBr) νmax: 3,432, 3,295 (indolic and amide N-H), 1,690, 1,637 (ketone and amide C=O) cm-1; 1H-NMR (CDCl3): δ 11.03 (1H, br.s, indole NH), 7.86 (1H, d, J = 8.8 Hz, H-5), 6.90 (1H, d, J = 8.8 Hz, H-6), 6.19 (1H, br.t, CONH), 4.02 (3H, s, OCH3), 3.51 (2H, q, J = 6.4 Hz, H-2'), 3.28 (2H, t, J = 6.4 Hz, H-1'), 3.08 (2H, t, J = 6.9 Hz, H-2'''), 2.62 (3H, s, 2-COCH3), 2.05 (2H, t, J = 6.7 Hz, H-2''), 1.70 (2H, quintet, J = 6.9 Hz, H-3'''´), 1.53 (2H, quintet, J = 6.7 Hz, H-3''´), 1.34 (2H, m, H-4'''), 1.28 (8H, m, H-5''' to H-8'''), 1.26 (10H, m, H-4'' to H-8''), 0.86 (3H, t, J = 6.9 Hz, H-9'''), 0.85 (3H, t, J = 6.7 Hz, H-9''); 13C-NMR (CDCl3): δ 203.1 (C-2'''), 190.7 (2-COCH3), 173.5 (C-1''), 161.0 (C-7), 137.2 (C-9), 132.4 (C-2), 128.1 (C-5), 123.7 (C-4), 121.4 (C-3), 109.7 (C-8), 106.8 (C-6), 56.4 (OCH3), 45.1 (C-2'''), 40.9 (C-2'), 36.8 (C-2''), 31.8 (C-7'' and C-7'''), 29.6 (C-4'''), 29.5 (C-5'''and C-6'''), 29.3 (C-4'' to C-6''), 28.1 (2-COCH3), 25.6 (C-1'), 24.5 (C-3'''), 24.3 (C-3''), 22.7 (C-8'' and C-8'''), 14.1 (C-9'' and C-9'''); EIMS m/z (rel. int.%): 512[M+] (15), 469 (20), 355 (100), 343 (8), 342 (6), 270 (8), 257 (12), 242 (14), 230 (12); HREIMS Calcd. for [C31H48N2O4]: 512.3602. Found: 512.3591.
2-Acetyl-3-(2-decanoylamidoethyl)-8-decanoyl-7-methoxyindole (31). Colorless crystalline solid (CHCl3-MeOH, 1:1); mp 201–202 °C; IR (KBr) νmax: 3,436, 3,295 (indolic and amide N-H), 1,691, 1,637 (ketone and amide C=O) cm-1; 1H-NMR (CDCl3): δ 11.03 (1H, br.s, indole NH), 7.86 (1H, d, J = 8.8 Hz, H-5), 6.89 (1H, d, J = 8.8 Hz, H-6), 6.18 (1H, br.t, CONH), 4.02 (3H, s, OCH3), 3.52 (2H, q, J = 6.4 Hz, H-2'), 3.28 (2H, t, J = 6.4 Hz, H-1'), 3.08 (2H, t, J = 7.0 Hz, H-2'''), 2.62 (3H, s, 2-COCH3), 2.05 (2H, t, J = 6.7 Hz, H-2''), 1.70 (2H, quintet, J = 7.0 Hz, H-3'''´), 1.51 (2H, quintet, J = 6.7 Hz, H-3''´), 1.34 (2H, m, H-4'''), 1.26 (22H, m, H-4'' to H-9'' and H-5''' to H-9'''), 0.86 (3H, t, J = 7.0 Hz, H-10'''), 0.85 (3H, t, J = 6.7 Hz, H-10''); 13C-NMR (CDCl3): δ 203.0 (C-2'''), 190.7 (2-COCH3), 173.4 (C-1''), 161.0 (C-7), 137.2 (C-9), 132.4 (C-2), 128.1 (C-5), 123.7 (C-4), 121.4 (C-3), 109.7 (C-8), 106.8 (C-6), 56.4 (OCH3), 45.1 (C-2'''), 40.9 (C-2'), 36.8 (C-2''), 31.9 (C-8'''), 31.8 (C-8''), 29.6* (C-4'''), 29.5** (C-5'''and C-6'''), 29.4* (C-7''), 29.3** (C-5'' and C-6''), 29.2* (C-4'' and C-7'''), 28.1 (2-COCH3), 25.6 (C-1'), 24.5 (C-3'''), 24.3 (C-3''), 22.7 (C-9'' and C-9'''), 14.1 (C-10'' and C-10'''); (*,** values may be interchanged for a given compound); EIMS m/z (rel. int.%): 540 [M+] (15), 497 (20), 370 (85), 369 (100), 357 (47), 356 (24), 270 (25), 257 (50), 242 (60), 230 (35); HREIMS Calcd. for [C33H52N2O4]: 540.3967. Found: 540.3977.
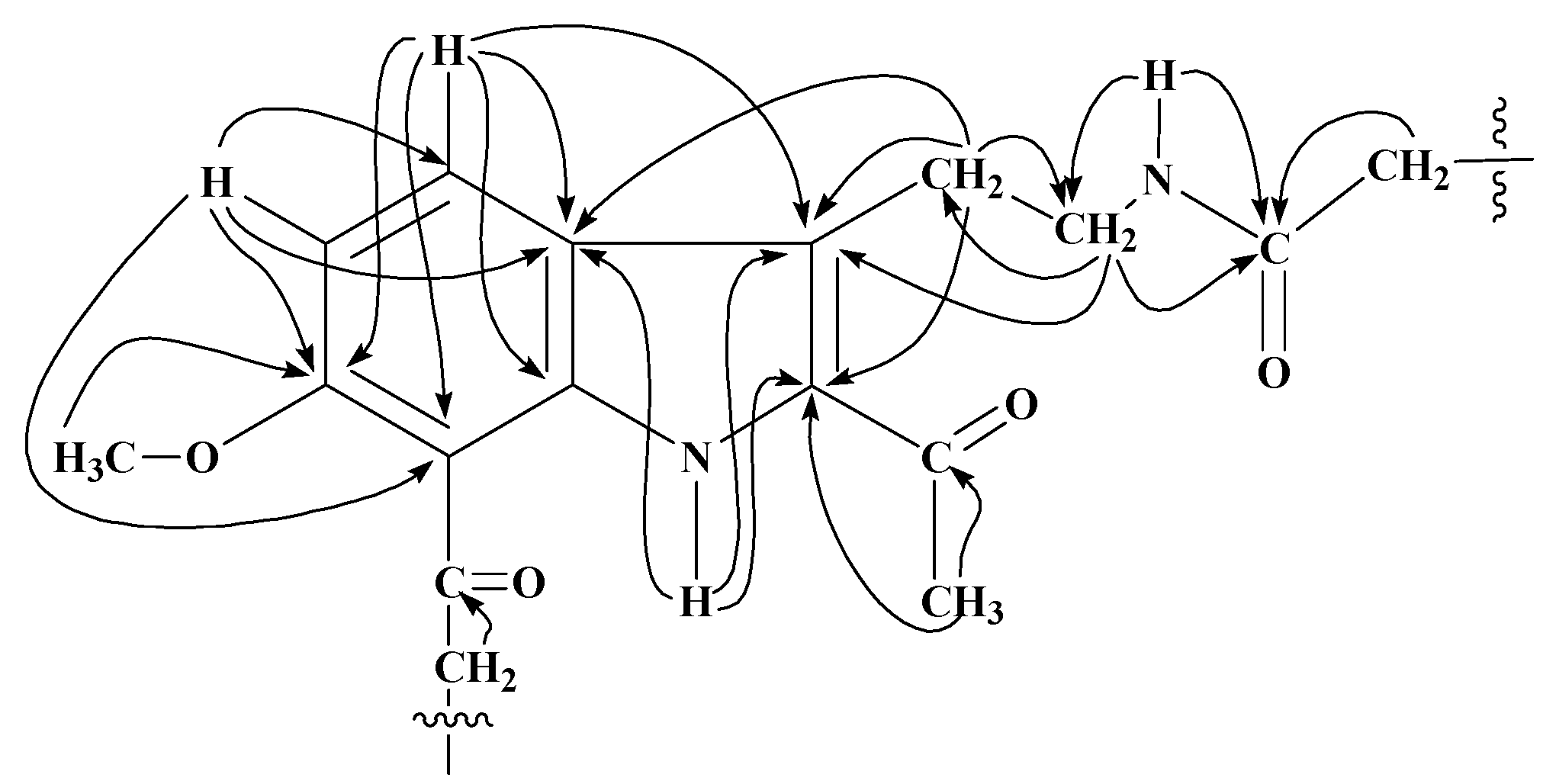
 13C) interactions of 10-acyl analogues of 1.
13C) interactions of 10-acyl analogues of 1.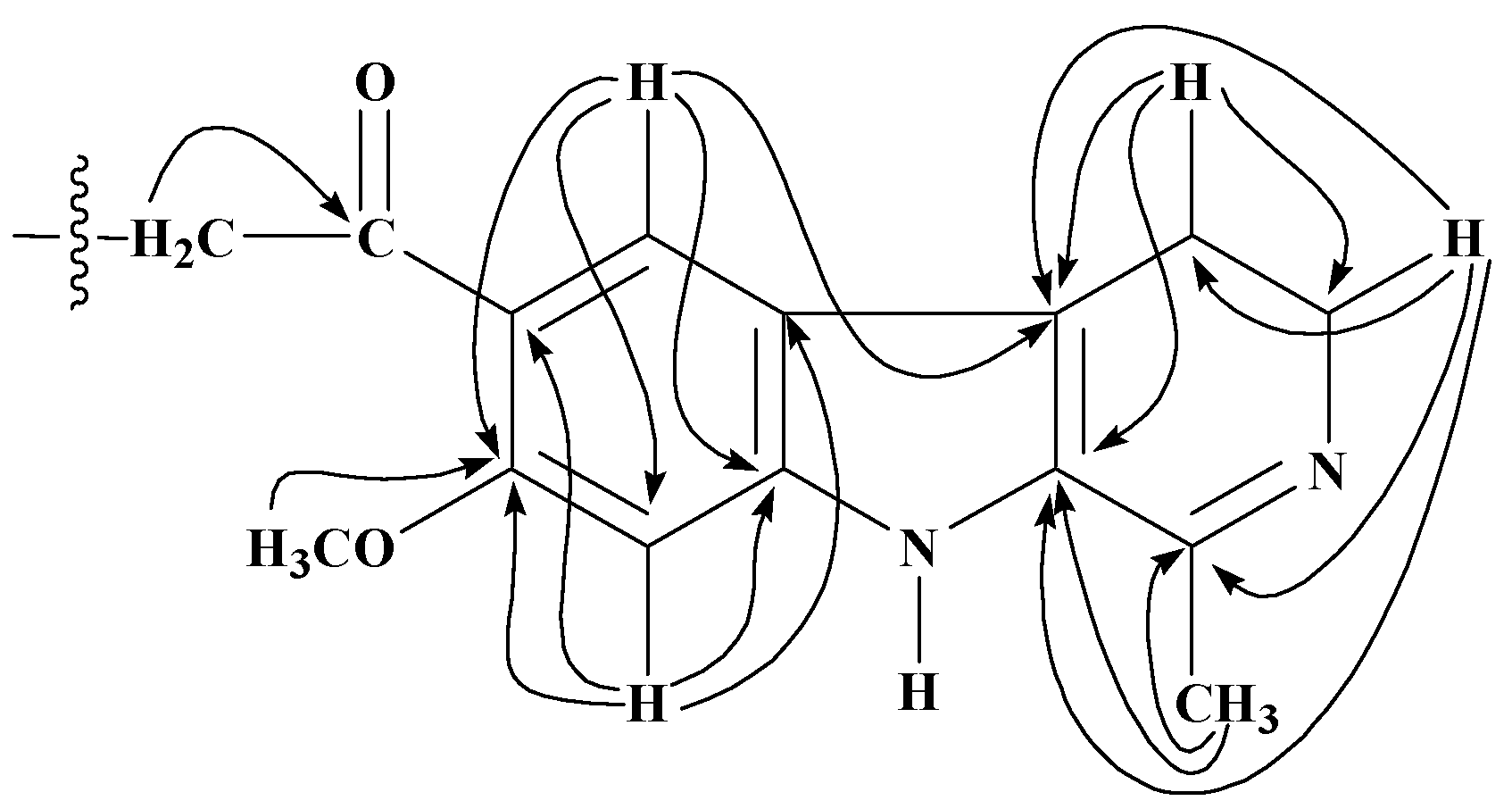
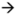 13C) interactions of 12-acyl analogues of 1.
13C) interactions of 12-acyl analogues of 1.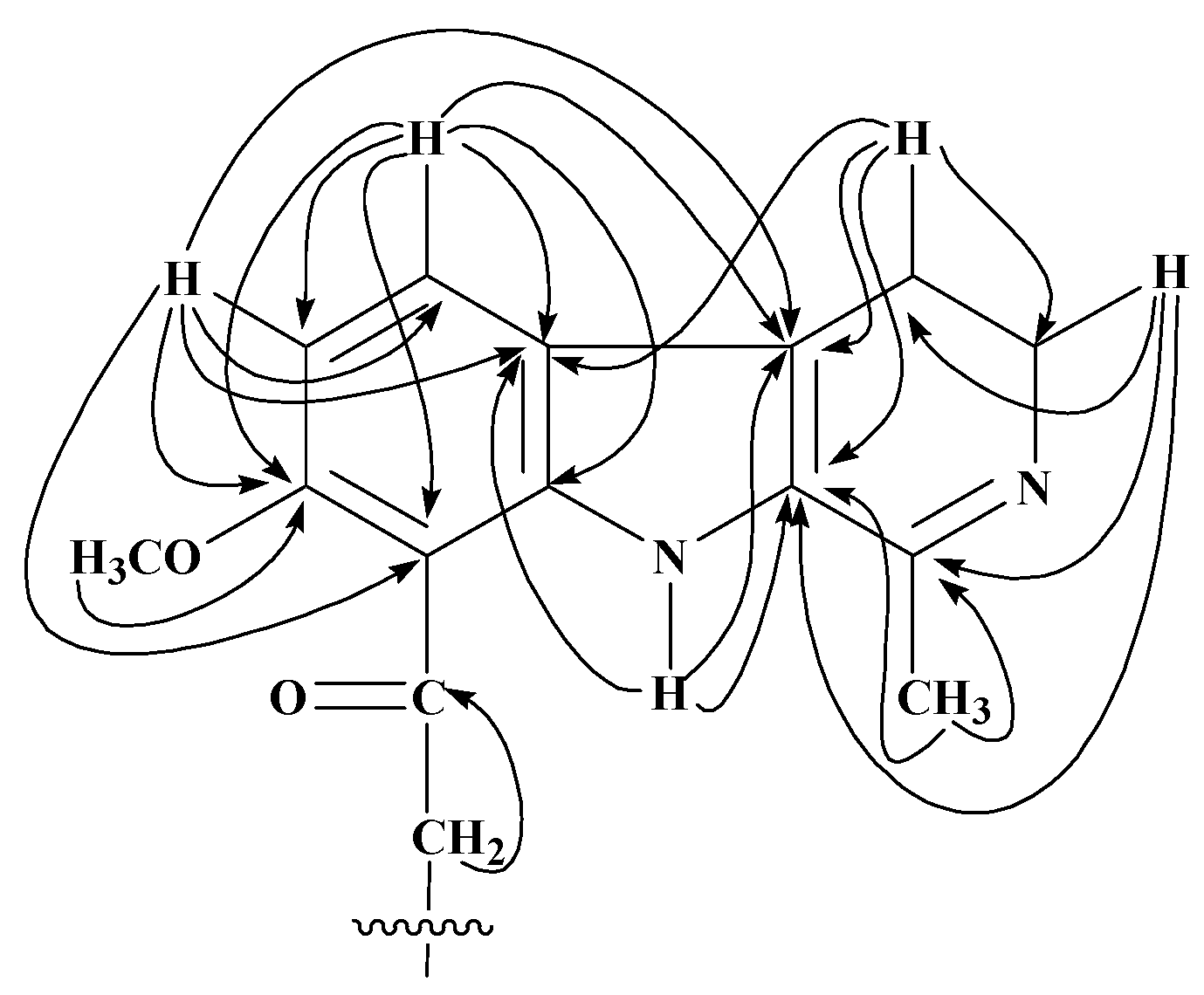
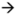 13C) interactions of N-acylated tryptamine analogues obtained from 1.
13C) interactions of N-acylated tryptamine analogues obtained from 1.
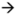 13C) interactions of N-acylated tryptamine analogues obtained from 1.
13C) interactions of N-acylated tryptamine analogues obtained from 1.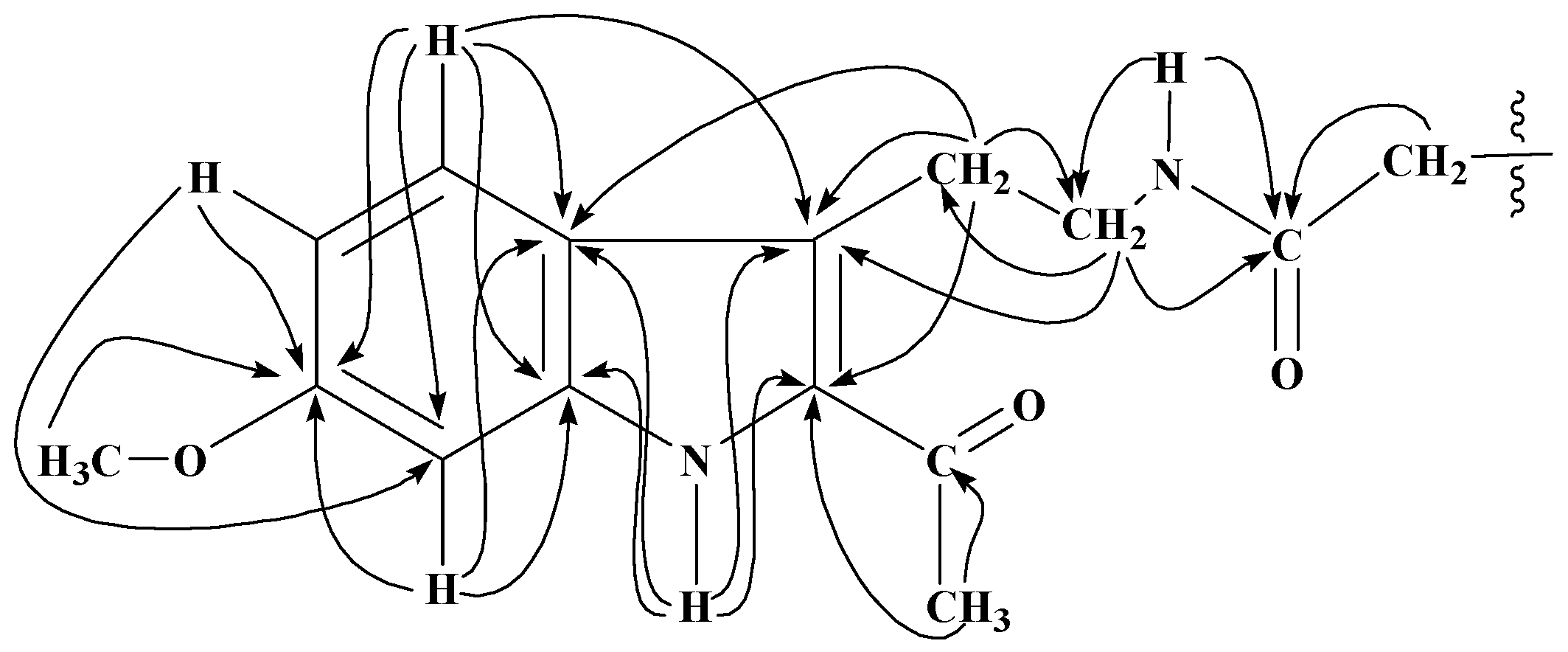
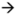 13C) interactions of 8, N-diacyl analogues of tryptamine obtained from 1.
13C) interactions of 8, N-diacyl analogues of tryptamine obtained from 1.
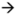 13C) interactions of 8, N-diacyl analogues of tryptamine obtained from 1.
13C) interactions of 8, N-diacyl analogues of tryptamine obtained from 1.